
Hemodynamics of Convergent Cavopulmonary Connection with
Ventricular Assist Device for Fontan Surgery: A Computational and
Experimental Study
Qiyuan Wu
1
, Vincent Cleveland
2
, Seda Aslan
1
, Xiaolong Liu
1
, Jacqueline Contento
2
, Paige Mass
2
,
Byeol Kim
1
, Catherine Pollard
1
, Pranava Sinha
3
, Yue-Hin Loke
2,4
, Laura Olivieri
2,5
and Axel Krieger
1
1
Department of Mechanical Engineering, Johns Hopkins University, Baltimore, MD, U.S.A.
2
Sheikh Zayed Institute of Pediatric Surgical Innovation, Children’s National Hospital, Washington, DC, U.S.A.
3
Department of Pediatric Cardiac Surgery, M Health Fairview University of Minnesota, Minneapolis, MN, U.S.A.
4
Division of Cardiology, Children’s National Hospital, Washington DC, U.S.A.
5
Division of Pediatric Cardiology, University of Pittsburgh Medical Center, Pittsburgh, PA, U.S.A.
{jcontento, pmass}@childrensnational.org, {bkim95, cpollar9}@jhu.edu, sinha228@umn.edu,
YLoke@childrensnational.org, olivierilj@upmc.edu, axel@jhu.edu
Keywords:
Convergent Cavopulmonary Connection, Ventricular Assist Device, Single Ventricle Heart Disease,
Computational Fluid Dynamics.
Abstract:
Fontan surgery is the clinical standard for single ventricle heart disease, with total cavopulmonary connection
(TCPC) as the current preferred configuration. Mechanical circulatory support (MCS) is often desired to im-
prove hemodynamics and reduce post-surgical complications. Convergent cavopulmonary connection (CCPC)
was recently proposed to solve the difficulty of integrating MCS in TCPC. In this study, we investigated the
hemodynamics of the CCPC conduit with a ventricular assist device (VAD) integrated and explored indexed
power jump (iPJ) and time-averaged wall shear stress (TAWSS) by computational fluid dynamics (CFD) with
assistance from flow loop experiments. Positive time-averaged iPJ was observed in the cases with limited
cardiac output, and regions with non-physiologic low TAWSS were significantly reduced for all cases. These
results could strengthen the feasibility of this novel CCPC Fontan configuration as a solution for MCS inte-
gration.
1 INTRODUCTION
Surgical management of patients with single ventri-
cle heart disease culminates into Fontan surgery. This
operation establishes passive pulmonary blood flow
by directing systemic venous blood flow to the pul-
monary artery (PA) bypassing the heart. The to-
tal cavopulmonary connection (TCPC) is the cur-
rent preferred Fontan configuration, which passively
routes venous flow into the pulmonary arteries. Al-
though life-saving, the Fontan operation has sub-
optimal long-term outcomes, including heart failure
(d’Udekem et al., 2014), protein losing enteropa-
thy (Atz et al., 2017), decreased exercise toler-
ance (Kempny et al., 2012), pulmonary arteriovenous
malformations (AVMs) (Pike et al., 2004), chronic
cyanosis (Deal and Jacobs, 2012), and increased risk
for venous thrombosis and stroke. Adding mechani-
cal circulatory support (MCS), such as a ventricular
assist device (VAD), can improve the hemodynam-
ics, reduce post-surgical complications, and lengthen
the lifespan of selected Fontan patients (Cedars et al.,
2021). However, MCS integration in TCPC is
anatomically challenging, and sometimes impossible,
due to the opposite directed inflows of the superior
vena cava (SVC) and inferior vena cava (IVC) and the
perpendicular outflows to the left and right pulmonary
arteries.
Long-term outcomes (Shah et al., 1997; Trusty
et al., 2018) of Fontan surgery such as pulmonary ar-
teriovenous malformations (PAVM) (Shinohara and
Yokoyama, 2001), decreased exercise capacity, un-
derdeveloped PA, and thrombosis are linked to hemo-
dynamics in postoperative Fontan geometries (Shino-
Wu, Q., Cleveland, V., Aslan, S., Liu, X., Contento, J., Mass, P., Kim, B., Pollard, C., Sinha, P., Loke, Y., Olivieri, L. and Krieger, A.
Hemodynamics of Convergent Cavopulmonary Connection with Ventricular Assist Device for Fontan Surgery: A Computational and Experimental Study.
DOI: 10.5220/0011633200003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 3: BIOINFORMATICS, pages 51-58
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
51

Figure 1: Schematic workflow of this study. (a) Anatomy of native TCPC Fontan, anterior view; (b) 3D digital model of
TCPC; (c) Design of CCPC Fontan; (d) Simplified internal geometry of VAD-integrated CCPC; (e) CFD simulation.
hara and Yokoyama, 2001). It has been demonstrated
that power loss (PL) is related to exercise capacity
(Khiabani et al., 2015) and hepatic flow distribution
(HFD) is related to the development of PAVM (Vet-
tukattil, 2002; Trusty et al., 2019), while wall shear
stress (WSS) is linked with thrombosis risk (Hath-
cock, 2006).
