
Artificial Intelligence and Numerical Methods Aided Design of
Patient-Specific Coronary Stents
William Solórzano-Requejo
1,2 a
, Carlos Aguilar
1b
, Rodrigo Zapata Martínez
1c
,
Oscar Contreras-Almengor
3d
, Isabel Moscol
2e
, Carlos Ojeda
2f
, Jon Molina-Aldareguia
1,3 g
and Andrés Díaz Lantada
1h
1
ETSI Industriales, Universidad Politécnica de Madrid, Madrid, Spain
2
Department of Mechanical and Electrical Engineering, Universidad de Piura, Piura, Peru
3
IMDEA Materials Institute, Getafe, Spain
Keywords: Machine Learning, Computational Design, Personalized Medicine, Automated Design, Additive
Manufacturing.
Abstract: The design of personalized medical devices, which are adapted to the patient’s needs, starts from a digital
model created from the advanced use of clinical imaging techniques such as magnetic resonance imaging or
computed tomography. However, this methodology has several sources of error related to the medical imaging
acquisition, segmentation and reverse engineering process, tessellation, and the selected additive
manufacturing technique. Therefore, this paper proposes a new design strategy that avoids medical image
segmentation. To demonstrate its feasibility, a patient-specific coronary stent was designed and manufactured
based on slices similar to medical images. Using artificial intelligence algorithms and numerical methods, the
ellipse that best fit the patient’s artery was obtained, and finally customized stent was generated from the
parameterization of unit cells, demonstrating that it is possible to semi-automate the design of biodevices by
removing some sources of error inherent to the conventional workflow.
1 INTRODUCTION
Cardiovascular diseases have become an increasingly
serious threat to human life. In particular, coronary
artery disease is a major health and economic burden,
and the third leading cause of death worldwide (Scafa
Udriște et al., 2021). It develops when fatty
substances, such as fat, calcium, and cellular debris,
are deposited in the arterial wall obstructing blood
flow and triggering an inadequate supply of oxygen
and nutrients to the myocardium, potentially inducing
a heart attack, brain haemorrhage or ischemic stroke
(Pan et al., 2021).
a
https://orcid.org/0000-0002-2989-9166
b
https://orcid.org/0000-0003-0291-3041
c
https://orcid.org/0000-0002-2611-7050
d
https://orcid.org/0000-0002-8166-4161
e
https://orcid.org/0000-0001-8959-9547
f
https://orcid.org/0000-0001-6163-5382
g
https://orcid.org/0000-0003-3508-6003
h
https://orcid.org/0000-0002-0358-9186
The most common treatment is the percutaneous
coronary intervention (PCI), a minimally invasive
and effective surgery used to clean the artery by
introducing a tubular metallic structure (stent) inside
it, whose objective is to restore the cardiovascular
system function through the expansion of the arterial
wall, decreasing the risk of restenosis (Saçlı et al.,
2018).
Top-class coronary stent must have mechanical
properties like high elasticity and flexibility to allow
coiling and expansion in the blood vessel; high radial
and fatigue strength to withstand periodic
physiological loads; good biocompatibility to reduce
the incidence of thrombosis and restenosis and good
Solórzano-Requejo, W., Aguilar, C., Zapata Martínez, R., Contreras-Almengor, O., Moscol, I., Ojeda, C., Molina-Aldareguia, J. and Diaz Lantada, A.
Artificial Intelligence and Numerical Methods Aided Design of Patient-Specific Coronary Stents.
DOI: 10.5220/0011639000003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 37-45
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
37
radiopacity on fluoroscopy for long-term follow-up
(Cockerill et al., 2021; Scafa Udriște et al., 2021;
Tomberli et al., 2018). Stents are prevalently
manufactured with standard dimensions and shapes.
Surgeons select the one that best fits the patient’s
anatomy. However, mismatch between the implant
and the anatomy affects its performance and lifespan.
Follow-up studies have proven that, one year after
implantation, the probability of restenosis is up to
40%, and around 10% of patients need a new
prosthesis (Pan et al., 2021; Schillinger et al., 2007).
This is the consequence of geometrical changes in a
coronary artery after surgery due to the difference in
size between the expanded stent and the blood vessel.
If the stent does not fit to the diseased artery, a strong
interaction force will be generated resulting in stress
concentration, damaging the artery, and eventually
leading to restenosis (Wang et al., 2018). This
pathology is a major challenge; therefore, the
combination of medical and mechanical design can
improve significantly the function of vascular
prostheses to meet the needs of patients and hospitals
worldwide.
Current trends around the production and design
of personalized medical devices have integrated
clinical information about the patient’s anatomy,
ranging from single-unit production using manual
techniques to fully automated manufacturing with a
rapid, non-invasive and more accurate approach
(Paxton et al., 2022).
