
Constrained ALS for Estimation of Human Upper Limb Synergies
Rabya Bahadur
1,2 a
and Saeed ur Rehman
2
1
Department of Electrical & Computer Engineering, COMSATS University Islamabad, Abbottabad Campus, Pakistan
2
Department of Electrical & Computer Engineering, SS CASE Institute of Information Technology, Multi Garden,
Keywords:
Non-Negative Matrix Factorization, Neuromuscular Synergies, Correlation, Regularization, Electromyogram.
Abstract:
Human body movement is a complex task that requires the control of multiple joints via a network of muscles.
The biological signals, particularly electromyography (EMG), are correlated by nature. The brain transmits
these signals through the neuromuscular transmission system of the body. The combination of muscular acti-
vation for each particular movement presents a set of weights known as synergies. The traditional alternating
least square (ALS) based non-negative matrix factorization (NMF) gets trapped in local minima for co-linear
data. Therefore, it is not a suitable method for extracting muscle synergies. This paper advocate using l2-norm
as an additional constraint for ALS-based NMF. The addition of l2-norm decorrelates the data, resulting in a
better estimation of synergistic weights. For our results, we acquired EMG signals from six healthy subjects.
Both plain and regularized NMF were used to extract the synergies. The synergies acquired via plain NMF
have a higher cross-correlation within and indicate the triggering of the same muscles irrespective of the tar-
geted isometric contraction. In contrast, the regularized NMF synergies targeted the correct muscular set for
a particular isometric contraction. Our results show that the synergies acquired via regularized NMF are also
more correlated with the physiologically inspired synergies.
1 INTRODUCTION
Human body movement is a complex task requir-
ing multiple joint control via a network of muscles.
The human hand movement is performed by a pair
of agonist/antagonist muscles in coordination with
assistance from adjacent muscles(Ayhan and Ayhan,
2020). The agonist muscle is a prime mover, while
the antagonist is a sleeping partner in that direction.
For the opposite direction of the same joint, the role
of the agonist and antagonist are switched. The par-
ticipation of muscles in any movement varies between
0 to 1 on a normalized scale. Where 0 refers to relax
state of a muscle while 1 refers to maximum contrac-
tion. The action of these muscles is controlled via
the neuromuscular translational system of the human
body. It is a hierarchical process; initiated by the mo-
tor neurons in the brain, ending up at synergistic level
activation (Bahadur and Rahman, 2018). Synergies
describe the level of muscle activation involved in a
particular movement. The concept of muscular syn-
ergies reduces the dimensionality of complex human
neuromuscular coordination systems.
a
https://orcid.org/0000-0002-1923-403X
Finding the muscle synergies can be challenging
as the only available data is a matrix of recorded
electromyographic (EMG) signals. Separating mus-
cle synergies from time-variant motor unit action po-
tentials using surface EMG is a blind source separa-
tion problem(Bahadur R. and Khan, 2021). There ex-
ist several algorithms in literature for the estimation
of muscle synergies, among which the most promi-
nent are principal component analysis (PCA), inde-
pendent component analysis (ICA), and non-negative
matrix factorization (NMF) (Rodrigues et al., 2022;
Rasool et al., 2016; Ma et al., 2021). PCA requires
the sources to be uncorrelated, while ICA requires in-
dependence of the sources (Naik and Nguyen, 2015;
Zhao et al., 2022). For biological systems, such as-
sumptions about the sources cannot be established;
therefore, the literature is more focused on NMF and
its variants (Roh J. and Bee, 2015; Naik and Nguyen,
2015; Lin C. and Farina, 2018; Bahadur R. and Khan,
2021). NMF is a non-convex optimization problem;
the implementation of the problem via an iterative
multiplicative update was a revival of this technique
(Lee and Seung, 2000). However, the convergence of
this algorithm is not guaranteed (Lin, 2007); there-
fore, several algorithms are adopted to implement
Bahadur, R. and Rehman, S.
Constrained ALS for Estimation of Human Upper Limb Synergies.
DOI: 10.5220/0011604400003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 4: BIOSIGNALS, pages 29-35
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
29

NMF. One very famous approach is the alternating
least square (ALS) method. The solution is faster and
convergent; however, it gets trapped in local minima,
especially becoming very slow for co-linear data (Ci-
chocki and Zdunek, 2007).
