
A Formal Probabilistic Model of the Inhibitory Control Circuit in the
Brain
Elisabetta De Maria
1
, Benjamin Lapijover
1
, Thibaud L’Yvonnet
2
, Sabine Moisan
2
and Jean-Paul Rigault
2
1
Universit
´
e C
ˆ
ote d’Azur, CNRS, I3S, Sophia Antipolis, France
2
INRIA, Sophia Antipolis, France
fi
Keywords:
Inhibitory Control, Biological Neural Networks & LI&F Model, Probabilistic Model, Model Checking.
Abstract:
The decline of inhibitory control efficiency in aging subjects with neurodegenerative diseases is due to anatom-
ical and functional changes in (pre)frontal regions of the brain. Among these regions, the basal ganglia play
a central role in the inhibitory control loop. We propose a probabilistic formal model of the biological neural
network governing the inhibitory control function and we study some of its relevant dynamic properties. We
also explore how parameter variations influence the probability for the model to display some key behaviors.
We model the different structures of the inhibitory control loop thanks to discrete Markov chains representing
Leaky Integrate and Fire neurons. The model is implemented and verified using the PRISM framework. The
final aim is to detect sources of pathological behaviors in the neural network responsible for inhibitory control.
1 INTRODUCTION
This work proposes a formal model of the neural net-
work governing the inhibitory control function of the
human brain and studies some of its dynamic prop-
erties. We chose this function because it has been
studied for a long time and several models already
exist (Verbruggen and Logan, 2009);moreover, it is
managed by a restricted amount of brain structures
(basal ganglia) which makes it easier to model than
other cognitive functions. The aim is to artificially
represent the behavior of inhibitory control in hu-
mans through a probabilistic model. The main ad-
vantage of probabilistic models is their ability to rep-
resent a wide variability of behaviour with a single
model. In the context of early onsets of neuropatholo-
gies, this approach is convenient as even healthy sub-
jects are not necessarily expected to ace clinical tests.
We model the different structures involved in the in-
hibitory control loop thanks to probabilistic discrete
Markov chains implementing a modified version of
Leaky Integrate and Fire neurons. Markov chains
are widely used in biology, especially for modeling
event driven probabilistic systems. In the case of
neurobiology, there have been various utilizations of
Markov chains to simplify neurons modeling (Saku-
mura et al., 2001; Nossenson and Messer, 2010; Inoue
et al., 2021). We rely on formal methods to implement
this model and on model checking techniques to vali-
date and explore it. More precisely, we use PRISM (a
state of the art probabilistic model checker) to imple-
ment the model and to perform model checking.
2 FORMAL MODELING AND
VERIFICATION
Model checking is a method developed in the eight-
ies (Clarke et al., 1986) for automatic verification of
software models. It helps identify software design
problems before implementation. In our case, we use
model-checking not to verify a software tool, but to
model the functioning of a brain structure and to ex-
plore all its possible behaviors. Hence probabilistic
models are well adapted.
Among the existing probabilistic model checkers,
we chose PRISM (Kwiatkowska et al., 2011) which is
well established in the literature, and compatible with
many other tools (parameter synthesis tools or other
model checkers). PRISM is a tool for formal modeling
and analysis of systems with random or probabilis-
tic behavior. It supports several types of probabilistic
models, discrete as well as continuous.
For the modeling formalism, we rely on discrete-
time Markov chains (DTMCs), which are transition
146
De Maria, E., Lapijover, B., L’Yvonnet, T., Moisan, S. and Rigault, J.
A Formal Probabilistic Model of the Inhibitory Control Circuit in the Brain.
DOI: 10.5220/0011625700003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 3: BIOINFORMATICS, pages 146-154
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

systems augmented with probabilities. Their set of
states represents the possible configurations of the
modeled system and the transitions between states
represent the evolution of the system, which occurs
in discrete-time steps. Probabilities to transit between
states are given by discrete probability distributions.
The dynamics of DTMCs can be specified thanks
to the PCTL
∗
(Probabilistic Computation Tree Logic)
temporal logic (Hansson and Jonsson, 1994). The
main PCTL
∗
state quantifiers that we use in this pa-
per are X (next time), F (sometimes in the future), G
(always in the future). The until operator, U, is such
that p1 U p2 means that property p1 remains true until
property p2 becomes true.