Soft tissue reconstruction from computed
tomography (CT) or magnetic resonance (MR) slices
provides an additional view of the patient’s
pathological state that helps to optimize stent fitting.
This whole process, including the reconstruction,
personalized design, and manufacturing to get the
final cardiovascular device entails a high
computational cost and has several sources of error
(Díaz Lantada et al., 2022).
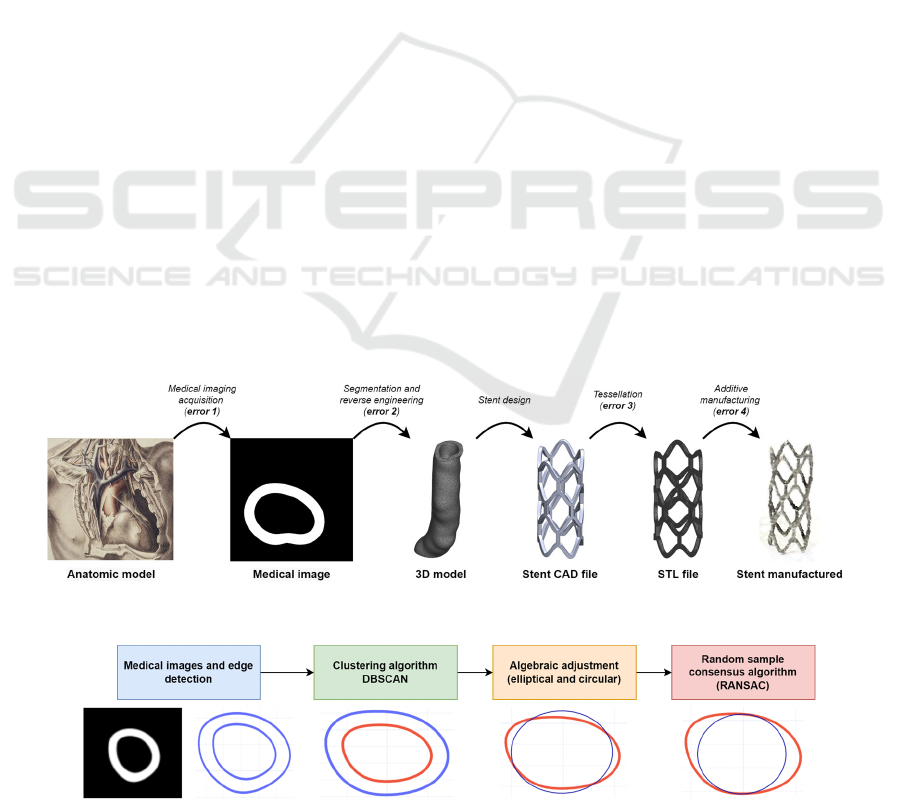
Around the slice acquisition, as consequence of
the resolution (pixels) and the thickness of the slices,
an error occurs because to reconstruct the 3D model
the designer must properly select the voxels that
compose it (segmentation). Then, it will be exported
as an STL file, which gives rise to the second error
resulting from the separation between voxels and
triangular facets due to the action of smoothing tools.
This digital artery allows the designer to model the
custom medical device and export it as an STL file,
generating the third error due to the tessellation
process (Figure 1).
Custom-made implants are generally produced
with additive manufacturing (AM) technology.
Depending on the type of AM, different layer heights
are required, creating a staircase effect. The error
associated with this effect is less significant compared
to the medical imaging acquisition, due to the
differences in the magnitudes of the layer thickness,
however, this generates the fourth source of error
(Figure 1).
All the errors described affect the final product.
Although the inaccuracy associated with medical
image and the selected manufacturing process is not
easy to avoid, the one related to segmentation can be
mitigated by capturing the patient’s physiologic
information from the image, using an algorithm that
extracts its edge and allows the designer to model on
it. This is the objective of this paper, focused on
Figure 1: Patient-specific stent workflow errors.
Figure 2: Roadmap.
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
38

coronary stents, which integrates artificial
intelligence, numerical methods, and computer-aided
design to produce personalized implants that reduce
the possibility of failure.
2 MATERIALS AND METHODS
This section details the basics of medical image edge
extraction (2.1), inner and outer edge separation (2.2),
algebraic fitting (2.3), and enhancement employing
the random sample consensus algorithm (RANSAC,
2.4) to obtain information from tomographic slices of
a coronary artery and design a stent that matches the
patient's anatomy (Figure 2).
2.1 Medical Images and Edge Detection
This study, as a proof of concept, does not intended
to focus on CT or MR image processing due to its
high complexity. Therefore, Chitubox
®
v1.9.0
(Chitubox, Zhongcheng Future Industrial Park,
Hangcheng Avenue, Baoan District, Shenzhen,
Guangdong, China 518128), an open-source program
for manufacturing 3D models with digital light
processing (DLP) technology, was used to obtain
axial tomographic images, properly setting
parameters such as number of pixels (resolution),
printing table dimensions and layer height. When the
STL file is imported into the software, black-and-
white images are generated from bottom to top, going
through the file layer by layer, producing medical-
like slices which in this research will be considered as
medical images (Moscol et al., 2022).