Biological signals in general and EMG data, in
particular, are naturally co-linear; traditional ALS
lacks to provide an optimal solution. Therefore, this
paper advocates using constrained ALS-based NMF
for synergy estimation from EMG signals. The pa-
per is organized in the following manner, section 2
presents the mathematical details, while the experi-
mental setup is presented in section 3. The 4
th
module
describes synergies for elbow & shoulder based on the
physiological studies. The methodology is provided
in section 5. Results and discussion are presented in
section 6, followed by a conclusion at the end.
2 MATHEMATICAL MODEL
In the initial part of this section, we will introduce the
plain NMF, followed by the recommended regulariza-
tion function.
2.1 NMF and Traditional ALS
A standard NMF problem is defined by eq(1).
Y = SΦ(t) + E, S, Φ(t) ≥ 0 (1)
Where Y ∈ R
[N×M]
is the root mean square (RMS)
of the recorded EMG signal for a length of
0
T
0
a
, N is
the total number of channels, and M is the total num-
ber of RMS values, S ∈ R
[N×P]
is the synergy matrix
and P is the estimated rank of matrix Y. Φ(t) ∈ R
[P×M]
is a matrix of P time variant signals (motor units) and
E ∈ R
[N×M]
is the additive noise. For error minimiza-
tion, consider calculating the Euclidean norm.
D(Y|SΦ(t)) = ||Y − SΦ(t)||
2
, S, Φ(t) ≥ 0 (2)
Since both the motor signals and synergies are un-
known, the overall problem is non-convex. ALS pro-
poses the use of an alternating procedure for estima-
tion of S and Φ(t), converting the non-convex prob-
lem into convex (eq. (3)).
Φ
k
(t) = arg min
φ(t)
||Y − S
k−1
Φ(t)||
2
, Φ(t) ≥ 0
(3a)
S
k
= arg min
S
||Y − SΦ
k−1
(t)||
2
, S ≥ 0 (3b)
S
0
and Φ
0
(t) are initialized randomly. S
k
and Φ
k
(t)
are the k
th
estimates while S
k−1
and Φ
k−1
(t) are the
estimates from the previous iteration. Gradient de-
scent is adopted to attain minimum error. For pNMF,
there is only one essential constraint: elements of all
decomposed matrices should be positive; there is no
additional constraint in the traditional ALS. The prob-
lem can quickly get caught in the local minimum; si-
multaneously, the algorithm can become much slower
to converge for co-linear data such as EMG signals
(Cichocki and Zdunek, 2007). Therefore, additional
penalty functions are required to address these issues.
2.2 Constrained ALS for Synergy
Estimation
Stochastic theory’s two famous optimization con-
straints are the l
1
− norm and the l
2
− norm. l
1
−
norm is used for sparse data while l
2
− norm (also
known as regularization) has the inherited property
of decorrelating the channels(Cichocki and Zdunek,
2007). Physiological signals, especially multiple
channel EMG from a specific limb, are correlated
with each other (Ma et al., 2021). Therefore, this pa-
per uses regularization as an additional penalty func-
tion to accelerate and obtain an optimal solution for
synergy extraction from EMG signals using the ALS-
based NMF algorithm. It is a kind of regression that
restricts the error towards zero; thus, the modified ver-
sion of eq.(3) would be:
Φ
k
(t) = arg min
φ(t)
||Y − S
k−1
Φ(t)||
2
+ α
φ
||Φ
k
(t) − Φ
k−1
(t)||
2
, Φ(t), α
φ
≥ 0
(4a)
S
k
= arg min
S
||Y − SΦ
k−1
(t)||
2
+ α
S
||S
k
− S
k−1
||
2
, S, α
S
≥ 0 (4b)
The regularization parameters α
φ
and α
S
are non-
negative values that compel the variation in the de-
composed matrices towards zero. The values of these
parameters are based on error variance. The sys-
tem’s noise variance (σ
2
n
) is calculated from the initial
state where the muscles are at rest. The regulariza-
tion values are iteratively updated using the formula
α
k
= cα
k−1
;0 < c < 1,. The smaller values of the
regularization constraint result in the imposition of a
full rank matrix providing the possible optimal solu-
tion. For the given problem, the iterations stop when
an optimal value is achieved, i.e., α
k
≈ σ
2
n
.
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
30
3 EXPERIMENTAL SETUP
3.1 Consent & Declarations
Arms + Hands SRA Labs provided a database for car-
rying out this study. The experiment was conducted
with the consent of participants following the Decla-
ration of Helsinki under the approval of the North-
western University Institutional Review Board.