The most important PCTL
∗
operator is P, to rea-
son about the probability of event occurrences. P is
used to replace the usual path quantifiers forall and
exists. A property P bound [prop] is true in a state
s if the probability that property prop holds in all the
paths from s satisfies the bound bound (a compari-
son operator followed by a probability value). For ex-
ample, the property P= 0.5 [X (y = 1)] holds in a
state if the probability that y = 1 is true in the next
state equals 0.5. All the above state quantifiers, ex-
cept X, have bounded variants, where a time bound is
imposed on the property. Furthermore, the P PRISM
operator can be used as P=? [prop] to compute the
probability for prop to occur.
PRISM also supports positive real value user-
defined ”rewards” which can be seen as counters that
do not impact the number of states and transitions of
the model nor its behavior. The R operator allows
to retrieve reward values. Additional operators deal
with reward: we mainly use C (cumulative-reward).
PRISM model checking algorithms automatically val-
idate DTMCs over PCTL
∗
or reward-based proper-
ties. They compute the actual probability of some be-
havior of a model to occur. In addition, PRISM offers
the possibility to run experiments which is a ”way of
automating multiple instances of model checking” ac-
cording to its authors. This feature allows users to ob-
tain curves displaying the evaluation results of a prop-
erty with respect to one or several variables. Besides,
PRISM also proposes statistical model-checking, a
way to test properties through several simulations.
3 COGNITIVE FUNCTIONS AND
BRAIN STRUCTURES
Cognitive functions is a broad designation for brain
processes necessary in the acquisition and process-
ing of information and in reasoning (e.g., learning,
awareness, decision making (Kiely, 2014)). The con-
cept of cognitive functions is the base of many exist-
ing models of the brain mechanisms. For instance, J.
Hopfield modeled the associative memory with neural
networks (Hopfield, 1982). More recently, (Schmidt
et al., 2019) used artificial neuron models to under-
stand the relations between the brain waves and cog-
nitive functions such as working memory or executive
control. One of the main challenges of neurocognitive
science is to better understand the cognitive functions
and their interactions, which may improve the clinical
assessment methods and the therapies.
3.1 Biological Neuron
Biological neurons allow inter cellular communica-
tion via electrical signals. They can have thousands
of ramifications called dendrites that receive nerve
(sensory and motor) information, named afferent sig-
nals. On the other hand the axon is usually unique and
sends information (efferent signals) to other cells.
The action potential that runs through the axon is
a nerve impulse caused by the difference in ion con-
centration between the inside and the outside of the
neuron. When a neuron does not receive any sig-
nal, the membrane maintains a resting potential which
constantly balance the concentration of the potassium
ions on both sides of the membrane. Nerve impulses
modulate the membrane potential and if this poten-
tial exceeds the threshold of excitability it triggers in
turn an action potential (a.k.a. spike). A spike is a
quick rise and fall of the membrane potential. It trav-
els along the neuron axon to reach the synaptic termi-
nals (Purves et al., 2019). Depending on the emitting
neuron, the spike either directly travels to the den-
drites of the receiving neurons or triggers the release
of neurotransmitters. These neurotransmitters reach
receptors on the dendrite side of the synaptic connec-
tion. In both cases, the receiving neuron follows the
same cycle as the neuron that sent the spike.
3.2 Inhibitory Control Circuit
According to the literature (Jahanshahi et al., 2015),
the brain regions involved in the inhibitory control
cognitive function are the cortex, the basal ganglia,
and the thalamus. Together, these anatomical struc-
tures make it possible to temporarily stop the action
of the motor cortex and therefore to inhibit an irrele-
vant action initially planned. A deficit in this circuit
can cause cognitive impairment (Braak et al., 2002).
The basal ganglia (figure 1) are considered as mo-
tor structures that allow movements to start and stop.
Basal ganglia are essentially a part of the motor loop
and thus of the inhibitory control circuit. They con-
A Formal Probabilistic Model of the Inhibitory Control Circuit in the Brain
147

tain several anatomical structures such as the stria-
tum (STr), composed of the putamen and caudate nu-
cleus. These two latter structures are distinct in the
circuit, but in our formal model we only represent
the STr. This simplification is advisable to limit the
size and complexity of the model and acceptable be-
cause the striatum can be considered as a whole func-
tional zone (Johns, 2014). Basal ganglia also contain
the globus pallidus separated into two structures: the
outer segment Gpe and the inner segment Gpi (related
to the substantia nigra pars reticulata noted SNpr,
that we represent together in our model); the subtha-
lamic nucleus (STN); and the substantia nigra pars
compacta (SNpc). The cortex (Cx) and the thalamus
(Th) are also part of the inhibitory control loop but not
of the basal ganglia (Purves et al., 2019).
Figure 1: Brain coronal section of basal ganglia and its com-
ponents (from A. Gillies, M. H
¨
aggstr
¨
om & P. J. Lynch).
