
Stylohyoid and Posterior Digastric Measurement with Intramuscular
EMG, Submental EMG and Swallowing Sound
Adrien Mialland
1 a
, Ihab Atallah
2
and Agn
`
es Bonvilain
1
1
Univ. Grenoble Alpes, CNRS, Grenoble INP, Gipsa-Lab, 3800 Grenoble, France
2
Otorhinolaryngology, CHU Grenoble Alpes, 38700 La tronche, France
Keywords:
Stylohyoid, Digastric, Electromyography, Swallowing Sound, Implantable Active Artificial Larynx.
Abstract:
The stylohyoid and the posterior digastric muscles have essentially been measured through indirect imaging
method because of the difficulty to measure them. They are small neck muscles, close to each other, that
cannot easily be accessed independently. Yet, they showed promising results for a robust and safe indwelling
detection of swallowing, both in terms of timing and recruitment. The possibility to thoroughly establish
their relevance through their direct functional analysis would enable the development of an implantable active
artificial larynx, that would protect the airway during swallowing detection. Therefore, we set up the first
standardized procedure that allows their direct measurement through intramuscular electromyography (EMG)
and that we report in this paper. We also used submental surface EMG and swallowing sound modalities
to access the major time points of the swallowing process. Finally, various exercises, along with swallowing,
were performed by the volunteers. 16 peoples were measured with our new procedure, and both the stylohyoid
and the posterior digastric could be measured independently with no difficulty. Timings and tasks comparison
are therefore ongoing.
1 INTRODUCTION
The past decades have provided detailed descriptions
of the swallowing mechanism, its anatomical struc-
tures and their complex interplay (Shaw and Martino,
2013). But swallowing studies still tend to mostly
report on muscles activity during swallowing tasks
only, most likely because of the need to better under-
stand the swallowing process for a clinical practice.
However, this does not allow to draw conclusions in
a broader perspective to get an extensive picture of
muscles timing and recruitment, with regard to vari-
ous tasks. Besides, the available measurement meth-
ods limit the possibilities. The neck muscles are usu-
ally small and close to each other, and the traditional
surface electromyography (EMG) approach may lead
to measurements that contain unwanted contamina-
tion from adjacent muscles (cross-talk). Therefore,
several muscles got little attention because of the dif-
ficulty to measure them independently (Steele, 2015).
Yet, we aim at the feasibility of an implantable ac-
tive artificial larynx through the real-time, robust and
safe indwelling detection of swallowing, which needs
a
https://orcid.org/0000-0001-5359-674X
high quality signals. This would be beneficial in the
context of laryngeal removal, known as total laryn-
gectomy, that requires the trachea to be sewn on the
anterior neck, to create a tracheostomy. Indeed, as
the laryngeal functions are lost, breathing can only be
safely performed through an isolated tract. But it adds
several adverse effects and the air no longer passes
through the nose and the mouth anymore, which al-
lowed filtration, warming, humidification, olfaction
and acceleration of the air for better tissue oxygena-
tion (Maclean et al., 2009). Therefore, these func-
tions could be restored if the trachea could be set back
in place and protected with an active closure mecha-
nism, during swallowing. This requires the develop-
ment of a robust and real-time swallowing detection
strategy, based on muscles that activate early, provide
stable and dedicated activation pattern, and are not al-
tered by the surgery.
In that regard, we focused on the stylohyoid and
the posterior digastric muscles that showed indirect
but appropriate results. Indeed, few studies hint to-
ward their importance. First, on animals the stylohy-
oid have been shown to activate at the beginning of
the swallowing, at the same time than the submental
muscles (German et al., 2009). On humans, imag-
48
Mialland, A., Atallah, I. and Bonvilain, A.
Stylohyoid and Posterior Digastric Measurement with Intramuscular EMG, Submental EMG and Swallowing Sound.
DOI: 10.5220/0011628100003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 4: BIOSIGNALS, pages 48-54
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

ing methods confirmed these results (Okada et al.,
2013) and the analysis of their anatomical structure
revealed a significant potential to move the larynx
(Pearson et al., 2011), but direct functional data are
lacking. Second, both muscles were studied as a
whole STH-PD complex, which was shown to mainly
activate during swallowing and jaw opening (Kurt
et al., 2006). The authors also studied their nerve
conduction in comparison to the mylohyoid, which
is part of the submental muscles. They argued that
”electrophysiological identification factors may make
easier to work on STH–PD muscles”. In addition,
contractile patterns from animals showed a repeatable
and stable activity of the stylohyoid (Thexton et al.,
2012). Third, these muscles are both directly acces-
sible during the surgery, with no further impairment
required. Alternatively, other muscle could have been
considered for a robust an safe detection of swallow-
ing, but several uncertainties mitigate their interest.