3.2 Participants
Six healthy subjects’ data is selected for this study.
The database comprised EMG signals using isomet-
ric contractions collected from two females and four
males aged 55 to 71. All subjects were right-hand
dominant with no neuromuscular impairment in the
upper limbs.
3.3 Setup
The participants were comfortably seated in a chair,
and the arm and hand movement was restricted using
a brace and strap mechanism; the setup is provided
in fig. (1a). Isometric contractions were applied in a
3D space, and forces generated were recorded using
the multi-axis cartesian-based arm rehabilitation ma-
chine (MARCARM). The subject grasped the MAR-
CARM’s handle to exert isometric contraction infor-
mally in a 3D space. Setup details can be further ex-
plored in (Roh J. and Bee, 2015) paper.
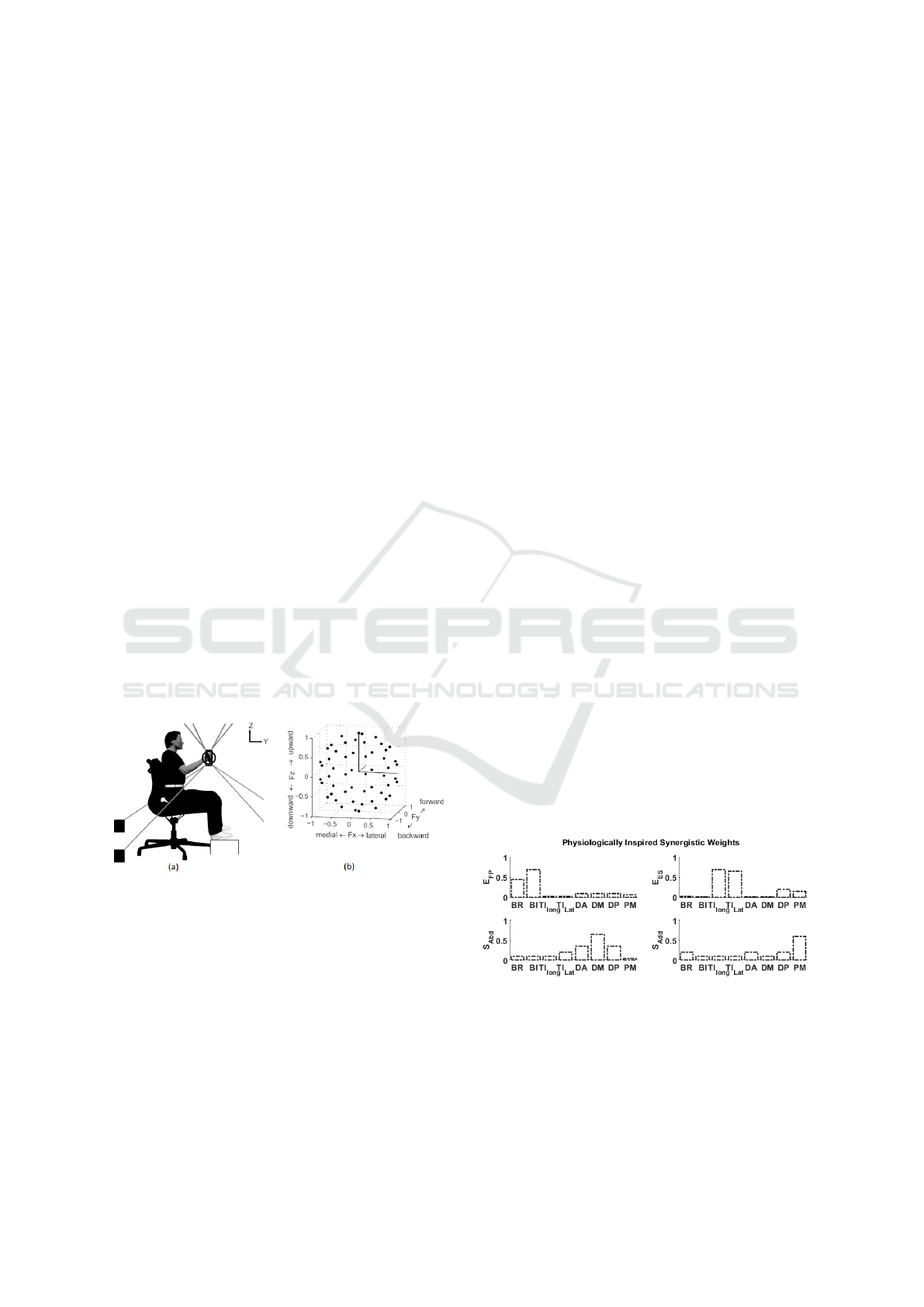
Figure 1: (a) Experimental brace and strap setup followed
using MARCARM. (b) Target force contractions applied in
multiple directions matching the exemplary target in 3D.