There are two pathways in the inhibitory control
loop. In the direct pathway, after receiving inputs
from the cortex, specialized STr neurons target spe-
cific neurons of the Gpi/SNpr complex with inhibitory
inputs (Mink, 1996). These inputs trigger the disinhi-
bition of the Th area, controlling the expression of the
desired motor program, leading to ”release” (Gray-
biel, 2000) the intended physical movement. In the
indirect pathway, STr neurons target the Gpe with in-
hibitory inputs leading to the dishinibition of STN. At
the same time, STN specialized neurons receive pow-
erful afferent signals from the cortex (Purves et al.,
2019) and target the Gpi/SNpr complex with excita-
tory inputs (Mink, 1996). These inputs trigger the in-
hibition of Th (Graybiel, 2000) in a way that removes
all the unwanted motor programs.
The decline of inhibitory control efficiency in
aging subjects is due to anatomical and functional
changes in (pre)frontal regions (Hu et al., 2014).
However, there are differences between healthy and
pathological aging. One of our goals is to differenti-
ate these two conditions. It has been shown (Crawford
et al., 2005) that in neurodegenerative diseases, such
as Parkinson’s disease, there is a degeneration of the
neurons of the substantia nigra pars compacta (SNpc).
The dopaminergic influx coming from the SNpc and
targeting STr is considerably reduced. STr is then less
inhibited which consequently reduces the inhibitory
emission of the basal ganglia, removes the inhibition
of the thalamus and therefore the motor inhibition.
4 COMPUTATIONAL MODELING
4.1 Inhibitory Control Models
Several models of the inhibitory control use the
go/no-go task to study and describe its underlying
mechanisms, for example, the horse-race models re-
viewed in (Verbruggen and Logan, 2009). They see
the inhibitory control function as a competition be-
tween a ”go” and a ”stop” process in the brain. This
modeling approach gave many insights on the mech-
anisms of the inhibitory control (Schall and Godlove,
2012). With the rise of computational simulation
techniques, the past decades have seen the develop-
ment of models based on neural networks with respect
to neuroanatomy (Schroll and Hamker, 2013).
The model presented in this work is inspired by
the work of (Wei and Wang, 2016) which attempted
to model the inhibitory control function with a set of
(heavy) biological ”Leaky Integrate and Fire” neuron
networks representing each brain structure involved
in this function.
4.2 Artificial Neural Network Models
The Leaky Integrate and Fire (LI&F) model is a good
compromise between biological fidelity and compu-
tational efficiency for mathematical analysis (Izhike-
vich, 2004).
4.2.1 Leaky Integrate and Fire Discrete Model
According to the LI&F model, the membrane of
a neuron can be represented as an electronic cir-
cuit (Brunel and Van Rossum, 2007). In this model
the intensity of the action potential is neglected,
but the instant of its occurrence plays an important
role (Paugam-Moisy and Bohte, 2012). We consider
a discrete version of the model where the membrane
potential u at time t is defined by
u(t) =
∑
n
i=1
w
i
· x
i
(t) + r · u(t − 1)
i f u(t − 1) < Tau
∑
n
i=1
w
i
· x
i
(t) otherwise
where x
i
(t) ∈ {0, 1} is the signal received at time t
by the neuron through its i
th
input synapse; w
i
is the
weight associated with the i
th
input synapse; r is the
leak factor; Tau is the excitability threshold beyond
which the neuron emits an action potential. If the
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
148

membrane potential at time t exceeds the threshold,
a spike is emitted and the membrane potential is reset
to zero. The neuron output function s(t) ∈ {0, 1} is
therefore defined by
s(t) = 1 i f u(t) ≥ Tau, 0 otherwise
4.2.2 Generalization to Neuron Boxes
Our goal is to model the interactions of structures
of the brain (made of thousands of neurons) while
keeping model checking tractable. As modeling each
and every neurons would make the model difficult to
check, we introduce a generalization of the LI&F neu-
ron to neuron boxes. We modified the equation of the
LI&F neuron to represent several neurons, each one
producing its own output at a given instant.
Each anatomical structure of the inhibitory con-
trol circuit is represented by a box of ten neurons of
LI&F type. This number is not proportional to the ac-
tual number of neurons in the brain structures. It is
a trade off between biological accuracy and compu-
tation capacity: the model checking experiments (see
5.4) show that this simplification is realistic enough
to represent these brain structures. Indeed, a network
of ten neurons cannot mimic the behavior of com-
plex networks with thousands of neurons. However,
it shows a ”firing ratio” (number of firing neurons out
of the ten ones) for each time step. Moreover, the
generalization allows to model these ten neurons with
only one entity that directly computes the firing ratio
at each time step. Thus boxes makes it possible to
have a behavior relatively close to a small network of
neurons, without requiring a lot of computing power.