Edge detection plays an important role in
determining the inner radius of the artery and
adapting the stent to it. One method to recognize the
edge consists of identifying pixels with different
intensities; however, the result was not adequately
adjusted (Figure 3). Therefore, a new edge detector,
based on the partial area effect that does not assume
continuity in pixel intensity values, was used to
achieve highly accurate extraction of edge position,
orientation and curvature in challenging conditions
such as images with noise, blurred edges, low contrast
areas or very close edges (Trujillo-Pino et al., 2013).
Figure 3: Edge detection at the (A) pixel and (B) subpixel
level.
To use this algorithm (Trujillo-Pino et al., 2013),
the medical images, a 3D matrix whose rows and
columns correspond to the number of pixels, were
converted to grayscale to get a 2D array whose pixel
intensities range from 0 to 255. In addition, three
hyperparameters were defined: the threshold, which
allows the algorithm to distinguish an edge if the
difference in intensities in two adjacent regions
exceeds 120; a zero-order filter, to detect the edge
without noise; and the order of the edge to be adjusted
(second order).
2.2 Clustering Algorithm
The coordinates of the points extracted from the edge
are represented in the pixel domain, but they must be
converted into millimetres for the biodevice design.
For this, the origin is defined in the centre of the
image and an equivalence is made between the
resolution and printing table dimensions configured
in Chitubox
®
.
The image is composed of two edges: internal and
external. However, the information required by the
designer is provided by the inner part, and to separate
them automatically the density-based spatial
clustering of application with noise (DBSCAN)
algorithm was used.
The main idea of this technique is that two points
are considered neighbours if the distance between
them is less than or equal to “eps” and the minimum
number of points to define a cluster is “min_sample”.
Hence, it is implemented by setting both
hyperparameters for the images, the values of
min_samples and eps were determined as 3 and 0.7
respectively.
2.3 Algebraic Fitting
2.3.1 Elliptical
The points of the inner edge, acquired with the
DBSCAN algorithm, allow a curve to be fitted for
Artificial Intelligence and Numerical Methods Aided Design of Patient-Specific Coronary Stents
39

automatically taking the measurement of the artery
and adjust the design to them. The ellipses, in
particular, adapt to the shapes and sizes of complex
anatomical sections (Solórzano-Requejo et al., 2022),
so the basic principles of their algebraic fitting are
detailed. The fitted ellipse is represented by an
implicit second-order polynomial (𝑄), defined by a
vector of coefficients (𝑣=[𝐴 𝐵 𝐶 𝐷 𝐸 𝐹]
):
𝑄
𝑝, 𝑣
=
[
𝑥
𝑥𝑦 𝑦
𝑥 𝑦 1
]
.
⎣
⎢
⎢
⎢
⎢
⎡
𝐴
𝐵
𝐶
𝐷
𝐸
𝐹
⎦
⎥
⎥
⎥
⎥
⎤
=0
(1)
If 𝑃=
𝑝
, 𝑝
,…,𝑝
is the set of points of the
inner edge, the vector of coefficients must be adjusted
to it, so the algebraic distance (𝐷
) is used because it
simplifies the calculations and requires less
computational resources (Fitzgibbon et al., 1999).
Mathematically, it is obtained by substituting the
coordinates of a point 𝑝
=
(
𝑥
, 𝑦
)
in the polynomial
𝑄, therefore, if 𝑝
belongs to the ellipse its distance
will be zero:
𝐷
(
𝑝
, 𝑣
)
= 𝐴𝑥
+ 𝐵𝑥
𝑦
+ 𝐶𝑦
+ 𝐷𝑥
+ 𝐸𝑦
+ 𝐹
(2)
The least-squares technique optimized the fit by
minimizing the square of the algebraic distance
between 𝑃 and the adjusted ellipse
𝑄, which can be
expressed as the squared norm of the product between
the design matrix 𝐷
, which contains information of
𝑃, and 𝑣.