3.4 EMG Acquisition
EMG signals are recorded using the Delsys Bagnoli
EMG acquisition unit at a sampling frequency of 1850
Hz. The signals are filtered via a built-in zero-lag 20-
450 Hz bandpass filter. Eight channel surface EMG
data are recorded from both arms while performing
isometric force contractions in multiple directions,
presented in fig. (1b). The targeted muscles of the up-
per arm are bicep brachii (BI), brachioradialis (BRD),
tricep longus (T I
long
), tricep lateral (TI
lat
), deltoid an-
terior (DA), deltoid medial (DM), and pectoralis ma-
jor (PM). The recording duration is 9 sec for each
contraction with a 2 sec initial baseline period. A suc-
cessful trial would mean maintaining the target force
at the sphere’s center for 0.8 sec. A total of 10 trials
were performed for each target sphere in a 3D space.
To avoid fatigue, a break of 1 min is given between
each trial.
4 SYNERGY & HUMAN
PHYSIOLOGY
Synergy is the interrelated actuation of several mus-
cles using a smaller number of activation patterns.
The human upper arm works by activating the shoul-
der and elbow joints. The two joints are controlled
by employing multiple muscles working as antago-
nistic pairs with assistive secondary muscles (Ayhan
and Ayhan, 2020). Each shoulder and elbow joint
requires two dedicated synergies activating the flex-
ion and extension of each joint (Inouye and Cuevas,
2016). Thus, four synergies (i.e., 2
2
) are required to
analyze the movement of the upper arm. The four
synergies of the human upper limb based on the two
joints (elbow and shoulder) can be categorized as el-
bow flexor-pronator/extensor-supinator and shoulder
abduction/adduction. Based on our study of the kine-
siology of elbow and shoulder complex (Yesilyaprak,
2020; Ayhan and Ayhan, 2020) fig. (2) presents the
physiologically inspired synergistic weights present-
ing the involvement of the targeted eight muscles in