To take into account the difference of sizes be-
tween biological structures, the weight of each con-
nection of the model was set to a value proportional
to the weight of the same connection and the number
of neurons connecting two structures in the model of
Wei and Wang. The dynamic of a box is defined by:
U(t) =
∑
n
i=1
w
i
· X
i
(t) + r ·U(t − 1) · (
N−S(t−1)
N
)
This formula is close to the usual LI&F one (see sec-
tion 4.2.1) except that Boolean x
i
is replaced by in-
teger X
i
ranging from 0 to 10 to mimic the possible
10 neuron inputs from another box. Another simplifi-
cation is that when a biological connection exists be-
tween two boxes A and B, all neurons of box A are
considered to be connected to all neurons of box B.
Thus, the new formula does not take Boolean in-
puts but input firing ratio. This simplification led to
a new parameter, N, that represents the number of
neurons in the whole box (N = 10 in the presented
model). As neurons are far less sensitive to stim-
uli after emitting a spike due to the ”refractory peri-
ods” (Purves et al., 2019), the last term of the formula
was introduced. In this term, S(t − 1) represents the
number of neurons which discharged at the previous
time step. More precisely, S(t) is defined by
S(t) =
U(t)
Tau
, where 0 ≤ S(t) ≤ N
To track the activity of a box in the model, one should
check the values of S(t) of this box in the time win-
dow of interest.
Since biological inputs from the cortex are ir-
regular, the input spikes are modeled by a Poisson
law (Heeger, 2000). To approximate this irregularity,
Wei and Wang modeled these inputs as Poisson spike
trains. Our model uses the same Poisson function to
compute the activation probabilities of the cortex and
SNpc neurons: P
(
k
)
=
e
−λ
λ
k
k!
, where k is the number
of cortex and SNpc neurons that send a spike.
5 MODEL AND VALIDATION
The first task was to provide a formal model of the
main interactions between the different basal gan-
glia nuclei. We developed a PRISM model to artifi-
cially mimic the behavior of human inhibitory con-
trol through a probabilistic model based on discrete-
time Markov chains. Second, we automatically tested
probabilistic temporal properties of this model thanks
to model-checking to explore potential sources of
pathological behavior in the inhibitory control circuit.
The complete code of the model and supplementary
materials can be found at https://gitlab.com/ThibLY/
inhibctrlformmodel.git.
5.1 Basal Ganglia Model Overview
Neuropsychologists and neurobiologists have theo-
rized several models of the functioning of the basal
ganglia which have already been integrated into soft-
ware systems. In (Wei and Wang, 2016) the authors
proposed a neural network model of LI&F neurons
for the functional structures of the basal ganglia. They
obtained diagrams to visualize e.g., the importance of
some specific connections in inhibitory control.
We keep the same division into functional struc-
tures. As mentioned, we mainly use model check-
ing techniques that allow the automated exploration
of each state of a model to validate or to reject a given
property. However, these methods imply to imple-
ment models with lower complexity than simulation
methods. Thus, our model follows the architecture of
Wei and Wang using LI&F neuron boxes and other
adaptations, resulting in the graph of figure 2.