Since these performance characteristics vary with
shape, our group has reported a novel configuration of
the Fontan called CCPC, and published that this con-
nection was feasible in 3D simulations in a variety of
patient sizes, with improvements in HFD as well as
provision of a large surgical target for VAD insertion
(Sinha et al., 2022). The CCPC graft was designed to
avoid the momentum counteraction between IVC and
SVC inflows since they have totally opposite direc-
tions in TCPC, and to create an access point of MCS
within a single inflow-single outflow system, thus in-
creasing Fontan efficiency and aiding in MCS incor-
poration. Based on the novel configuration of CCPC,
a VAD can be integrated into the Fontan graft, with an
occlusion plug preventing local recirculation around
the VAD and holding the device in position. With in-
tegrated VAD, flow from IVC and SVC is fully mixed
and HFD would be more balanced following the out-
flow distribution. But power jump/loss and WSS have
not been studied for VAD integrated CCPC Fontans.
Computational Fluid Dynamics (CFD) has been
applied to analyze the postoperative hemodynamic
performance of patient-specific grafts (Aslan et al.,
2022; Liu et al., 2022c; Liu et al., 2022b). Compared
with in vitro experiments and in vivo measurement
such as 4D MRI, CFD provides detailed full profile of
the flow field, which would not only create more de-
tailed results, but also allow explainable analysis over
flow patterns. Though modern CFD methods and sim-
ulation techniques have good accuracy and resolution,
the reliability of CFD simulation is dependent on the
accuracy of boundary conditions and model assump-
tions. In vitro experiments have been used to validate
the fidelity of simulations (Liu et al., 2022a).
In this study, we created VAD integrated CCPC
graft models on three patients with relatively small,
medium and large cardiac output. Hemodynamics of
VAD integrated graft were studied by CFD, to pre-
dict the indexed power jump (iPJ) and time averaged
wall shear stress (TAWSS), along with in vitro exper-
iments performed as comparison. This work studies
the local hemodynamics and evaluates the feasibility
of integrating VADs within Fontan grafts.
2 METHODS
2.1 3D Modeling of the Original Fontan
Cardiovascular magnetic resonance (CMR) imaging
was used to acquire cardiac geometry of three sin-
gle ventricle patients. Contrast-enhanced magnetic
resonance angiography (MRA) acquired in the late
phase with spatial resolution 1.4 x 1.4 mm and phase
contrast imaging for the cavae (SVC, IVC) and the
pulmonary arteries (RPA, LPA) were anonymized
and proceeded for modeling the geometry of original
Fontan. Software (Mimics; Materialise, Leuven, Bel-
gium) was used for segmenting the three-dimensional
(3D) geometry of Fontan, including the SVC, IVC,
RPA and LPA. This digital Fontan model was then
made hollow and smoothed, for either CFD study or
3D printed in vitro experiments.
2.2 Convergent Cavopulmonary
Connection Conduits with the
Integration of VAD
Partnered with clinical input and constrained by
patient-specific anatomy obtained in the previous step
(Section 2.1), surgically feasible CCPC shapes were
created by iterative CAD as illustrated in our previous
work (Sinha et al., 2022). The CCPC design was di-
vided into 3 parts or so-called limbs; the superior limb
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
52
(from SVC to the common limb), the inferior limb
(from the IVC to the common limb), and the common
limb (from the convergence of the superior and infe-
rior limbs to the pulmonary arteries), with regard to
anatomical constraints imposed by the chest wall, air-
ways, lungs, and cardiac structures such as the aorta,
pulmonary arteries, pulmonary veins, and atria. All
patients had levocardia and normal systemic and pul-
monary venous anatomy.
Patient models were divided into three size cat-
egories based on body surface area (BSA): small
(<0.75 m
2
), medium (0.75-1.5 m
2
), and large (>1.5
m
2
). Three patients, one from each category, were
chosen to be included in this study. For bench top
testing, the models were modified to include pressure
ports at the vessel inlets and outlets, providing at-
tachment points for pressure transducers. The CCPC
models were cut into two sections to allow for the
placement of the VAD inside the shared conduit. The
ends of the vessels were extended to allow attachment
of flexible PVC tubing. The models were 3D printed
in Nylon 12 material (Xometry, USA).
An Impella RP (Abiomed, USA) was modified to
fit within the common conduit of the CCPC mod-
els. A custom, 3D printed, occlusion plug was placed
around the VAD within the conduit to prevent re-
circulation between the VAD inlet and outlet. The
power and control cable was passed through the IVC
and connected to the controller by way of a sealed
gasket.
2.3 CFD Simulation
ANSYS (Canonsburg, PA, USA) software was used
to perform CFD simulations. Fluid domain was
meshed by tetrahedral cells, with prismatic bound-
ary layers created near the graft wall. We solved
3D continuity and Navier Stokes equation based on
the following assumptions: the blood is Newtonian
fluid with a density of 1060kg/m3 and the viscosity
of 0.00371Pa·s. Standard k-epsilon model was used
as the viscous model.