The mylohyoid is part of the submental muscles and
demonstrates the greatest potential in laryngeal supe-
rior movements (Pearson et al., 2011). But it is also
part of the floor of the mouth and activate for var-
ious tasks, to support the tongue. Also, infrahyoid
muscles are easily accessible during the surgery but
could be removed with the larynx, and may exhibit
a large range of activation for various tasks (German
et al., 2009). Finally, the pharyngeal muscles are ei-
ther impaired during the surgery or would require to
place the sensors deep inside the neck, and therefore
to further damage the remaining functional anatomy
(Lippert et al., 2016).
Besides, the swallowing process have been char-
acterized through various methods that give access to
the major events. In particular, the submental muscles
are acknowledged to lead the reflexive part of swal-
lowing and their activation assists the tongue in the
propulsion of the bolus posteriorly (Park et al., 2017).
These muscles are commonly measured through sur-
face electromyography and are considered mostly free
of contamination from adjacent muscles (cross-talk)
(McKeown et al., 2002). Also, the forces applied to
the bolus produce various sound components that are
linked to bolus locations and anatomical events. Es-
pecially, the passage of the bolus through the upper
esophageal sphincter (UES) generates a specific burst
and is the only sound component to be found 100% of
the time (Morini
`
ere et al., 2008). Recently, the begin-
ning of that event has been suggested to be linked to
the closure of the laryngeal vestibule (Kurosu et al.,
2019), which protects the airway from bolus aspi-
ration. Therefore, these tow measurement methods
would allow to correlate the stylohyoid and posterior
digastric muscles activity with key swallowing events.
So, this paper intends to describe the measurement
protocol we set up to enable the evaluation of the sty-
lohyoid and the posterior digastric muscles with intra-
muscular EMG. Submental surface EMG and swal-
lowing sound measurement methods were also used
to respectively access the beginning of the swallow-
ing and the moment the bolus goes through the UES.
2 MATERIALS AND METHODS
2.1 Subjects
Twelve healthy adults (8 males/ 8 females) with no
history of dysphagia, neck surgery, immune defi-
ciency or any neurological impairment participated in
this study. The mean age was 36.1 ± 13, 6 and all
enrolled participant met the following inclusion cri-
teria: age of 18 years or higher and a body mass
index (BMI) of 25 or lower. Participants with BMI
higher than 25 were intentionally excluded from this
study to avoid a potentially excessive amount of fat
in the region of the neck that would make the proper
placement of the sensors more complex. Each par-
ticipant received a detailed explanation of the study
and were made aware of their rights, including the
possibility to withdraw at any time. Each participant
returned a written informed consent prior to the par-
ticipation. Each participant underwent a standardized
examination conducted by a registered otolaryngolo-
gist to confirm the absence of any sign of dysphagia.
Each participant were anonymized and no identifying
information were collected. All participant accom-
plished the full protocol. This study was approved
by the research ethics committee of Sud-M
´
editerran
´
ee
III of N
ˆ
ımes in France (Protocol ID: 38RC22.0096).
This study has been carried out in accordance with
the Declaration of Helsinki of the World Medical As-
sociation revised in 2013 for experiments involving
humans.