𝐷
=
⎣
⎢
⎢
⎡
𝑥
𝑥
𝑦
𝑦
𝑥
𝑦
1
𝑥
𝑥
𝑦
𝑦
𝑥
𝑦
1
⋮ ⋮ ⋮ ⋮ ⋮ ⋮ ⋮
𝑥
𝑥
𝑦
𝑦
𝑥
𝑦
1
⎦
⎥
⎥
⎤
(3)
𝑚𝑖𝑛𝐷
(
𝑝
, 𝑣
)
= 𝑚𝑖𝑛
‖
𝐷
𝑣
‖
(4)
To avoid the trivial solution of 𝑣 = 0
, 𝑣 is
bounded with a constraint of
‖
𝑣
‖
= 1, preventing
that all coefficients are zero (Paton, 1970). Lagrange
multipliers allow to minimize the distance
considering this condition:
𝐿= 𝑣
𝐷
𝐷
𝑣 − 𝜆(𝑣
𝑣−1)
(5)
To minimize 𝐿, its gradient with respect to 𝑣 is set to 0:
𝛻
𝐿=0 ⟺ 2𝐷
𝐷
𝑣 −2𝜆𝑣=0
(6)
𝐷
𝐷
𝑣 =𝜆𝑣
(7)
Equation (7) leads to the eigenvector problem,
then λ and 𝑣 must be an eigenvalue and eigenvector
of 𝐷
𝐷
. If 𝐷
𝐷
𝑣= 𝜆𝑣, equation (4) will be:
𝑚𝑖𝑛 𝑣
𝐷
𝐷
𝑣 =𝑚𝑖𝑛 𝜆
‖
𝑣
‖
= 𝑚𝑖𝑛 𝜆
(8)
Consequently, the coefficient vector ( 𝑣) that
minimizes the distance will be the eigenvector of
𝐷
𝐷
corresponding to the smallest eigenvalue (𝜆).
2.3.2 Circular
For cardiovascular prosthesis, it may be more
interesting to fit a circle to the inner wall of blood
vessels. Therefore, this subsection describes the
theory related to its algebraic fitting (Černov, 2011).
Starting from the general equation of the circle (𝑓)
and developing it:
𝑓=
[
𝑥
𝑥𝑦 𝑦
𝑥 𝑦 1
]
.
⎣
⎢
⎢
⎢
⎢
⎡
1
0
1
−2𝑘
−2𝑚
𝑘
+ 𝑚
−𝑟
⎦
⎥
⎥
⎥
⎥
⎤
=0
(9)
Linking equation (1) to (9), it is determined that
𝐴= 𝐶=1, 𝐵= 𝑂, 𝐷= −2𝑘, 𝐸= −2𝑚 and 𝐹=
𝑘
+ 𝑚
−𝑟
. Therefore, 𝐷
from a point 𝑝
=
(
𝑥
, 𝑦
)
to the fitted circle is:
𝐷
(𝐷, 𝐸, 𝐹, 𝑝
)=𝑥
+ 𝑦
+ 𝐷∙𝑥
+ 𝐸∙𝑦
+ 𝐹
(10)
To find the values of 𝐷, 𝐸 and 𝐹 defining the
fitted curve, the algebraic distance squared of the “𝑛”
points composing the inner edge is minimized:
𝐷
= 𝑚𝑖𝑛
(
𝑥
+ 𝑦
+ 𝐷∙𝑥
+ 𝐸∙𝑦
+ 𝐹
)
(11)
For this purpose, 𝐷
is partially derived with
respect to 𝐷, 𝐸 and 𝐹, equating to 0 and rewritten in
matrix form:
⎣
⎢
⎢
⎢
⎢
⎡
𝑥
𝑥
∙𝑦
𝑥
𝑥
∙𝑦
𝑦
𝑦
𝑥
𝑦
𝑛
⎦
⎥
⎥
⎥
⎥
⎤
.
𝐷
𝐸
𝐹
=
⎣
⎢
⎢
⎢
⎢
⎡
−
(
𝑥
+ 𝑦
)
∙𝑥
−
(
𝑥
+ 𝑦
)
∙𝑦
−
(
𝑥
+ 𝑦
)
⎦
⎥
⎥
⎥
⎥
⎤
(12)
Solving the linear system, the coefficients of the
fitted circumference are obtained.
2.4 RANSAC Algorithm
The random sample consensus algorithm
(RANSAC), published by Fischler and Bolles
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
40
(Fischler & Bolles, 1981), is an iterative method for
estimating the parameters of a mathematical model
from a dataset containing outliers. RANSAC
proposes to create a cost function that sums the
distance of the points to the fitted curve and
iteratively select some of them to fit again and choose
the one that produces the lowest cost.
Due to the iterative nature of the algorithm, it is
not deterministic, and the model obtained may not be
the best. Nevertheless, it is a useful tool to integrate
with the elliptical and circular fitting, presented in the
previous sections, since in personalized implant
design it is required that the fitted curve adapts as well
as possible to the inner edge without exceeding it. If
only the algebraic fitting is used, the result does not
meet this condition, so it is necessary to integrate this
procedure to RANSAC (Figure 2).
Compared to the algebraic fitting that squares the
distance to make the function convex and ensure that
a minimum exists, the modified RANSAC algorithm
takes advantage of the sign of the algebraic distance
(cost function) to determine whether the adjusted
curve is inside or outside the edge. This innovate
approach is interesting and can be applied to image
processing and computer vision tasks.