For boundary conditions, since previous studies
(Esmaily-Moghadam et al., 2015) showed only small
differences of WSS between rigid wall model and
fluid-structure interaction wall models, all walls were
modeled as rigid in this study. Inlet boundary condi-
tions were set as mass flow inlets, with patient specific
pulsatile profiles obtained by phase contrast imaging
from cardiac MRI, while outlet boundary conditions
were set as outflows, with flow split calculated from
time averaged flow. Extensions of 10 times the di-
ameter length were applied at inlets and outlets to de-
velop the necessary realistic velocity profile over the
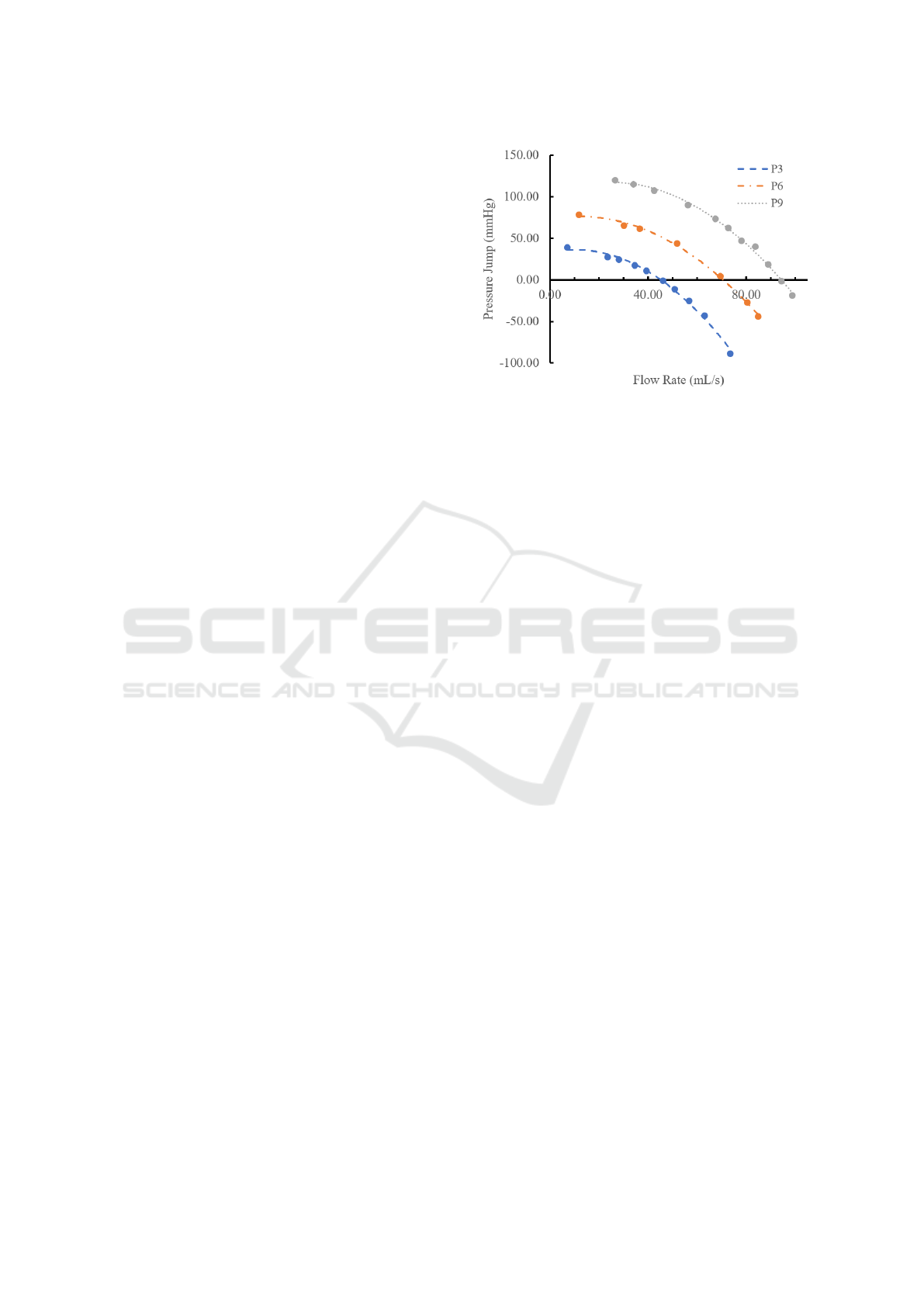
Figure 2: Pressure jump profile of the Impella RP VAD,
under P3, P6 and P9 speed settings. Curves were regressed
from experimental data shown in solid dots.
cross sections. A fan model was applied on the inter-
nal boundary to simulate the effect of the VAD, with a
2nd order polynomial pressure jump curve as pressure
jump profile, whose coefficients were regressed from
experimental data with 3 speed settings of the Impella
RP VAD (Abiomed,Inc), marked as dots in Figure 2.
Those experimental data were obtained as illustrated
in the second paragraph of Section 2.4. The tangential
velocity was calculated from the axial velocity at the
fan boundary, using a linear coefficient of 0.3, which
is a standard estimation for the VAD.