2.2 Sensors Placement
Prior to the sensor placement, the beard must be
shaved, and the neck area must be cleared from any
jewelry and clothing items that could interfere. Dur-
ing any sensor placement, the participant was asked
for any discomfort and a swallowing was performed
to confirm the absence of pain. The participants were
asked to comfortably sit on a chair and each sensors
were placed (Figure 2) in the following order:
SWALLOWING SOUND: The cricoid cartilage
was first located by the otolaryngologist and the ac-
celerometer was placed at its center, as it was sug-
Stylohyoid and Posterior Digastric Measurement with Intramuscular EMG, Submental EMG and Swallowing Sound
49
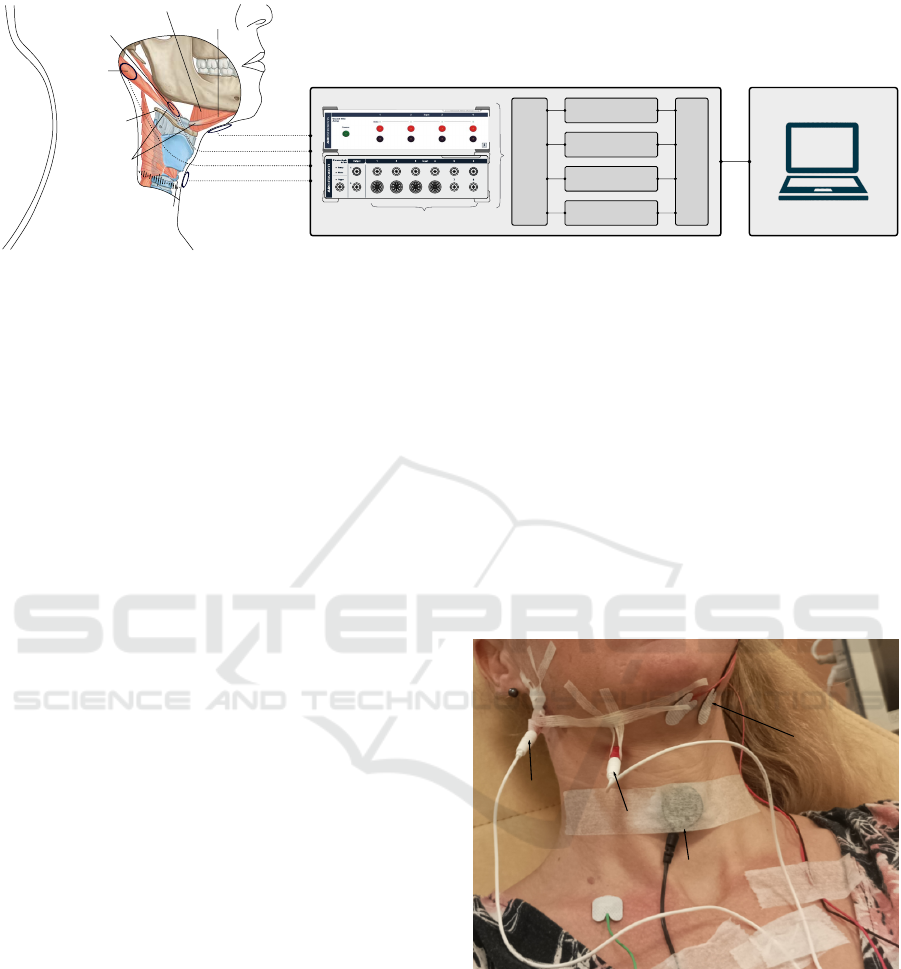
cricoid cartilage
hyoid bone
stylohyoid
posterior
digastric
intermediate
tendon
mylohyoid
anterior
digastric
sEMG
iEMG
iEMG
ACC
Analog input connectors
16 bits ADC, 4 kHz
BP 10-500 Hz
BP 10-1000 Hz
BP 10-1000 Hz
LP 2000 Hz
Intrumentation
amplifier x4
Figure 1: Sensors locations, anatomical structures and acquisition setup. EMG: electromyography, sEMG: surface EMG,
iEMG: intramuscular EMG, ACC: accelerometer, BP: band-pass, LP: low-pass. ADC: analogue digital converter. The targeted
anatomical regions are shown with ellipses for each sensors. 1 needle is inserted in the stylohyoid at the level of the lesser
horn of the hyoid bone, next to the intermediate tendon which do not generate myoelectric signal. 1 needle is inserted in
the posterior digastric, where it separates from the stylohyoid. 1 surface EMG measures the submental muscles activity. 1
accelerometer measures the swallowing sound from the top of cricoid cartilage. Each signals are then passed through an
instrumentation amplifier, band-pass filtered and digitized at 4kHz by a 16 bits ADC, before being sent to the laptop.
gested to act as a resonator for the sound (Takahashi
et al., 1994; Cichero and Murdoch, 2002). The ac-
celerometer was fixed with a hypoallergenic paper
tape, so as to record anterior-posterior vibrations. The
length of the tape was chosen so as to span the neck
horizontally from one lateral side to the opposite side.
The tension was adjusted to get a clear event when a
swallowing is performed.
SURFACE EMG: The 2 differential electrodes
were placed under the left part of the submental area,
with their center approximately 2cm apart, and the
ground electrode was placed over the right clavicle.
Both areas were first cleaned with a dedicated abra-
sive and conductive paste to reduce the electrode-skin
impedance.