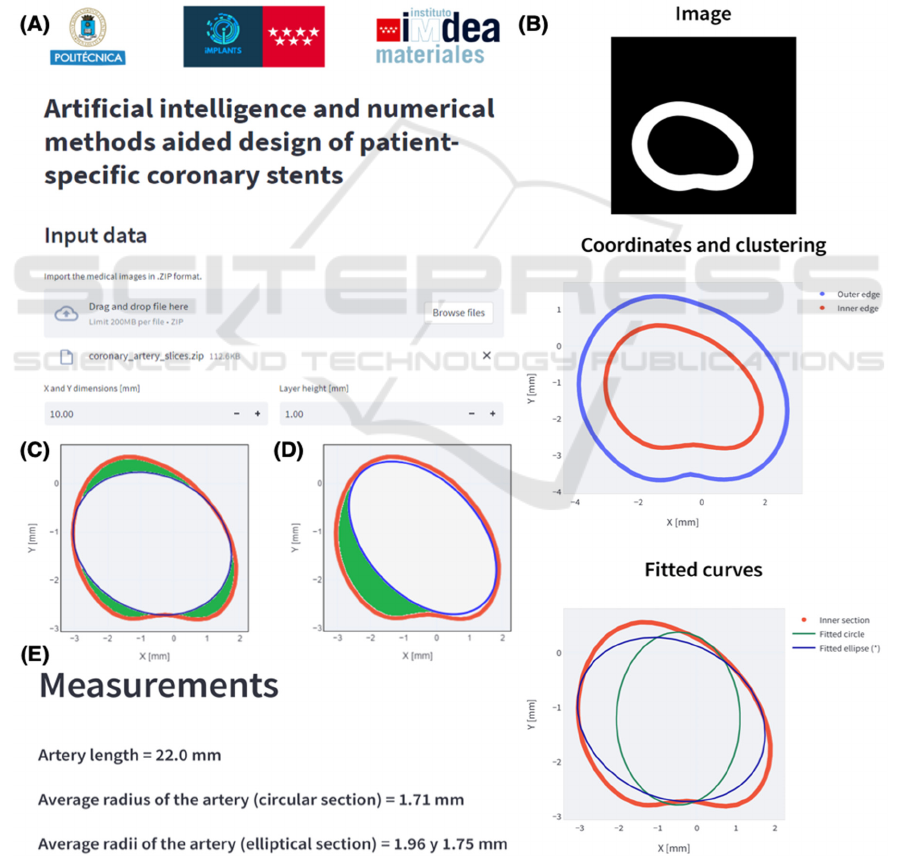
Figure 4: (A) Web application. (B) Coronary artery slice fits. Comparison between (C) the fit provided by the application and
(D) another possible solution. (E) Output of the web application.
Artificial Intelligence and Numerical Methods Aided Design of Patient-Specific Coronary Stents
41
3 RESULTS AND DISCUSSION
3.1 Web Application and Virtual
Model
In order to integrate the explained algorithms and to
encourage the symbiotic use of computer aided
design, artificial intelligence and numerical methods
in the design of personalized biodevices, a web app
has been developed with the Streamlit library
(Streamlit, n.d.) of Python
®
3.7.14 (Phyton Software
Foundation) as it allows the deployment of apps in a
simple way.
As mentioned above, two different approaches
were used to assist the parametric design of
personalized coronary stents. The circular algebraic
fitting which, in most cases, does not appropriately
adjust the patient’s coronary topology and a new
innovative and more suitable approach, the elliptical
one.
To prove the effectiveness of both methods, a
virtual STL model of a patient's artery has been used.
The STL file (Model ID 3DPX-012589), based on a
CT scan and segmented by researchers at the
University of Toronto and Toronto General Hospital,
has been downloaded from the NIH 3D Print
Exchange repository (Phantom Coronary Artery
Models | NIH 3D Print Exchange, n.d.) and imported
into Chitubox
®
by setting up the resolution, print plate
dimensions and layer height as 512 512 pixels,
101022 mm and 1 mm respectively. Finally, the
3D model was sliced, and each image has been stored
in a general .ZIP file.
The .ZIP archive has been uploaded in the web
application (Figure 4A) by introducing the
parameters configured in Chitubox
®
to transform
pixel into coordinates and compute the algebraic
distance, used as cost function to get the circle or
ellipse that best fits the arterial wall to cause minimal
long-term restenosis (Figure 4C & D). The app
outputs, for each slice, the circular and elliptical
algebraic fitting (Figure 4B), the average radius or
radii and length of the artery, parameters that will be
introduced into the parametric design (Figure 4E).