A Formal Probabilistic Model of the Inhibitory Control Circuit in the Brain
149
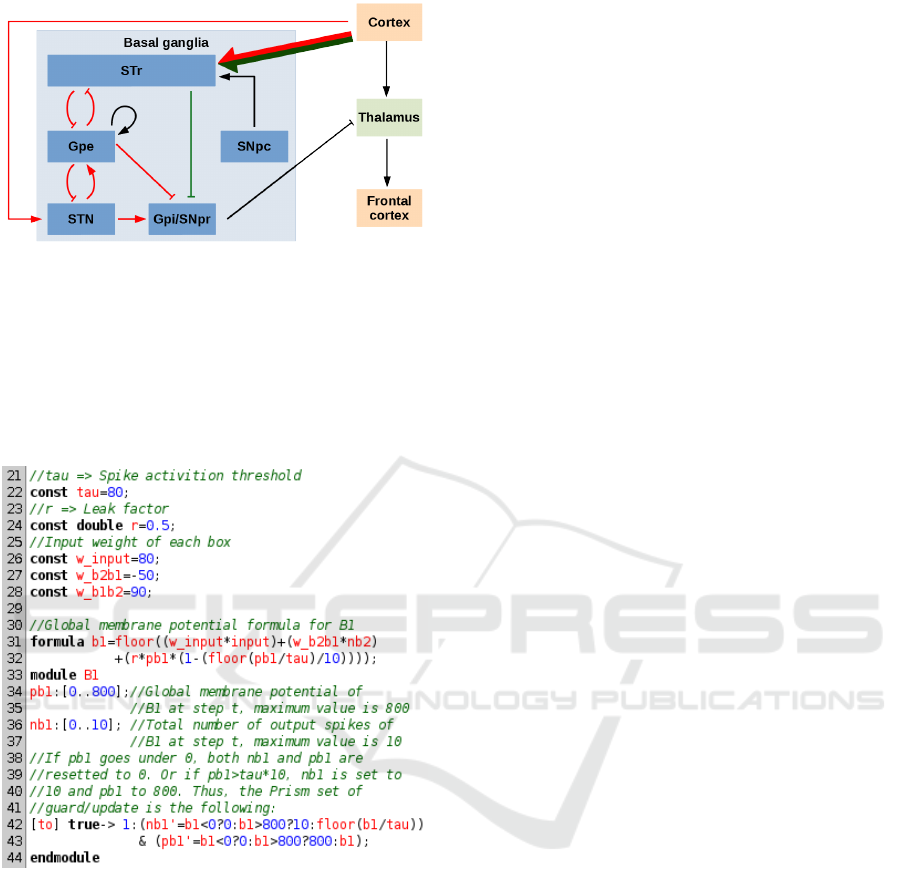
Figure 2: Inhibitory control circuit diagram. In green: di-
rect pathway, in red: indirect one. A classic arrow corre-
sponds to an excitation, a flat-tipped arrow to an inhibition.
The PRISM code for a single box of neurons fol-
lows the formula in section 4.2.2. We added condi-
tions to limit the output firing ratio of boxes to val-
ues between 0 and 10 as each box represents 10 neu-
rons (see figure 3). The whole model contains all the
Figure 3: Example of PRISM code of a box of neurons re-
ceiving inputs from another box and the inputs of the model.
biological structures represented in the basal ganglia
square of figure 2 plus the thalamus. We introduced
excitatory and inhibitory connections between them
and we add connections from the cortex as inputs;
these inputs follow a Poisson law determining the
number of spikes sent to the STr and Th boxes (sec-
tion 4.2.2). Finally, we implemented an additional
Delay box between the STN and the SNpr boxes to
enforce a discrete loop and we integrated alternative
connections between the SNpc and STr to differenti-
ate a healthy brain from a brain with a degenerated
SNpc. For this we defined 2 different formulas (see
PRISM code in figure 4): one takes into account both
the SNpc and the cortex inputs and the second one
only takes inputs from the cortex. Since (Cheng et al.,
2010) showed that the death of dopaminergic neu-
rons reaches 70% in the later phases of Parkinson’s
disease, we considered that a pathological brain has
a 30% probability to follow the first formula and a
70% probability to follow the second. In a healthy
brain, dopaminergic neurons are functional so the
brain model will always follow the first formula.
5.2 PRISM Model Implementation
The model was implemented and verified with the
PRISM framework. Each neuron box is implemented
by one PRISM module. All the neuron box mod-
ules have a common structure with two variables (for
global membrane potential and firing ratio) and one
set of guards and updates.
5.2.1 Healthy Inhibitory Control Model
The implementation in PRISM of a healthy inhibitory
control contains six modules: Entry (partial repre-
sentation of cortex and SNpc), STr, GPe, STN, SNpr
(representing the Gpi/SNpr complex considered as a
single functional structure), and Th. These modules
have an associated formula for their global membrane
potential and constants for their connection weights,
except Entry that generates input spikes following a
Poisson law. Their connections follow the architec-
ture of the basal ganglia shown in figure 2. However,
we add two modules. First, the Delay module is a
simplified neuron box that sends the same amount of
spikes that it receives but at the next instant. This
supplementary module is necessary as our model is a
discrete system. It allows the SNpr to receive sig-
nals from GPe and STN simultaneously, by delay-
ing signals from STN to make them reach SNpr at
the same instant as the ones from GPe. Second, the
Inhibitor module generates no-go signals at regular
intervals (every ten counts) to simulate external in-
hibitory events. In the model, it sends stop signals
to the ST N module. As shown in figure 2, the T h
module receives the final inhibition signal; hence, if
its output firing ratio is low, it indicates a successful
inhibition.