INTRAMUSCULAR EMG: Both the stylohyoid
and the posterior digastric muscles are directly acces-
sible behind the skin, but are close to each other. To
isolate their activity, they are targeted close to their
origin and insertion points: the stylohyoid muscle was
targeted next to its insertion point, at the level of he
junction between the body and the greater horn of the
hyoid bone. This point could be identified by pal-
pation of the lesser horn of the hyoid, which is at-
tached by its base to the angle formed by the junc-
tion between the body and greater horn. The EMG
needle punctures the skin perpendicularly at the in-
sertion point of the stylohyoid bone and then directed
upward and backward. The posterior digastric mus-
cle was targeted in its posterior portion, next to its
origin from the mastoid notch. The EMG needle is
inserted perpendicularly behind the vertical ramus of
the mandible and in front of the anterior border of
the sternocleidomastoid muscle. It is then directed
upward, medially and backward. The anatomical re-
gions of both the site of puncture are located through
palpation and were marked on the skin before inser-
tion. Besides, while the otolaryngologist was slowly
inserting the needles, a second trained operator mon-
itored the EMG activity on the computer to look for
muscle activities. It could be requested to the partici-
pant to swallow to elicit an event. Once an event were
visible on the signal, the needles were no further in-
serted. They were then secured with a Steri-Strip so
that they cannot come out of the muscles.
submandibular
accelerometer
posterior
digastric
stylohyoid
Figure 2: Sensor placement. The stylohyoid and the pos-
terior digastric muscles are measured with intramuscular
EMG, the submental muscles with surface EMG, and the
swallowing sound with an accelerometer. The needles are
secured with steri strips so that they cannot come out.
2.3 Signal Acquisition
From each participant 4 signals were recorded: 2 in-
tramuscular EMG with concentric needle electrodes
(27-gauge, 30 mm), 1 surface EMG with 3 surface gel
electrodes (2 differential and one ground), and 1 swal-
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
50

lowing sound signal with an single axis accelerom-
eter (Pulse Transducer TN1012/ST, 1600Hz, ADIn-
strument). The 3 EMG signals were fed to a Bio-Amp
(FE234, 4 channels, ADInstrument) pre-Amplifier
with differential inputs, which is in turn connected
to a PowerLab (35 series, 4 channels, ADInstrument)
data acquisition system. The accelerometer were di-
rectly connected to the PowerLab with a built-in ded-
icated connector. On the computer, the LabChart
ADInstrument data acquisition and analysis software
receives the signals from the PowerLab. This set up
allows to have all 4 signals synchronized in time.
Then, the Bio-Amp filters the 2 intramuscular EMG
signals with a 10 − 1000Hz analogue band-pass fil-
ter and the surface EMG signal with a 10 − 500Hz
analogue band-pass filter. The swallowing sound sig-
nal is also filtered, with a 2000Hz low-pass analogue
filters. Finally, each channel uses a 16 bits analogue-
digital-converter (ADC) and all signals were sampled
at 4000Hz, which simplified the file format and al-
lowed down-sampling if required.
2.4 Protocol
Both swallowing and non-swallowing tasks were per-
formed by participants. The task is first prepared by
asking the participant to take the bolus into the mouth
and/or to adopt a relaxed position. Then, the signal
acquisition is launched and 2 second with no motion
is recorded. The participant is then asked to perform
the task and 2 more second with no motion are ac-
quired. This ensure to record a clear event. Also,
when a task is performed, the keyboard is pressed to
place a marker on the event, which facilitates its loca-
tion in post-processing.
SWALLOWING TASKS: Participants were asked to
perform 5 swallowing of Saliva, water (10ml), thick
liquid (compote) and solid bolus (madeleine).
NON-SWALLOWING TASKS: Participant were
asked to perform (1) 3 times mouth opening, lips
purseing, teeth clenching, smiling, whistling, cough-
ing, blowing through a straw, counting from 1 to 10,
saying ”iii” in ascending and descending order. (2)
3 times movement tasks being jaw movements, lat-
eral head movements and head extension and flexion.
They are performed in neutral - right - left - neutral
or neutral - extension - flexion - neutral order depend-
ing on the task. (3) 5 times chewing, that are actually
recorded at the moment the solid bolus swallowing
tasks is performed. To separate the two, the partic-
ipant is asked to hold the bolus in the mouth once
chewed, ready to swallow, before being asked to swal-
low it. All tasks are performed to a comfortable full
extent and at a natural pace.