the movement of elbow and shoulder joints. The mus-
cles on x-axis are organized in such a manner that the
left most muscles start with the elbow activation while
the right most is pectoralis major of the shoulder.
Figure 2: Synergistic distribution involved in upper limb
movements according to human physiology. E
FP
: El-
bow Flexion-Pronation; E
ES
: Elbow Extension-Supination;
S
Add
: Shoulder Adduction; S
Abd
: Shoulder Abduction.
To understand the statistical behavior of the phys-
iologically inspired synergies, table (1) presents the
auto-correlation of the synergies. Elbow flexion and
Constrained ALS for Estimation of Human Upper Limb Synergies
31
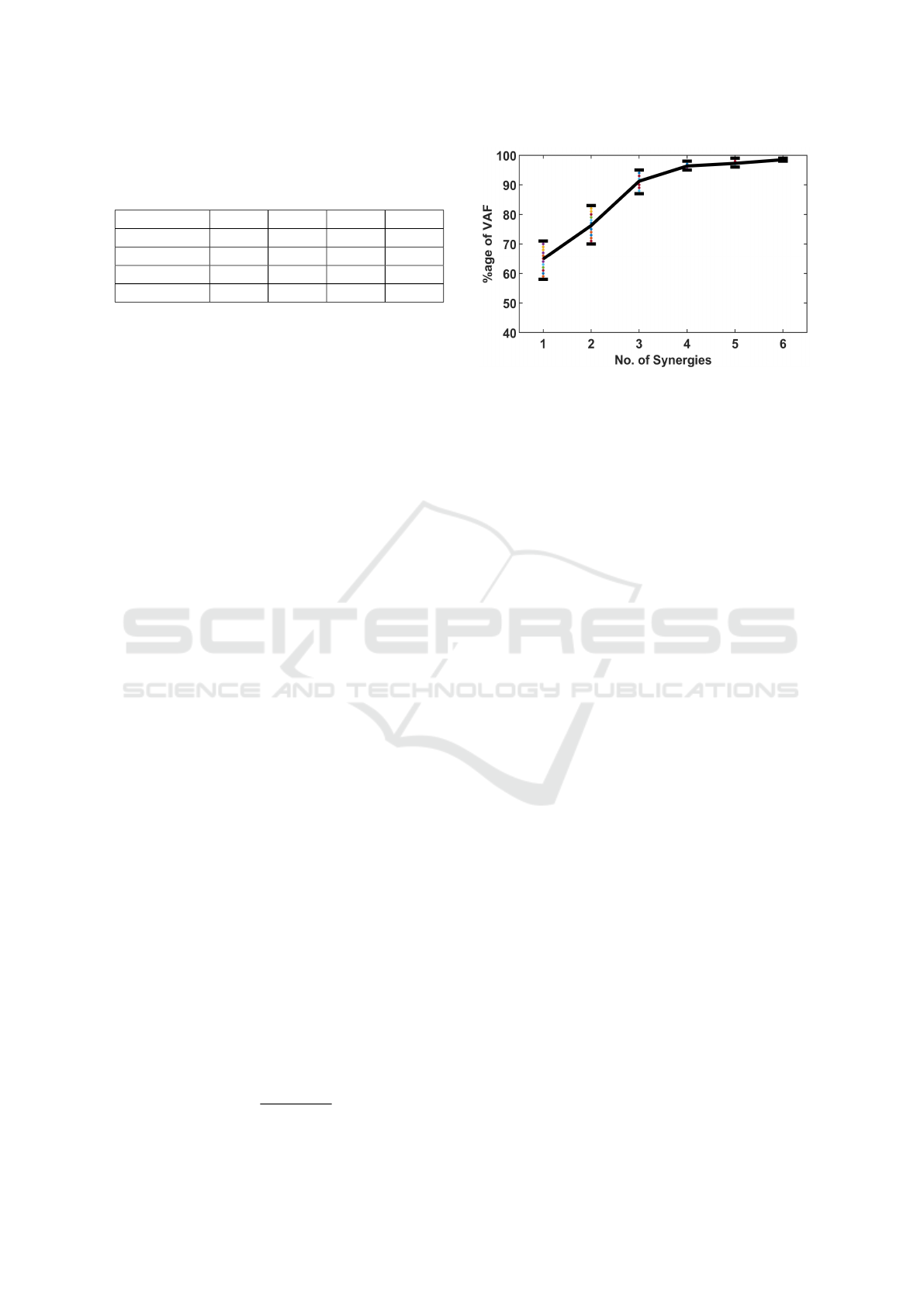
Table 1: Auto-correlation matrix of synergies based on hu-
man physiology. E
F
: Elbow Flexion; E
E
: Elbow Extension;
S
Add
: Shoulder Adduction; S
Abd
: Shoulder Abduction.
Movements E
FP
E
ES
S
Abd
S
Add
E
FP
1 -0.629 -0.380 -0.220
E
ES
-0.629 1 -0.111 -0.131
S
Abd
-0.380 -0.111 1 -0.350
S
Add
-0.220 -0.131 -0.350 1
extension result from the bicep-triceps muscles work-
ing as agonist/antagonist pair. Therefore, a minimum
correlation is observed between the two synergies.
The correlation among other cross synergies is neg-
ative or close to zero because the assistive muscles in
all movements are the same with variation in intensi-
ties, while prime movers are the exact opposite.
5 METHODOLOGY
5.1 Pre-processing
Matlab R2020a is used to analyze the data. The re-
spective mean value is subtracted from each surface
EMG signal for pre-processing, and the baseline was
removed. The active areas are selected based on sig-
nal segmentation and recorded timings. RMS of the
segmented surface EMG was calculated with a win-
dow length of 0.1 sec (T
a
= 180 samples) and an over-
lapping window, T
ol
= 10 samples. EMG signals from
multiple trials for each subject were concatenated to
acquire the synergies.