5.2.2 Parkinsonian Inhibitory Control Model
To model the behavior observed in Parkinson’s dis-
ease, we proposed an alternative formula for the
global membrane potential of STr (see figure 4). The
STr neuron box module has one more update in its set
of guards and updates. It has a probability of 0.3 to
update its membrane potential with the ”healthy” for-
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
150

Figure 4: Code excerpt: brain with Parkinson syndrome.
mula and of 0.7 to update it with the ”pathological”
one, according to (Cheng et al., 2010).
5.3 Properties of Individual Boxes and
of Box Synchronization
The first step was to validate the boxes individu-
ally. Each neuron box must respect the specifications
stated in section 4.2.2. In particular, at the box level,
the model must verify the conditions concerning the
global membrane potential and the firing ratio: the
maximum and minimum global membrane potential
must not be exceeded; the maximum and minimum
number of spikes must be respected; as long as the
global membrane potential is not greater than or equal
to a threshold, there should be no spike. Thus, we ver-
ified that the corresponding PCTL
∗
properties, which
are invariants of the boxes, hold for all boxes. In the
property below, X denotes the current box under test,
n X is the number of spikes emitted by box X, and
potential X is the global membrane potential of X.
As a reminder, P =? is the PCTL operator to com-
pute a probability and F = nprop indicates whether
property prop is true at time step n.
Property 1. What is the probability for the number
of spikes to always be 0 until the potential equals or
exceeds tau = 80?
P =?[n X = 0 U potential X ≥ 80]
The resting potential of a neuron is −10mV and
the biological threshold is 70mV; since the resting po-
tential in our model is 0 we chose a threshold value of
80. The PRISM model checker gives a positive answer
(P=1) for all properties and all boxes.
The next step is to validate the synchronization
of the boxes which must be connected to respect the
known properties about the connections of the corre-
sponding biological structures, as shown in figure 2.
As examples, the next properties check both the direct
and indirect pathways from the instant the stop signal
is sent (arbitrarily chosen to be the tenth).
Property 2. What is the probability for the stop
signal to rise at the 10
th
instant?
P =?[F = 10 n
ST N > 3]
Property 3. What is the probability for GPe and the
Delay box to be activated at the 11
th
instant?
P =?[F = 11 n GPe > 3 & n Delay > 3]
Property 4. What is the probability for STr to be
inhibited and SNpr to be activated at the 12
th
instant?
P =?[F = 12 n STr < 5 & n SN pr > 3]
PRISM explicit model checking engine gives the
expected valid answers (P=1). The model shows the
disinhibition and activation of Gpi/SNpr nuclei that
trigger the inhibition of the thalamus and thus the in-
hibition of an action. Moreover, the simulation graph
in figure 5 (obtained with the ”run experiment” and
statistical model-checking tools of PRISM) showing
spiking activity also confirms the inhibition of STr
and Th following a stop signal. Though they are not at
the same scale level, these activities can approximate
the simulation results of (Wei and Wang, 2016).
Figure 5: STN-SNpr-Th path. The stop signal reaches the
STN, enables the SNpr activation (through the STr inhibi-
tion at the same time (t = 13)), and finally the thalamus
inhibition, and therefore of the behavioral response; in red:
STN, in blue: SNr, in green: Th.
5.4 Property on Thalamus Inhibition
To deduce the probability that a movement is actually
inhibited, we defined a property evaluating the
probability to observe a low number of firing neurons
in the thalamus box. We arbitrarily consider that the
Th box is inhibited when it releases less than 4 spikes
(40% of the maximal ”firing ratio” which is 10 in our
implementation). In PCTL* this property is written:
A Formal Probabilistic Model of the Inhibitory Control Circuit in the Brain
151
Property 5. What is the probability for Th to be
inhibited at the first stop trial (13
th
time-step)?
P =?[F = 13(n T h < 4)]
We compared our model checking results with the
simulation results of Wei and Wang. The behavior of
our formal inhibitory control model is close to the re-
sults presented in (Wei and Wang, 2016). This behav-
ior is still described as a race in which the stop signal
information has to transit from STN to SNpr before
the STr inputs inhibit SNpr (Schmidt et al., 2013).
Wei and Wang conducted an experiment on their
model by modulating the network weights. The goal
was to determine if the inhibitory control behavior
is more sensitive to the modulation of some connec-
tions than to others. To reproduce this experiment on
our model, we doubled the weight of all the connec-
tions one at a time and we computed the probability
to reach a state where the number of spikes emitted
by the thalamus is less than 4. The results, shown in
figure 6, go slightly beyond those of (Wei and Wang,
2016). In this figure a + indicates that increasing the
Figure 6: Inhibitory control circuit with modification of
connection weights.
weight of the associated connection increases the Stop
Signal Reaction Time (SSRT) (lower probability for
Th inhibition), and a − indicates that increasing the
weight decreases SSRT (higher probability for Th in-
hibition). This experiment highlights two categories
of connections: those which facilitate thalamus inhi-
bition and those which prevent it. The STr-GPe, STN-
SNpr, and SNpr-Th connections belong to the first
category while GPe-GPe, STN-GPe, Gpe-SNpr, and
STr-SNpr connections belong to the second one (see
figure 6). To the best of our knowledge these specific
connections are yet to be studied in vivo. Such results
need further biological experiments for validation.