3.2 Parametric Design of Coronary
Stents
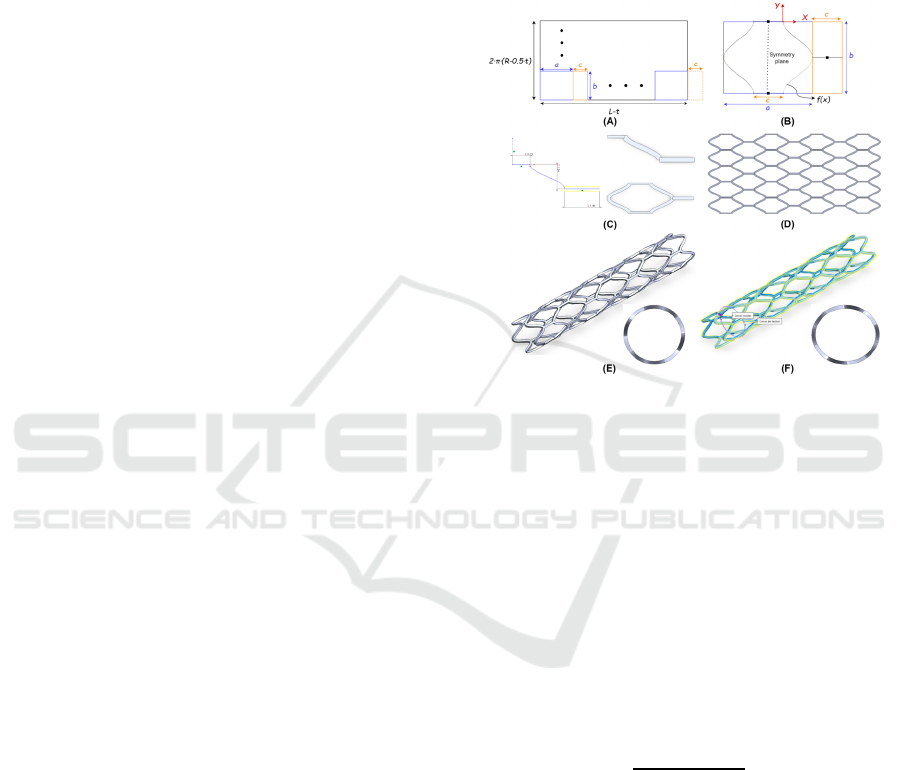
The stent designs are based on a geometry unit cell
(Figure 5C) that is repeated bidirectionally, resulting
in a 2D mesh (Figure 5D) that folds back on itself to
form the stent (Figure 5E) and then deforms to obtain
the elliptical cross-section (Figure 5F). The modelling
of these unit cells is parameterised according to the
web app outputs, the average radius (𝑅) and length
( 𝐿), in addition, for the elliptical adjustment, the
major (𝑅
) and the minor average radius (𝑅
). The
number of unit cell repetitions (𝑁
and 𝑁
) in the
transversal (𝑋) and radial (𝑌) axes, and the thickness
(𝑡) can be modified as the designer requires.
Figure 5: (A) Macroscopic and (B) microscopic view of
coronary stent model. (C) Unit cell and (D) mesh
construction. (E) Circular and (F) elliptical parametrized
stent.
As an example, the entire parametrized design
workflow can be seen in Figure 5. Macroscopically,
the unit cell, in most cases, is a 𝑎𝑏 rectangle that
will repeat 𝑁
and 𝑁
times in the 𝑋 and 𝑌 axis,
respectively. When figuring 𝑎 and 𝑏 measures, the
thickness of the stent must be considered. In this
example, unit cells connectors must be also taken into
consideration, resulting in a
(
𝑎+ 𝑐
)
𝑏 rectangle.
Therefore, the width of the mesh will be 2 𝜋
(
𝑅−0.5 ⋅𝑡
)
and the dimension of 𝑏:
𝑏=
2𝜋
(
𝑅−0.5𝑡
)
𝑁
(13)
The relation between the mesh and unit cell length is
(Figure 5A):
𝐿−𝑡= 𝑁
(
𝑎+ 𝑐
)
−𝑐
(14)
Microscopically, the unit cell has a specific shape
bounded by the 𝑎𝑏 rectangle, hence, the
mathematical relation between macroscopic and
microscopic parameters must be established. To
simplify the parametrization, the length of the unit
cell ( 𝑎) and the connector length (𝑐) was set as
follows (Figure 5B):
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
42

𝑐=
𝑎
3
(15)
Introducing (15) into (14), the value of 𝑎 is defined as
a function of 𝐿, 𝑁
and 𝑡:
𝑎=
𝐿−𝑡
4
3
𝑁
−
1
3
(16)
The curve 𝑓
(
𝑥
)
used in the unit cell follows the
sinusoidal equation parametrized as a function of 𝑏
and 𝑐. Where
is the amplitude and the vertical
offset,
the period and
the phase shift (Figure 5B).