Computation was performed using the semi-
implicit method for pressure linked equations (SIM-
PLE), with first order time discretization, second or-
der pressure and momentum discretization, and first
order turbulent kinetic energy and dissipation rate dis-
cretization.
Four transient simulation cases were studied. Case
1, 2 and 3a have geometries and physiological condi-
tions of patient 1, 2 and 3 respectively, and case 3b
has a geometry of patient 3 but with half of the cardiac
output, since the cardiac output of patient 3 is already
sufficient with no clinical need for VAD support. So
we created case 3b for mimicking the situation of this
patient with insufficient circulatory function. Details
of cases are shown in Table 1.
2.4 Experimental Setup for Steady Flow
Condition
A bench top flow loop was designed to provide steady
flow testing of the VAD within the CCPC models, as
a comparison to CFD. A solution of 40% glycerin and
60% water was used as a blood mimicking fluid. To
observe the impact of the impella VAD in a continu-
ous flow environment, the VAD was installed in a 5/8”
PVC tube with an occlusion plug. Pressure transduc-
Hemodynamics of Convergent Cavopulmonary Connection with Ventricular Assist Device for Fontan Surgery: A Computational and
Experimental Study
53

Table 1: Information and cardiac outputs of patients included in the study.
Patient # Case # Patient Weight (kg) BSA Cardiac Output (L/min) Cardiac Index
1 1 15 0.64 1.564 2.44
2 2 46 1.44 4.29 2.98
3 3a 66 1.73 5.31 3.07
3 3b 66 1.73 2.65 1.53
ers (Utah Medical, USA) were connected to the pres-
sure posts at the inlet and outlets of the CCPC model.
The flow distribution was controlled using ball valves
at the inlet and outlet of the model. The pressure and
cardiac output were measured as the Impella VAD
was operated through its speed levels (P3, P6 and P9)
without additional input from the diaphragm pump.
To obtain the pressure jump profile of the VAD, a
12v DC diaphragm pump (Flojet, USA) was used to
generate continuous flow rates through the loop. Pres-
sure transducers were connected upstream and down-
stream of the VAD. The VAD alone (without CCPC
models) was tested at three fixed speed settings (P3,
P6, and P9) respectively under external controlled
flow rates. The inlet and outlet pressures were moni-
tored as the flow rate generated by the DC pump was
increased.
2.5 Hemodynamic Metrics
The hemodynamic parameters studied include in-
dexed power jump (iPJ) across the Fontan, and time-
averaged wall shear stress (TAWSS) distribution on
conduit walls.
Indexed power loss (iPL) characterises the energy
loss of blood flow in patient-specific physiological
conditions. High iPL would exacerbate cardiac func-
tion (Khiabani et al., 2015). In the case of VAD as-
sisted Fontan, the conduit is actually obtaining extra
power instead of losing power, so hereby we used in-
dexed power jump (iPJ) as the substituted term for
iPL to characterize the energy amelioration of VAD
assisted Fontan. It is defined the same as iPL, shown
in Equation (1), in which ρ is the density of blood, Q
s
is the systemic venous flow, BSA is the body surface
area of the patient. PJ is the power jump calculated as
Equation (2), where A is the boundary area, p is the
static pressure, and v is the velocity.
iPJ =
PJ
ρQ
3
s
/BSA
2
(1)
PJ =
∑
inlets
Z
A
p +
1
2
ρv
2
v × dA
−
∑
outlets
Z
A
p +
1
2
ρv
2
v × dA
(2)
Wall shear stress is linked to thrombus formation
by affecting how quickly reactive components are de-
livered and how rapidly the reaction products are dis-
seminated. The physiologic range of wall shear stress
in large veins is 0.1-1 Pa (Hathcock, 2006). In this
study, a TAWSS distribution was calculated by aver-
aging wall shear stress distribution over time, as an
indicator of thrombosis risk.
Table 2: Comparison of CFD to experimental data for static
pressure under steady flow condition. P3-P9: VAD speed
setting, CFD: simulation, EXP: flow loop experiment, P
IVC
,
P
SVC
, P
LPA
, P
RPA
: static pressure at IVC, SVC inlet and
LPA, RPA outlet, respectively, PJ: pressure jump.
(mmHg) P
IVC
P
SVC
P
LPA
P
RPA
PJ
P3
CFD -3.64 -3.64 6.34 6.12 9.87
EXP -3.64 -3.19 2.88 2.22 5.96
P6
CFD -8.03 -8.04 7.84 7.37 15.64
EXP -8.03 -6.99 5.64 4.68 12.67
P9
CFD -12.59 -12.65 14.54 13.82 26.81
EXP -12.59 -11.54 8.18 7.18 19.75
3 RESULTS
3.1 Comparison of CFD and
Experiments on Steady Flow
Conditions
Pressure jumps of case 3 under VAD speed setting P3,
P6 and P9 from CFD simulations and experimental
measurements were compared to indicate the devia-
tion between them, and to give an insight on what the
trend of deviation is and where the errors come from.