Table 1 shows results and computation times for
the verification of property 5 for the model with-
out modification and for some connection modifica-
tions. The decrease of the number of firing neurons
in the STr-GPe, STN-SNpr, and SNpr-Th connections
causes a decrease in the probability of inhibition and
Table 1: Model checking results (explicit engine) for prop-
erty 5 of the model without modifications (Original) and
after weight modification of specific connections.
Connection Result Time (s)
Original 0.8527 0.288
Gpe-SNpr 0.5251 0.303
STr-SNpr 0.4889 0.300
STr-GPe 0.9432 0.310
STN-SNpr 1 0.267
SNpr-Th 1 0.250
consequently a decrease in behavioral inhibition. A
decrease in the inhibition probability in the model
means a longer SSRT leading to the fulfillment of an
unwanted action. More bibliographical research may
be necessary to evaluate the relevance of these results.
5.5 Comparison of Impaired versus
Healthy Brain Models
In a healthy brain all SNpc neurons are present. As
the number of SNpc neurons depletes in Parkinson’s
disease (Cheng et al., 2010), with about 30% neu-
rons remaining in SNpc of advanced Parkinson’s, an
unhealthy brain only has 30% probability of taking
SNpc neurons into account. Still with property 5 we
Table 2: Results of property 5 for the model without modi-
fications (Healthy) and the Parkinsonian model.
Result Time (s)
Healthy 0.8527 0.288
Parkinson 0.7651 2.42
found that there is a greater chance of getting a thala-
mus inhibition in the case of a healthy brain (table 2).
The second formula in figure 4 and its associated 30%
probability are enough to lead to smaller chances of
action inhibition (of the order of 10
−1
).
6 CONCLUSION AND
PERSPECTIVES
A better understanding of the mechanisms of in-
hibitory control could allow targeted treatments for
different classes of patients with dementia. This work
proposed a discrete probabilistic model of the in-
hibitory control circuit of the brain and its formal val-
idation. This model reproduces known biological be-
haviors from the literature such as the pathway race.
Our model also faithfully represents the importance
of some connections in the pathways (e.g., SNr and
STr connections). These behaviors were translated in
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
152

PCTL properties to check the adequacy of the model.
We also ran an experiment to explore the sensitivity
of inhibitory control to the modulation of some con-
nections. The modified model complies with Parkin-
son’s disease. Further modifications to represent, e.g.,
Alzheimer’s disease are planned as future work.
Probabilistic formal models can represent a wide
variety of behaviors while enabling model checking.
To check our model with standard tools, it was neces-
sary to brought up a new generalization of the LI&F
classical neuron model to represent small networks
behavior with a single module. This work opens new
avenues for the formal modeling of cognitive func-
tions. Moreover, it has proven the feasibility of such
model exploration using only off the shelf laptops.
In the future, the model will be coupled with the
activity model of a patient playing a serious game tar-
geting the inhibitory control function. The goal is to
explore modifications in the brain neural network that
may generate a patient behavior characteristic of neu-
rocognitive disorders.
REFERENCES
Braak, H., Tredici, K. D., Bratzke, H., Hamm-Clement, J.,
Sandmann-Keil, D., and R
¨
ub, U. (2002). Staging of
the intracerebral inclusion body pathology associated
with idiopathic Parkinson's disease (preclinical and
clinical stages). Journal of Neurology, 249.
Brunel, N. and Van Rossum, M. C. (2007). Lapicque’s 1907
paper: from frogs to integrate-and-fire. Biological cy-
bernetics, 97.
Cheng, H. C., Ulane, C. M., and Burke, R. E. (2010). Clin-
ical progression in Parkinson disease and the neurobi-
ology of axons. Annals of Neurology, 67.
Clarke, E. M., Emerson, E. A., and Sistla, A. P. (1986).
Automatic verification of finite-state concurrent sys-
tems using temporal logic specifications. ACM Trans-
actions on Programming Languages and Systems
(TOPLAS).