5.2 Variance Accounted For Test
We infer from eq. (1 & 2) that the pseudo rank ‘P’
of the matrix Y is substantial for effectively decom-
posing the EMG signals into motor signals and in-
tended synergistic weights. To determine the mini-
mum number of synergies (P) using NMF, we use the
Variance Accounted For (VAF) test, eq. (5). The tra-
ditional and regularized NMF were used to perform
VAF; from fig. (3) it can be observed that the knee-
curve is attained near 4 synergies with an average ac-
curacy of 96.5%. The findings of number of synergies
‘P’ is in accordance with the physiological study as
mentioned in section (4). Therefore, the dimension of
the synergy matrix S would be [8 × 4], where N = 8
presents the number of channels and P = 4 is the min-
imum number of synergies to effectively reconstruct
the database.
VAF =
1 −
var(Y −
ˆ
Y)
var(Y)
!
× 100 (5)
Figure 3: VAF test to determine the number of minimum
synergies required. Each dotted color presents percentage
the VAF score for the reconstructed EMG signal from its
decomposed matrices using NMF.
5.3 Synergy Alignment
As per our findings, the knee curve is obtained around
four synergies with an accuracy of 96.5% using the
VAF test. Therefore, four synergies were acquired us-
ing plain NMF and regularized NMF. As synergistic
weights are the coefficients associated with the tem-
poral motor signals; therefore, temporal motor signals
acquired via the NMF are used to align the muscle
synergies. For this purpose, a pivot signal is ran-
domly selected from an arbitrary subject. The correla-
tion between estimated motor signals across different
subjects is calculated. Maximum correlated value is
used in a nested procedure to align the four synergis-
tic weight patterns for the entire database.
6 RESULT & DISCUSSION
6.1 Synergy Estimation Via Traditional
ALS
This section provides a detailed discussion on the syn-
ergy estimation using the plain NMF, i.e., ALS with-
out additional constraints. The traditional ALS is an
iterative procedure; the estimations stop when a count
limit is reached. For this study, the count limit is set to
300. Synergistic muscle activation involves identify-
ing specific muscles engaged in a particular contrac-
tion. However, in fig. (4), we do not observe such
unique muscle activation patterns; in fact, different
synergies indicates the activation of similar muscles.
For example, on average same pattern is observed in
synergy 1 and 2 (with a correlation coefficient, r =
0.6), while synergy 2 and 3 are also closely correlated
(r = 0.647). Table (2) presents the auto-correlation
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
32

matrix for the four synergistic weights estimated via
plain NMF. It can also be inferred that the estimated
synergies lack the recognition of specific antagonistic
muscular pairs. Synergy 2 (fig.(4)-top right) refers to
the activation of BI and TI muscles simultaneously,
which is not feasible in case of a healthy subject. The
behavior observed in the cross-correlation of syner-
gies using plain NMF is very opposite to that assessed
by actual human physiology. These observations refer
to the inaccurate or non-optimal synergy estimation of
traditional ALS.
Figure 4: Synergistic estimated via traditional ALS-
based NMF. E
FP
: Elbow Flexion-Pronation; E
ES
: Elbow
Extension-Supination; S
Add
: Shoulder Adduction; S
Abd
:
Shoulder Abduction.
Table 2: Correlation matrix of synergies estimated using
plain NMF. E
F
: Elbow Flexion-Pronation; E
E
: Elbow
Extension-Supination; S
Add
: Shoulder Adduction; S
Abd
:
Shoulder Abduction.
Movements E
FP
E
ES
S
Abd
S
Add
E
FP
1 0.6 0.298 -0.414
E
ES
0.6 1 0.647 -0.4336
S
Abd
0.298 0.647 1 0.136
S
Add
-0.414 -0.4336 0.136 1
6.2 Synergy Estimation via Regularized
ALS
Regularized NMF refers to the adaptation of the
ALS algorithm with additional Tikhonov constraint.
The mathematical details are already discussed in
section(2.2). The algorithm converges when regu-
larization parameter reaches its optimal value α
∗
≈
σ
2
n
. Synergies extracted via regularized NMF are pre-
sented in fig. (5), and the correlation table is presented
in table (3;). The first synergy indicates activation of
bicep BI and BR, referring to elbow flexion. In com-
parison, the second synergy identifies elbow exten-
sion with the tricep (T I
lat
and T I
long
) as the most ac-
tive identified muscle, followed by PM and BR. Syn-
ergy 3 presents a low composite activation of BR, BI,
and shoulder muscles, referring to shoulder abduc-
tion. Finally, synergy 4 with dominant deltoid ante-
rior followed up by PM, T I
long
, and the BR as shoul-
der adduction. The behavior of synergies estimated
via the proposed algorithm and human-inspired phys-
iological synergies are closely related, p=0.753. The
cross-correlation among the synergies is negative in
the majority of cases (table(3)); a correlation of 0.38
is observed in the case of elbow flexion and extension
due to the secondary muscles involved. Unlike plain
NMF, regularized NMF does not simultaneously pro-
vide multiple antagonistic pair activation in a single
synergy.