The pressure jump was calculated by subtracting the
mean static pressure at IVC and SVC inlets from the
mean static pressure at LPA and RPA outlets. Relative
deviations were calculated on the CFD results with
experimental data as references. The simulations had
the exact same boundary conditions as the settings
in the flow loop experiment mentioned in the first
paragraph of Setion 2.4. Pressure results from CFD
were aligned with experimental data by IVC pressure.
Comparison of pressure data from CFD and experi-
ment at SVC inlet, LPA and RPA outlets are shown in
Table 2. Results from simulation show higher pres-
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
54
sure jumps than experimental data, probably due to
the less viscous energy loss caused by the simplified
geometry in simulation and extra energy dissipation
in experiments.
3.2 Hemodynamics Under Pulsatile
Conditions
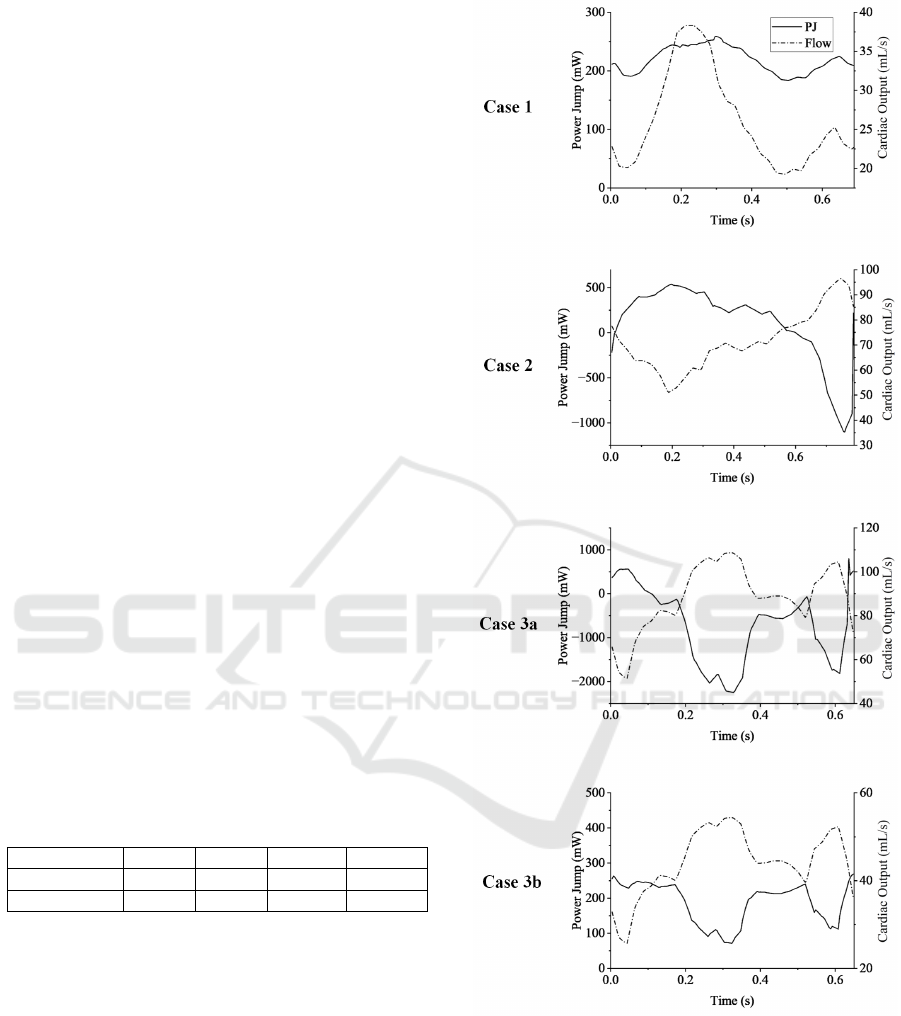
The power jump of four cases are shown in Figure 3,
with cardiac outputs shown as reference in dash-dot
lines. The time averaged iPJ of these cases along
with corresponding power loss are presented in Ta-
ble 3. For cases with smaller cardiac output (case 1
and case 3b), power jump stayed in the positive range
over time. For the case with medium cardiac out-
put (case 2), power loss occurred in the period with
high flow rate, but the VAD was still adding energy
to the system when averaged over time. For case 3a,
which has a cardiac output higher than the maximum
capacity of the VAD (5.31L/min vs 5L/min), signifi-
cant power loss was observed during more than 80%
of the cardiac cycle. The Fontan was consuming sig-
nificant energy over time due to the inadequacy of the
VAD speed. Since the VAD has a fixed setting of
speed over the cardiac cycle, the pressure jump that
VAD provides is subject to the flow rate. The occlu-
sion plug prevents any local recirculation or bypass,
forcing the total systemic flow to go through the VAD
and thus creates extremely high flow rates across the
VAD during part of the cardiac cycle. Instead of pro-
viding additional power to the circulatory system, the
VAD functions as a power drain when its speed fails
to catch up to the blood flow speed.
Table 3: Time averaged power jump and iPJ. TA: time-
averaged, PJ: power jump.
Case 1 Case 2 Case 3a Case 3b
TA-PJ (mW) 219 111 -747 187
TA-iPJ 4.81 0.168 -3.08 0.771
The TAWSS distribution is shown in Figure 4.