2.5 Signal Processing
Offline analysis was performed with a custom piece
of software. A 2
nd
order high-pass Butterworth dig-
ital filters with a cut-off frequencies of 20Hz is ap-
plied on the EMG signals and a 4
th
order Butterworth
notch filters is applied to all signals to eliminate the
50Hz line noise. The baselines are then delineated to
include 1 second of recording that contains no event
of any sort. Next, all signals are transformed using
the Teager-Kaiser energy operator (TKEO) Ψ[x(n)] =
x(n)
2
− x(n− 1)x(n +1), which has been shown to im-
prove the SNR (Li et al., 2007). Then, the following
are extracted:
MUSCLE ONSET/OFFSET: We used a method
based on generalized likelihood ratio (GLR) (Xu
et al., 2013). Using a 200ms sliding window of size n
samples, it continuously computes the likelihood ra-
tio of a contracted muscle state hypothesis H
1
against
a relaxed muscle state hypothesis H
0
. This allows to
estimate the time instant r of muscle onset and offset,
based on a series of observations y
n
0
= [y(0), ..., y(n)]
from the current window. A probability density func-
tion (PDF) P
1
(y(t)) and P
0
(y(t)) is associated with
each hypothesis, and the likelihood that y
n
0
is drawn
from one of these PDF is expressed in Equation 1.
L
i
(y
n
0
) = p
i
(y
n
0
) =
n
∏
t=0
P
i
(y(t)) (1)
Where i is the type of hypothesis. The likelihood
ratio is then expressed in Equation 2.
L
1
(r, y
n
0
)
L
0
(y
n
0
)
=
p
0
(y
r−1
0
)p
1
(y
n
r
)
p
0
(y
n
0
)
=
n
∏
t=r
P
1
(y(t))
P
0
(y(t))
(2)
Where L
1
(r, y
n
0
) is the likelihood that y
n
0
is drawn
from both PDF, with H
1
starting from time instant r.
The likelihood ratio is then maximize over the possi-
ble transition time r of the current window. Therefore,
this requires to know the distribution followed by the
data and we opted for an exponential PDF. Indeed, on
the basis of empirical evidences, it closely represents
the TKEO transformed EMG, as long as its absolute
value is considered, which is in line with recent inves-
tigations (Selvan et al., 2018). An adaptive Threshold
is then applied and is chosen to be 10% of the max-
imum current GLR values. The onset and offset are
then located manually according to the threshold, to
avoid any false positive detection.
BOLUS THROUGH UES: the moment the bolus
enters the UES corresponds to the major sound burst
and is suggested to be linked to the closure of the la-
ryngeal vestibule (Kurosu et al., 2019). This allows
to locate the ultimate time point where the airway
Stylohyoid and Posterior Digastric Measurement with Intramuscular EMG, Submental EMG and Swallowing Sound
51
must be protect. The transformation of the signal with
TKEO allows to bring this event out and facilitate the
manual location of its beginning.
3 RESULTS AND DISCUSSION
The goal of this paper was to describe the protocol
we set up that made feasible the measurement and the
evaluation of the stylohyoid and the posterior digas-
tric muscles. Very few studies focused on those mus-
cles and none enabled the investigation of both their
timing and contraction patterns.
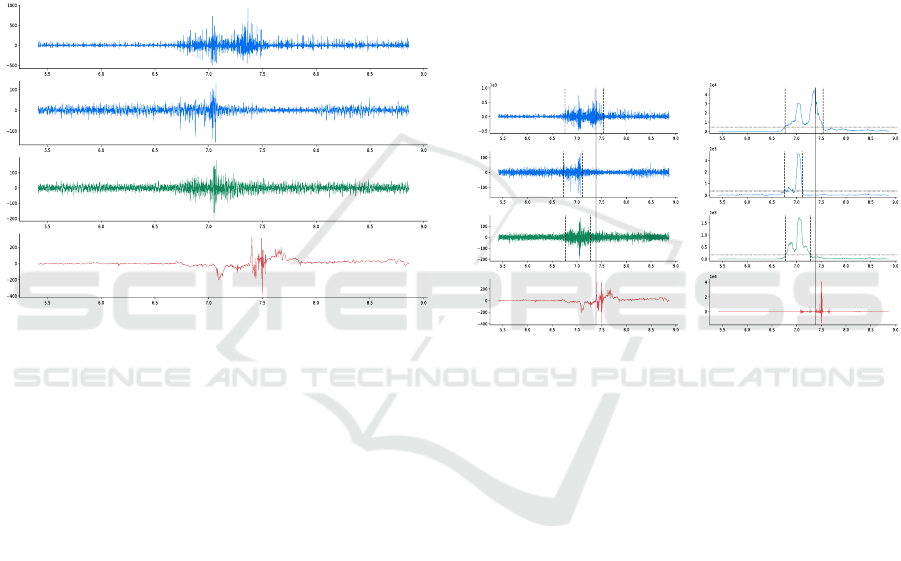
Figure 3: Raw signals of a saliva swallowing. SH: sty-
lohyoid, PD: posterior digastric, SUB: submental, ACC:
accelerometer. All signals provide distinguishable events.