Figure 5: Synergistic estimated via regularized NMF.E
FP
:
Elbow Flexion-Pronation; E
ES
: Elbow Extension-
Supination; S
Add
: Shoulder Adduction; S
Abd
: Shoulder
Abduction.
For better evaluation of synergies estimated via
plain and regularized NMF, this paper investigates
the cross-correlation of estimated weights with phys-
iological synergies, presented in fig.(6). Correla-
tion between estimated synergy and physiologically-
inspired synergy is called ‘auto’ if they belong from
the same order. Correlation between different or-
ders will be referred to as ‘cross’. In majority cases
regularized NMF provides maximum auto correlation
with physiologically-inspired synergy as compared
to plain NMF. Secondly, the synergies estimated via
plain NMF indicates a higher cross correlation; shoul-
der abduction synergy is comprised on the activation
of BR and BI as opposed to the actual deltoid muscles.
This synergy estimated via regularized NMF is also
having a consideraly low correlation (r = 0.6637),
yet it is able to detect the correct activation of deltoid
muscles. Also in case of elbow extension-supination
synergy a very low correlation factor (r = 0.1848 was
observed when comparing physiologically inspired
synergy to plain NMF. In this particular case, the syn-
ergy estimated via plain NMF shows an almost uni-
form activation of all muscles. In contrast, regular-
ized NMF targets the activation of tricep muscles in
assistance with PM (r = 0.8321).
It can be overall concluded that the synergy es-
timated via regularized NMF are rigorous and pro-
vides much similarity to the actual physiological data.
Therefore, this paper recommends using the proposed
regularized NMF compared to plain NMF.
Constrained ALS for Estimation of Human Upper Limb Synergies
33
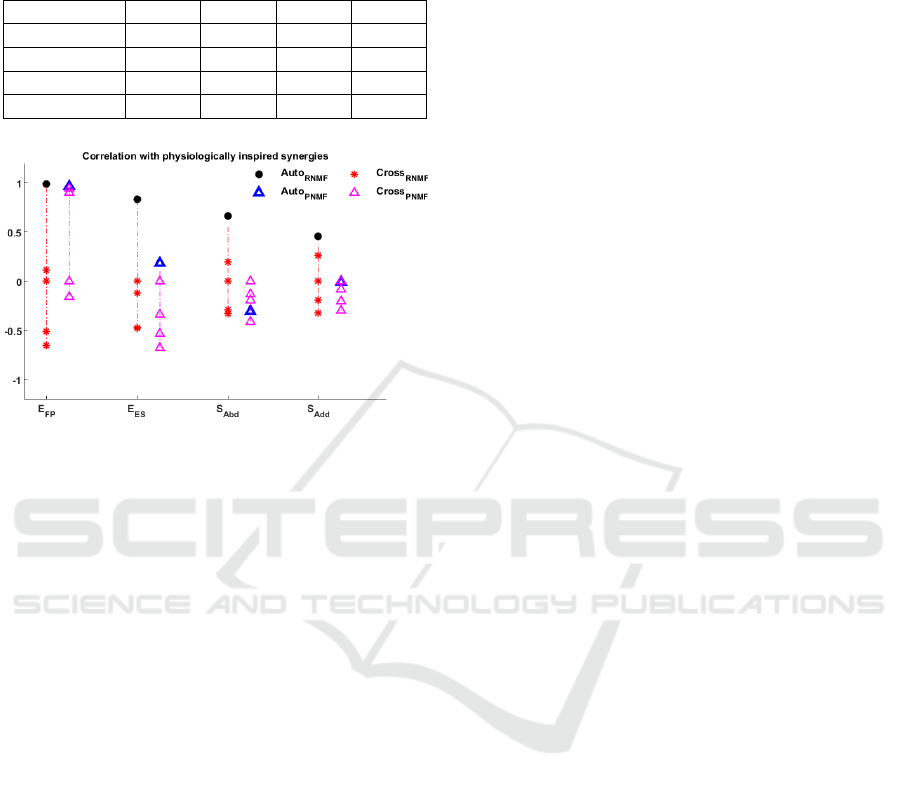
Table 3: Correlation matrix of synergies estimated using
regularized NMF. E
F
: Elbow Flexion; E
E
: Elbow Exten-
sion; S
Add
: Shoulder Adduction; S
Abd
: Shoulder Abduc-
tion.