High magnitudes of TAWSS were observed in the
downstream region of VAD (upper part of common
limb and the pulmonary arteries). This is reasonable
since the diameter of VAD is much smaller than the
lumen of the conduit, which would create a jet flow
in the center of the conduit lumen. The maximum
TAWSS reached 20, 57, 40, and 16 Pa and the min-
imum reached 0.025, 0.21, 0.19, and 0.048 Pa for
case 1, 2, 3a and 3b, respectively. Areas with non-
physiologic low TAWSS (<0.1 Pa) were fully elimi-
nated in case 2 and 3a, compared to 0.25% and 0.33%
of the total conduit wall area in the same CCPC con-
duit without VAD assistance (Sinha et al., 2022).
Figure 3: Power jumps and cardiac outputs during the car-
diac cycle for all four cases.
4 DISCUSSION
This study gave an overview of the local hemody-
namics of this novel design of VAD-integrated CCPC
Fontan. We compared CFD with experiments under
steady flow and investigated iPJ with regard to pul-
Hemodynamics of Convergent Cavopulmonary Connection with Ventricular Assist Device for Fontan Surgery: A Computational and
Experimental Study
55
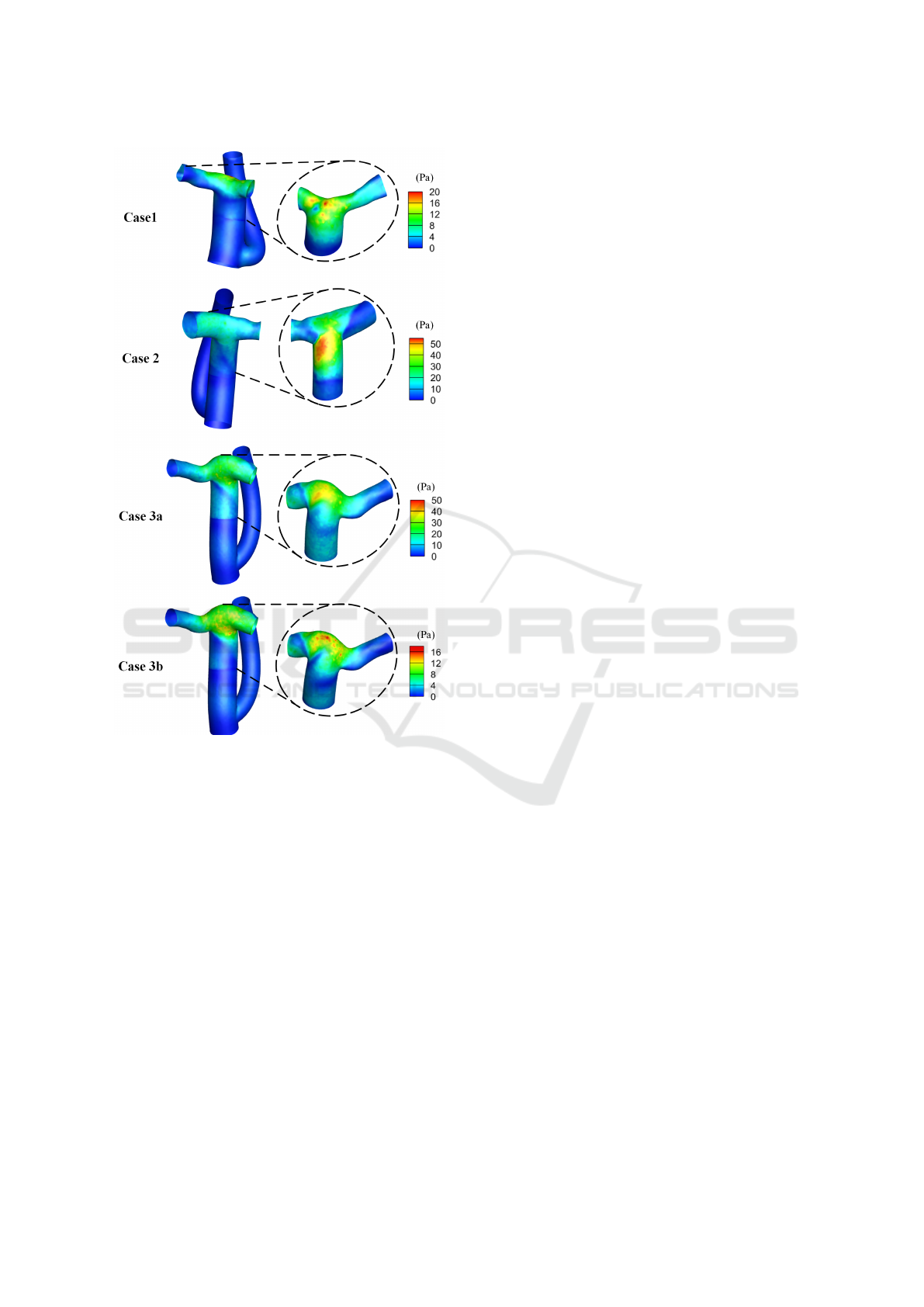
Figure 4: TAWSS distribution for all four cases. Left col-
umn: full Fontan from posterior view, Right column: the
part of Fontan downstream to VAD from anterior view.