𝑓
(
𝑥
)
=
𝑐
2
⋅sin
2𝜋
𝑏
𝑥−
𝑏
4
+
𝑐
2
;
𝑥 ∈
[
0, 0.5𝑏
]
(17)
This geometry has been designed and
parametrized in Solidworks
®
2021 (Dassault
Systèmes SolidWorks Corporation, UK). To validate
its suitability, the web outputs has been introduced in
the parametric file described above where 𝑁
, 𝑁
and
𝑡 has been set as 4, 5 and 0.2 mm respectively. Then,
the patient-specific stent has been exported in STL
format to manufacture prototypes using AM
techniques such as stereolithography (Figure 6B),
selective laser sintering (Figure 6C) and selective
laser melting (Figure 6D).
Figure 6: (A) Library of parameterized coronary stents.
Prototypes manufactured by (B) stereolithography, (C)
selective laser sintering and (D) melting.
The main objective of this methodology is to
promote the creation of a parametrized coronary stent
library (Figure 6A). Therefore, physicians will have
several options to select the one that best suits to the
clinical and mechanical patient needs (Martínez
Cendrero et al., 2022). Moreover, it will ensure a
more personalized and simplified workflow by
directly using the medical image and avoiding its
segmentation.
4 CONCLUSIONS
The increasing demand of personalized biomedical
solutions poses a major healthcare challenge. This
proposal is a useful tool that aids custom stent
modelling by adapting its cross-section to the internal
artery cavity improving its fit and filling.
Furthermore, this proof-of-concept avoids
segmentation, as the arterial edge is extracted directly
from the medical image, reducing the inherent errors
of conventional workflow.
The integration of artificial intelligence and
numerical methods in the web application has shown
that elliptical sections are better adapted to the
coronary artery and, from these fitted curves patient-
specific stents can be designed. The web application
transforms the designer`s work into an inspector to
ensure that the solution proposed by the application
meets quality standards.
The main problems of personalized medicine are
time-consumption and high economic cost.
Therefore, the web application and parametric
models, automatization tools, could be the solution
related to the first issue, as they reduce the delivery
times being the personalized protheses competitive in
emergency situations. Moreover, by using 3D
printing, the price of personalized medical devices
will be decreased significantly. Consequently, the
combination of medical imaging, automatization
tools and 3D printing could lead to new competitive,
affordable, and accessible high-quality patient-
specific implants.
5 FUTURE LINES
To balance the medical demand for personalized
solutions, advances in computer vision,
computational design and artificial intelligence will
be critical to achieve full automatization of the
implant’s workflow (Figure 1). This will help
designers produce a more aesthetic morphology and
precise fit, as well as a higher level of customization
and a comforting patient experience. This symbiosis
of computational, mechanical, and biomedical
Artificial Intelligence and Numerical Methods Aided Design of Patient-Specific Coronary Stents
43

technologies will result in a new generation of
customized prostheses that will improve clinical
outcomes for millions of patients worldwide. This
new methodology could be extrapolated to many
clinical cases, such as aortic valves and hip prostheses
(Solórzano Requejo et al., 2022).
ACKNOWLEDGEMENTS
The research presented has been supported by the
project: "iMPLANTS-CM: impresión de
metamateriales empleando aleaciones con memoria
de forma y gradientes funcionales para una nueva
generación de implantes inteligentes", funded by the
"Convocatoria 2020 de ayudas para la realización de
proyectos sinérgicos de I+D en nuevas y emergentes
áreas científicas en la frontera de la ciencia y de
naturaleza interdisciplinar" financed by Comunidad
Autónoma de Madrid (ref. del proyecto: Y2020/BIO-
6756).
The authors express their gratitude to Mar
Cogollo, Adrián Martinez, Francisco Franco, Miguel
Clavijo, Pedro Ortego, Javier Tuesta, Nicolás Kuroki,
Leandro Velásquez and Daira Mena for inspiring and
supporting us to conduct research that benefits
society.
REFERENCES
Černov, N. (2011). Circular and linear regression: Fitting
circles and lines by least squares. CRC.
Cockerill, I., See, C. W., Young, M. L., Wang, Y., & Zhu,
D. (2021). Designing Better Cardiovascular Stent
Materials: A Learning Curve. Advanced Functional
Materials, 31(1), 2005361. https://doi.org/10.1002/
adfm.202005361
Díaz Lantada, A., Solórzano, W., Martínez Cendrero, A.,
Zapata Martínez, R., Ojeda, C., & Munoz-Guijosa, J.
M. (2022). Methods and Technologies for the
Personalized Design of Open-Source Medical Devices.