The burst of higher frequency in the ACC signal corre-
sponds to the moment where the bolus goes through the up-
per esophageal sphincter.
The challenging aspect of our protocol is the
proper placement of the concentric needles, to record
the stylohyoid and the posterior digastric muscles sep-
arately. Even though concentric needles are very se-
lective, both muscles are thin and close to each other,
which may lead to the wrong muscle to be measured.
Therefore, we chose to target the stylohyoid close to
its insertion point, at the level of the lesser horn of
the hyoid bones. In that region, the posterior digastric
is composed of its intermediate tendon, which can-
not produce EMG signal and therefore cannot be mis-
taken for the stylohyoid EMG signal. Besides, the
posterior digastric muscle was targeted close to its ori-
gin at the level of the mastoid notch. In that region, it
separates from the stylohyoid, which originates from
the styloid process upper in the neck. In addition,
these anatomical regions were found through palpa-
tion, and the decision to exclude the participants with
a BMI upper than 25 limited the amount of fat and
allowed to find them with no difficulty. Finally, these
muscles are the first to be accessible behind the skin.
Once an activity were visible on the screen, the nee-
dle were not inserted any further and were secured
with steri strips. The needles were, therefore, fixed in
depth but could follow the anatomical movement. The
present paper therefore provide the first standardized
procedure that allows to measure both the stylohy-
oid and the posterior digastric muscles independently.
In the past, only one study provided direct functional
analysis of those muscle through intramuscular EMG
(Kurt et al., 2006), but they were only measured as a
whole STH-PD complex, with no possibility to differ-
entiate their activity, both in terms of timing and re-
cruitment. Also, the authors mainly focused on nerve
conduction and did not directly seek to evaluate their
timing. We therefore expend those points with our
new procedure, which provide independent signals.
Figure 4: Signal processing results. Left: raw signals.
Right: processed signals. Dashed lines: onset and offset.
Solid lines: the moment when the bolus goes through the
upper esophageal sphincter (UES). The dashed lines loca-
tions are found with the generalized likelihood ratio (GLR)
method, applied on the EMG (top 3) signals. The solid line
location is found with the Teager-Kaiser energy operator
(TKEO), applied on the accelerometer (bottom) signal.
With regard to the swallowing sound, we focused
on its major component. Early investigation of that
event showed its rise in frequency content compared
to the rest of the signal (Hamlet et al., 1990). The
authors suggested that this was the result of the in-
crease in pressure generated by the contraction of
the surrounding muscles that forces the bolus through
the UES. More recently, modern data analysis meth-
ods confirmed the particular frequency content of that
event (Lee et al., 2008) and formerly linked its oc-
currence with the bolus that passes through the UES
(Morini
`
ere et al., 2008). This event was also the only
one to occur 100% of the time. Finally, the begin-
ning of that event is suggested to be temporal linked
to the closure of the laryngeal vestibule (Kurosu et al.,
2019). This later event is of prime interest to define
the ultimate moment when the airway are physiolog-
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
52

ically required to be closed. This would in turn de-
fine the timing for an active closure mechanism to be
closed as well. Therefore, the use of TKEO is jus-
tified by its ability to highlight the high frequency
contents, and were particularly effective in this study
(Figure 4). However, one may argue about the lack of
bandwidth analysis of the swallowing sounds. Indeed,
only its upper limits have been investigated and no
recommendation allows to formerly delineate a par-
ticular sound component. Yet, these considerations
usually arise when it comes to the precise analysis
of the swallowing process. But the main UES sound
component clearly stands out and the TKEO essen-
tially magnifies its frequency content. Besides, the
swallowing sound modality allows to avoid the use
of videofluoroscopy, which requires X-ray exposure.