Views are not accurately aligned as anterior and posterior.
satile blood flow and TAWSS distribution of four dif-
ferent patient cases.
Comparison between CFD and benchtop experi-
ments was carried out, focusing on pressure under
steady flow condition, which showed an overestima-
tion of pressure jump by CFD compared to the exper-
iments. This might result from the simplified geomet-
ric model in simulation and extra power loss in the ex-
perimental setup. To solve this overestimation, model
tuning can be performed with more experimental data
in future work.
With the occlusion plug inside the Fontan conduit,
no blood can bypass or recirculate around the VAD,
which makes the Fontan a large source of energy con-
sumption when VAD capacity is unable to catch up to
the cardiac output. Specifically, for cases with smaller
cardiac outputs (case 1, CO=1.564L/min; case 3b,
CO=2.65L/min), the VAD capacity is significantly
strong enough and iPJ stays in the positive range.
For the medium case (case 2, CO=4.29L/min), neg-
ative iPJ occurs in part of cardiac cycle when flow
rate reaches the peak, but time averaged iPJ is still
positive, with VAD speed setting at P9 (maximum
speed). For the large case (case 3a, CO=5.31L/min),
even when the VAD is at the maximum speed (P9),
time averaged iPJ is negative. But in those conditions
when CO is large, VAD assistance would clinically be
unnecessary. The results on iPJ show improvement of
energy brought by VAD for all cases that need MCS
(usually with limited cardiac output).
Regions with large TAWSS are created with the
existence of VAD. Comparing to the scenario with-
out VAD, overall TAWSS is larger in both region up-
stream and downstream to the VAD. In case 2 and
case 3a, regions with non-physiologic low TAWSS
are eliminated. While the VAD brings extra energy to
the system, it also brings complex flow patterns and
instabilities, increasing the shear of the flow and on
the wall.
There are limitations of this study in respect to
the modeling aspect, including the geometric simplic-
ity of the VAD and using the ‘fan’ model as an ap-
proximation of fluid dynamic character of the VAD.
We adopted a simple cylinder as the VAD wall and a
planar surface as the plug, as well as using the ‘fan’
model to approximate pressure jump and flow pattern
of the VAD outlet, which would ignore all internal
flow field of the VAD and lose some detailed patterns
in the region near the VAD and plug. We used experi-
mental data to regress the pressure jump profile of the
‘fan’ model to minimize the deviation, and only ana-
lyzed TAWSS on the graft wall and iPJ over the entire
Fontan, avoiding the detailed region of the VAD.
Another limitation of this study is the fixed bound-
ary condition of inflow and outflows. The circulatory
system of a patient with single ventricle heart disease
is originally a hydraulic system with the left ventri-
cle as the only power source, and the inlet and outlet
flow profile of vena cava and PAs would change when
there is a second power source added. In future work,
multi-scale simulation can be carried out to model
the entire circulatory system or provide changeable
boundary conditions for Fontan.
This study performed CFD simulation and exper-
imental testing for a VAD-integrated CCPC Fontan.
iPJ and TAWSS were analyzed on four cases. For fu-
ture work, reduced-order models could be integrated
to model the cardiovascular system, in order to ad-
dress the strong assumptions of the flow rate boundary
conditions. Also, a more detailed geometric model of
the VAD, including structures in the nozzle region and
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
56

the complex shape outline of the VAD, can be adopted
in the future.
5 CONCLUSIONS
This study investigated iPJ and TAWSS of the CCPC
Fontan conduits with VAD integration. We showed
that a power jump over the Fontan was generated
for cases with limited cardiac output, and regions
with high TAWSS were created and regions with non-
physiologic low TAWSS were significantly reduced
for all cases. The result of this study verified the feasi-
bility of CCPC configuration as a solution for Fontan
MCS integration.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of
Health under grant R33HD090671 and American
Heart Association under grant 20IPA35320267.
REFERENCES
Aslan, S., Liu, X., Wu, Q., Mass, P., Loke, Y.-H., Hib-
ino, N., Olivieri, L., and Krieger, A. (2022). Virtual
planning and simulation of coarctation repair in hy-
poplastic aortic arches: Is fixing the coarctation alone
enough? In BIOINFORMATICS, pages 138–143.
Atz, A. M., Zak, V., Mahony, L., Uzark, K., D’agincourt,
N., Goldberg, D. J., Williams, R. V., Breitbart, R. E.,
Colan, S. D., Burns, K. M., et al. (2017). Longitudi-
nal outcomes of patients with single ventricle after the
fontan procedure. Journal of the American College of
Cardiology, 69(22):2735–2744.
Cedars, A., Kutty, S., Danford, D., Schumacher, K., Auer-
bach, S., Bearl, D., Chen, S., Conway, J., Dykes, J.,
Jaworski, N., et al. (2021). Systemic ventricular as-
sist device support in fontan patients: a report by ac-
tion. The Journal of Heart and Lung Transplantation,
40(5):368–376.