In A. Ahluwalia, C. De Maria, & A. Díaz Lantada (Eds.),
Engineering Open-Source Medical Devices: A Reliable
Approach for Safe, Sustainable and Accessible
Healthcare (pp.191–218). Springer International Pu-
blishing.https://doi.org/10.1007/978-3-030-79363-0_9
Fischler, M. A., & Bolles, R. C. (1981). Random sample
consensus: A paradigm for model fitting with
applications to image analysis and automated
cartography. Communications of the ACM, 24(6), 381–
395. https://doi.org/10.1145/358669.358692
Fitzgibbon, A., Pilu, M., & Fisher, R. B. (1999). Direct least
square fitting of ellipses. IEEE Transactions on Pattern
Analysis and Machine Intelligence, 21(5), 476–480.
https://doi.org/10.1109/34.765658
Martínez Cendrero, A., Franco Martínez, F., Solórzano
Requejo, W. G., & Díaz Lantada, A. (2022). Open-
source library of tissue engineering scaffolds. Materials
& Design, 223, 111154. https://doi.org/10.1016/
j.matdes.2022.111154
Moscol, I., Solórzano-Requejo, W., Ojeda, C., &
Rodríguez, C. (2022). Personalized Hip Replacement:
State of the Art and New Tools Proposals: Proceedings
of the 15th International Joint Conference on
Biomedical Engineering Systems and Technologies,
46–57. https://doi.org/10.5220/0010823100003123
Pan, C., Han, Y., & Lu, J. (2021). Structural Design of
Vascular Stents: A Review. Micromachines, 12(7),
770. https://doi.org/10.3390/mi12070770
Paton, K. (1970). Conic sections in chromosome analysis.
Pattern Recognition, 2(1), 39–51. https://doi.org/10.
1016/0031-3203(70)90040-3
Paxton, N. C., Nightingale, R. C., & Woodruff, M. A.
(2022). Capturing patient anatomy for designing and
manufacturing personalized prostheses. Current
Opinion in Biotechnology, 73, 282–289. https://doi.org/
10.1016/j.copbio.2021.09.004
Phantom Coronary Artery Models | NIH 3D Print
Exchange. (n.d.). Retrieved 11 April 2022, from
https://3dprint.nih.gov/discover/3dpx-012589
Saçlı, H., Kara, İ., & Kırali, M. K. (2018). Focus on
Coronary Atherosclerosis. In L. Gianturco (Ed.),
Atherosclerosis—Yesterday, Today and Tomorrow (1st
ed.). InTech. https://doi.org/10.5772/intechopen.77301
Scafa Udriște, A., Niculescu, A.-G., Grumezescu, A. M., &
Bădilă, E. (2021). Cardiovascular Stents: A Review of
Past, Current, and Emerging Devices. Materials,
14(10), 2498. https://doi.org/10.3390/ma14102498
Schillinger, M., Sabeti, S., Dick, P., Amighi, J., Mlekusch,
W., Schlager, O., Loewe, C., Cejna, M., Lammer, J., &
Minar, E. (2007). Sustained Benefit at 2 Years of
Primary Femoropopliteal Stenting Compared With
Balloon Angioplasty With Optional Stenting.
Circulation, 115(21), 2745–2749. https://doi.org/
10.1161/CIRCULATIONAHA.107.688341
Solórzano Requejo, W., Martínez Cendrero, A., Aguilar,
C., Zapata Martínez, R., Ojeda, C., & Díaz Lantada, A.
(2022). Diseño de dispositivos médicos personalizados
asistido por inteligencia artificial y métodos numéricos:
Aplicación a prótesis articulares. Congreso
Iberoamericano de Ingeniería Mecánica-CIBIM 2022.
XV Congreso Iberoamericano de Ingeniería Mecánica.
https://doi.org/10.5944/bicim2022.094
Solórzano-Requejo, W., Ojeda, C., & Díaz Lantada, A.
(2022). Innovative Design Methodology for Patient-
Specific Short Femoral Stems. Materials, 15(2), Article
2. https://doi.org/10.3390/ma15020442
Streamlit. (n.d.). Retrieved 3 October 2022, from https://
streamlit.io/
Tomberli, B., Mattesini, A., Baldereschi, G. I., & Di Mario,
C. (2018). Breve historia de los stents coronarios.
Revista Española de Cardiología, 71(5), 312–319.
https://doi.org/10.1016/j.recesp.2017.11.016
Trujillo-Pino, A., Krissian, K., Alemán-Flores, M., &
Santana-Cedrés, D. (2013). Accurate subpixel edge
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
44

location based on partial area effect. Image and Vision
Computing, 31(1), 72–90. https://doi.org/10.1016/
j.imavis.2012.10.005
Wang, Q., Fang, G., Zhao, Y.-H., & Zhou, J. (2018).
Improvement of Mechanical Performance of
Bioresorbable Magnesium Alloy Coronary Artery
Stents through Stent Pattern Redesign. Applied
Sciences, 8(12), 2461. https://doi.org/10.3390/app812
2461
Artificial Intelligence and Numerical Methods Aided Design of Patient-Specific Coronary Stents
45