Therefore, these elements make the swallowing sound
and the TKEO operator, an effective and safe combi-
nation to define the ultimate closing time, from the
perspective of an implantable active artificial larynx.
The related time marker were then set manually with
no difficulty and is visible Figure 4.
Besides, we looked for an semi-automated muscle
activity segmentation to reliably determine their onset
and offset points. But the literature provides a wide
range of algorithm and none has shown to be effec-
tive for all the various bio-mechanical application that
uses EMG (Tenan et al., 2017). So, we used the GLR
for its ability to modelize the signals and therefore
its robustness to various background noise (Xu et al.,
2013). As an example, intramuscular EMG requires
the insertion of a needle inside the muscles through-
out the whole acquisition. The presence of this in-
vasive foreign body may cause the reflexive contrac-
tion of relatively few muscle fibers, which can be vis-
ible on the signal as spurious background spikes (Fig-
ure 3). Besides, it allows to adapt to the channel being
analyzed so that every muscle activity are compared
on the same basis, which limits possible biases.
Finally, the various tasks performed by the partic-
ipants allowed to access a large range of every day
contraction pattern. However, this obviously does not
represent free-living signals because we seek to for-
merly characterize the stylohyoid and the posterior di-
gastric, with regard to the criteria described section 1.
Therefore, each tasks were performed with no addi-
tional motion to get a clear event on the signals. First
sight on the data confirmed the literature on the ac-
tivation of the stylohyoid and the posterior digastric
during swallowing. Further analysis are currently car-
ried out to provide extensive results.
4 CONCLUSION
Very few studies focused on the functional analy-
sis of the stylohyoid and the posterior digastric mus-
cle because of their location. Also, none sought
to formerly characterize both their timings and con-
traction patterns in various tasks, including swallow-
ing. Yet, those muscles showed promising results
for the development of an implantable active artifi-
cial larynx, through the robust and safe real-time de-
tection of swallowing. Therefore, to formerly evalu-
ate their potential independently, we described in this
paper the precise acquisition protocol that we set up
with the use of intramuscular EMG, submental sur-
face EMG and swallowing sound measurement meth-
ods. Intramuscular EMG were used to obtain the
activity of both the stylohyoid and the posterior di-
gastric independently, with the insertion of the nee-
dles following the localization of precise anatomical
structure. In addition, submental surface EMG and
swallowing sound allowed to locate the beginning
of the swallowing and the moment the bolus passes
through the upper esophageal sphincter, respectively.
All these modalities give access to various key swal-
lowing events for a comprehensive characterization.
16 people underwent the full procedure and the volun-
teers were asked to perform a large variety of activity,
along with swallowing, to evaluation the recruitment
specificity of the stylohyoid and the posterior digas-
tric muscles. Besides, timing investigation requires
the localization of onset and offset of muscle activ-
ity and we proposed the use of a generalized likeli-
hood ratio approach. It allowed to effectively abstract
from background noise, following the modelisation of
baseline and muscle activities. This paper therefore
allowed us to access specific swallowing signals, to
investigate the potential utility of both the stylohyoid
and posterior digastric muscles in the real-time de-
tection of swallowing. Further work are on-going to
provide extensive characterization.
ACKNOWLEDGMENTS
This research has been carried out with funding from
the R
´
egion Auvergne Rh
ˆ
one Alpes.
REFERENCES
Cichero, J. A. and Murdoch, B. E. (2002). Detection of
Swallowing Sounds: Methodology Revisited. Dys-
phagia, pages 40–49.
German, R. Z., Crompton, A. W., and Thexton, A. J. (2009).
Stylohyoid and Posterior Digastric Measurement with Intramuscular EMG, Submental EMG and Swallowing Sound
53

Integration of the Reflex Pharyngeal Swallow Into
Rhythmic Oral Activity in a Neurologically Intact Pig
Model. Journal of Neurophysiology, pages 1017–
1025.
Hamlet, S. L., Nelson, R. J., and Patterson, R. L. (1990).
Interpreting the Sounds of Swallowing: Fluid Flow
through the Cricopharyngeus. Annals of Otology, Rhi-
nology & Laryngology, 99:749–752.
Kurosu, A., Coyle, J. L., Dudik, J. M., and Sejdic, E. (2019).