Crawford, T., Higham, S., Renvoize, T., Patel, J., Dale, M.,
Suriya, A., and Tetley, S. (2005). Inhibitory control of
saccadic eye movements and cognitive impairment in
Alzheimer’s disease. Biological Psychiatry, 57.
Graybiel, A. M. (2000). The basal ganglia. Current biology,
10.
Hansson, H. and Jonsson, B. (1994). A logic for reasoning
about time and reliability. Formal aspects of comput-
ing.
Heeger, D. (2000). Poisson model of spike generation.
Handout, University of Standford, 5.
Hopfield, J. J. (1982). Neural networks and physical sys-
tems with emergent collective computational abilities.
Proceedings of the national academy of sciences, 79.
Hu, S., Chao, H. H.-A., Zhang, S., Ide, J. S., and Li, C.-
S. R. (2014). Changes in cerebral morphometry and
amplitude of low-frequency fluctuations of bold sig-
nals during healthy aging: correlation with inhibitory
control. Brain Structure and Function, 219.
Inoue, H., Hukushima, K., and Omori, T. (2021). Esti-
mation of Neuronal Dynamics of Izhikevich Neuron
Models from Spike-Train Data with Particle Markov
Chain Monte Carlo Method. Journal of the Physical
Society of Japan, 90.
Izhikevich, E. M. (2004). Which model to use for cortical
spiking neurons? IEEE transactions on neural net-
works, 15.
Jahanshahi, M., Obeso, I., Rothwell, J. C., and Obeso,
J. A. (2015). A fronto–striato–subthalamic–pallidal
network for goal-directed and habitual inhibition. Na-
ture Reviews Neuroscience, 16.
Johns, P. (2014). Chapter 3 - functional neuroanatomy.
In Johns, P., editor, Clinical Neuroscience. Churchill
Livingstone.
Kiely, K. M. (2014). Cognitive function. In Michalos, A. C.,
editor, Encyclopedia of Quality of Life and Well-Being
Research. Springer Netherlands, Dordrecht.
Kwiatkowska, M., Norman, G., and Parker, D. (2011).
PRISM 4.0: Verification of probabilistic real-time sys-
tems. In Proc. 23rd Int. Conf. on Computer Aided Ver-
ification (CAV’11).
Mink, J. W. (1996). The basal ganglia: Focused selec-
tion and inhibition of competing motor programs.
Progress in Neurobiology, 50.
Nossenson, N. and Messer, H. (2010). Modeling neuron fir-
ing pattern using a two state markov chain. In 2010
IEEE Sensor Array and Multichannel Signal Process-
ing Workshop.
Paugam-Moisy, H. and Bohte, S. M. (2012). Computing
with spiking neuron networks. Handbook of natural
computing, 1.
Purves, D., Augustine, G.-J., Fitzpatrick, D., and Hall, W.
(2019). Neurosciences. Broch
´
e.
Sakumura, Y., Konno, N., and Aihara, K. (2001). Markov
chain model approximating the hodgkin-huxley neu-
ron. In Dorffner, G., Bischof, H., and Hornik, K.,
editors, Artificial Neural Networks — ICANN 2001,
Berlin, Heidelberg. Springer Berlin Heidelberg.
Schall, J. D. and Godlove, D. C. (2012). Current advances
and pressing problems in studies of stopping. Current
Opinion in Neurobiology, 22. Decision making.
Schmidt, R., Herrojo Ruiz, M., Kilavik, B. E., Lundqvist,
M., Starr, P. A., and Aron, A. R. (2019). Beta oscilla-
tions in working memory, executive control of move-
ment and thought, and sensorimotor function. Journal
of Neuroscience, 39.
Schmidt, R., Leventhal, D. K., Mallet, N., Chen, F., and
Berke, J. D. (2013). Canceling actions involves a
race between basal ganglia pathways. Nature neuro-
science, 16.
Schroll, H. and Hamker, F. (2013). Computational models
of basal-ganglia pathway functions: focus on func-
tional neuroanatomy. Frontiers in Systems Neuro-
science, 7.
Verbruggen, F. and Logan, G. D. (2009). Models of re-
sponse inhibition in the stop-signal and stop-change
A Formal Probabilistic Model of the Inhibitory Control Circuit in the Brain
153

paradigms. Neuroscience & Biobehavioral Reviews,
33. Translational Aspects of Stopping and Response
Control.
Wei, W. and Wang, X.-J. (2016). Inhibitory control in the
cortico-basal ganglia-thalamocortical loop: Complex
regulation and interplay with memory and decision
processes. Neuron, 92.
BIOINFORMATICS 2023 - 14th International Conference on Bioinformatics Models, Methods and Algorithms
154