Deal, B. J. and Jacobs, M. L. (2012). Management of the
failing fontan circulation. Heart, 98(14):1098–1104.
d’Udekem, Y., Iyengar, A. J., Galati, J. C., Forsdick, V.,
Weintraub, R. G., Wheaton, G. R., Bullock, A., Justo,
R. N., Grigg, L. E., Sholler, G. F., et al. (2014). Re-
defining expectations of long-term survival after the
fontan procedure: twenty-five years of follow-up from
the entire population of australia and new zealand.
Circulation, 130(11 suppl 1):S32–S38.
Esmaily-Moghadam, M., Murtuza, B., Hsia, T.-Y., and
Marsden, A. (2015). Simulations reveal adverse
hemodynamics in patients with multiple systemic to
pulmonary shunts. Journal of biomechanical engi-
neering, 137(3):031001.
Hathcock, J. J. (2006). Flow effects on coagulation and
thrombosis. Arteriosclerosis, thrombosis, and vascu-
lar biology, 26(8):1729–1737.
Kempny, A., Dimopoulos, K., Uebing, A., Moceri, P.,
Swan, L., Gatzoulis, M. A., and Diller, G.-P. (2012).
Reference values for exercise limitations among
adults with congenital heart disease. relation to ac-
tivities of daily life—single centre experience and
review of published data. European heart journal,
33(11):1386–1396.
Khiabani, R. H., Whitehead, K. K., Han, D., Restrepo, M.,
Tang, E., Bethel, J., Paridon, S. M., Fogel, M. A., and
Yoganathan, A. P. (2015). Exercise capacity in single-
ventricle patients after fontan correlates with haemo-
dynamic energy loss in tcpc. Heart, 101(2):139–143.
Liu, X., Aslan, S., Kim, B., Warburton, L., Jackson, D.,
Muhuri, A., Subramanian, A., Mass, P., Cleveland,
V., Loke, Y.-H., Hibino, N., Olivieri, L., and Krieger,
A. (2022a). Computational Fontan analysis: Pre-
serving accuracy while expediting workflow. World
Journal for Pediatric and Congenital Heart Surgery,
13(3):293–301. PMID: 35446218.
Liu, X., Hibino, N., Loke, Y.-H., Kim, B., Mass, P., Fuge,
M., Olivieri, L., and Krieger, A. (2022b). Surgical
planning and optimization of patient-specific Fontan
grafts with uncertain post-operative boundary condi-
tions and anastomosis displacement. IEEE Transac-
tions on Biomedical Engineering, pages 1–1.
Liu, X., Kim, B., Loke, Y.-H., Mass, P., Olivieri, L.,
Hibino, N., Fuge, M., and Krieger, A. (2022c).
Semi-automatic planning and three-dimensional elec-
trospinning of patient-specific grafts for Fontan
surgery. IEEE Transactions on Biomedical Engineer-
ing, 69(1):186–198.
Pike, N. A., Vricella, L. A., Feinstein, J. A., Black, M. D.,
and Reitz, B. A. (2004). Regression of severe pul-
monary arteriovenous malformations after fontan re-
vision and “hepatic factor” rerouting. The Annals of
thoracic surgery, 78(2):697–699.
Shah, M. J., Rychik, J., Fogel, M. A., Murphy, J. D., and
Jacobs, M. L. (1997). Pulmonary av malformations
after superior cavopulmonary connection: resolution
after inclusion of hepatic veins in the pulmonary cir-
culation. The Annals of thoracic surgery, 63(4):960–
963.
Shinohara, T. and Yokoyama, T. (2001). Pulmonary arteri-
ovenous malformation in patients with total cavopul-
monary shunt: what role does lack of hepatic venous
blood flow to the lungs play? Pediatric cardiology,
22(4):343–346.
Sinha, P., Contento, J., Kim, B., Wang, K., Wu, Q., Cleve-
land, V., Mass, P., Loke, Y., Krieger, A., and Olivieri,
L. (2022). The convergent cavopulmonary connection
(ccpc): A novel and efficient configuration of fontan
to accommodate mechanical support. The Journal of
Thoracic and Cardiovascular Surgery. submitted.
Trusty, P. M., Slesnick, T. C., Wei, Z. A., Rossignac,
J., Kanter, K. R., Fogel, M. A., and Yoganathan,
A. P. (2018). Fontan surgical planning: previous
accomplishments, current challenges, and future di-
Hemodynamics of Convergent Cavopulmonary Connection with Ventricular Assist Device for Fontan Surgery: A Computational and
Experimental Study
57

rections. Journal of cardiovascular translational re-
search, 11(2):133–144.
Trusty, P. M., Wei, Z. A., Slesnick, T. C., Kanter, K. R.,
Spray, T. L., Fogel, M. A., and Yoganathan, A. P.
(2019). The first cohort of prospective fontan surgi-
cal planning patients with follow-up data: How accu-
rate is surgical planning? The Journal of thoracic and
cardiovascular surgery, 157(3):1146–1155.
Vettukattil, J. (2002). Pathogenesis of pulmonary arteriove-
nous malformations: role of hepatopulmonary inter-
actions.
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
58