Detection of Swallow Kinematic Events From Acous-
tic High-Resolution Cervical Auscultation Signals in
Patients With Stroke. Archives of Physical Medicine
and Rehabilitation, pages 501–508.
Kurt, T., G
¨
urg
¨
or, N., Sec¸il, Y., Yıldız, N., and Ertekin, C.
(2006). Electrophysiologic identification and evalu-
ation of stylohyoid and posterior digastricus muscle
complex. Journal of Electromyography and Kinesiol-
ogy, 16:58–65.
Lee, J., Steele, C. M., and Chau, T. (2008). Time and
time–frequency characterization of dual-axis swal-
lowing accelerometry signals. Physiological Mea-
surement, page 1105.
Li, X., Zhou, P., and Aruin, A. S. (2007). Teager–Kaiser
Energy Operation of Surface EMG Improves Muscle
Activity Onset Detection. Annals of Biomedical Engi-
neering, pages 1532–1538.
Lippert, D., Hoffman, M. R., Britt, C. J., Jones, C. A.,
Hernandez, J., Ciucci, M. R., and McCulloch, T. M.
(2016). Preliminary Evaluation of Functional Swal-
low After Total Laryngectomy Using High-Resolution
Manometry. Annals of Otology, Rhinology & Laryn-
gology, 125:541–549.
Maclean, J., Cotton, S., and Perry, A. (2009). Post-
Laryngectomy: It’s Hard to Swallow. Dysphagia,
pages 172–179.
McKeown, M. J., Torpey, D. C., and Gehm, W. C. (2002).
Non-invasive monitoring of functionally distinct mus-
cle activations during swallowing. Clinical Neuro-
physiology, pages 354–366.
Morini
`
ere, S., Boiron, M., Alison, D., Makris, P., and Beut-
ter, P. (2008). Origin of the Sound Components Dur-
ing Pharyngeal Swallowing in Normal Subjects. Dys-
phagia, pages 267–273.
Okada, T., Aoyagi, Y., Inamoto, Y., Saitoh, E., Kagaya, H.,
Shibata, S., Ota, K., and Ueda, K. (2013). Dynamic
change in hyoid muscle length associated with trajec-
tory of hyoid bone during swallowing: analysis using
320-row area detector computed tomography. Journal
of Applied Physiology, pages 1138–1145.
Park, D., Lee, H. H., Lee, S. T., Oh, Y., Lee, J. C.,
Nam, K. W., and Ryu, J. S. (2017). Normal contrac-
tile algorithm of swallowing related muscles revealed
by needle EMG and its comparison to videofluoro-
scopic swallowing study and high resolution manom-
etry studies: A preliminary study. Journal of Elec-
tromyography and Kinesiology, pages 81–89.
Pearson, W. G., Langmore, S. E., and Zumwalt, A. C.
(2011). Evaluating the Structural Properties of
Suprahyoid Muscles and their Potential for Moving
the Hyoid. Dysphagia, pages 345–351.
Selvan, S. E., Allexandre, D., Amato, U., and Yue, G. H.
(2018). Unsupervised Stochastic Strategies for Ro-
bust Detection of Muscle Activation Onsets in Surface
Electromyogram. IEEE Transactions on Neural Sys-
tems and Rehabilitation Engineering, 26:1279–1291.
Shaw, S. M. and Martino, R. (2013). The Normal Swallow:
Muscular and Neurophysiological Control. Otolaryn-
gologic Clinics of North America, pages 937–956.
Steele, C. M. (2015). The Blind Scientists and the Elephant
of Swallowing: A Review of Instrumental Perspec-
tives on Swallowing Physiology. Journal of Texture
Studies, pages 122–137.
Takahashi, K., Groher, M. E., and Michi, K.-i. (1994).
Methodology for detecting swallowing sounds. Dys-
phagia, pages 54–62.
Tenan, M. S., Tweedell, A. J., and Haynes, C. A. (2017).
Analysis of statistical and standard algorithms for de-
tecting muscle onset with surface electromyography.
PLOS ONE, page e0177312.
Thexton, A. J., Crompton, A. W., and German, R. Z. (2012).
EMG activity in hyoid muscles during pig suckling.
Journal of Applied Physiology, pages 1512–1519.
Xu, Q., Quan, Y., Yang, L., and He, J. (2013). An Adaptive
Algorithm for the Determination of the Onset and Off-
set of Muscle Contraction by EMG Signal Processing.
IEEE Transactions on Neural Systems and Rehabilita-
tion Engineering, pages 65–73.
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
54