Movements E
FP
E
ES
S
Abd
S
Add
E
FP
1 0.380 -0.353 0.388
E
ES
0.380 1 -0.633 -0.136
S
Abd
-0.353 -0.633 1 0.079
S
Add
0.388 -0.136 0.079 1
Figure 6: A comparison of synergies estimated for healthy
subjects with Synergistic activation based on human phys-
iology. The red ‘*’ refers to cross correlation of regular-
ized NMF based synergistic weight with physiologically in-
spired synergies; the black dots present auto correlation for
similar order synergies. The purple ‘∆’ refers to the one
estimated via plain NMF and the blue ‘∆’ are the correla-
tion between similar order synergies E
FP
: Elbow Flexion-
Pronation; E
ES
: Elbow Extension-Supination; S
Add
: Shoul-
der Adduction; S
Abd
: Shoulder Abduction.
7 CONCLUSION
Human body movements are based on the synergis-
tic activation of muscles. This paper investigates the
number of synergies and the approriate muscular acti-
vation involved in isometric contraction of the human
upper arm. According to our findings four synergies
are sufficient for upper limb movement identification.
Plain ALS-based NMF algorithm is insufficient for
synergy estimations as the method fails to provide op-
timal solution for co-linear data. Therefore, this paper
proposes using a constrained ALS-based NMF; the
regularization constraint decorrelates the EMG sig-
nals to attain the synergies behind the particular con-
tractions, thus, resolving the issue. Our statement is
supported by the results presented in section (6). In
the future, we look forward to analyzing the patholog-
ical disorder in the upper limbs of post-stroke subjects
using muscle synergies.
ACKNOWLEDGEMENTS
For carrying out this research work, we are very
thankful to Dr. Zev Rymer and his team at Shirley
Ryan Ability Lab, Chicago, USA, for providing us
with the entire database.
REFERENCES
Ayhan, E. and Ayhan, C. (2020). Kinesiology of the elbow
complex. In Comparative Kinesiology of the Human
Body. Elsevier Inc.
Bahadur, R. and Rahman, S. (2018). A robust and adaptive
algorithm for real-time muscle activity interval detec-
tion using emg signals. In 11th International Joint
Conference on Biomedical Engineering Systems and
Technologies. INSTICC.
Bahadur R., Rahman S., R. G. and Khan, M. (2021). Syn-
ergy estimation method for simultaneous activation of
multiple dofs using surface emg signals. Frontiers in
Neuroscience, 16:1–8.
Cichocki, A. and Zdunek, R. (2007). Regularized Alter-
nating Least Squares Algorithm for Non-negative Ma-
trix/Tensor Factorization, volume 4493. Springer,
Berlin.
Inouye, J. M. and Cuevas, F. J. V. (2016). Muscle synergies
heavily influence the neural control of arm endpoint
stiffness and energy consumption. PLOS: Computa-
tional Biology, pages 1–24.
Lee, D. and Seung, H. (2000). Algorithms for non-negative
matrix factorization. In Neural Information Process-
ing Systems, pages 556–562. NIPS.
Lin, C.-J. (2007). On the convergence of multiplicative up-
date algorithms for nonnegative matrix factorization.
IEEE Transactions on Neural Networks, 18(6):1589–
1596.
Lin C., Wang B., J. N. and Farina, D. (2018). Robust ex-
traction of basis functions for simultaneous and pro-
portional myoelectric control via sparse non-negative
matrix factorization. Journal of Neural Engineering,
15:26017(9pp).
Ma, Y., Shi, C., Xu, J., Ye, S., Zhou, H., and Zuo, G. (2021).
A novel muscle synergy extraction method used for
motor function evaluation of stroke patients: A pilot
study. Sensors, 21:11:3833.
Naik, G. R. and Nguyen, H. T. (2015). Non negative matrix
factorisation for the identification of emg finger move-
ments: Evaluation using matrix analysis. IEEE Jour-
nal of Biomedical and Health Informatics, 19:478–
485.
Rasool, G., Iqbal, K., Bouaynaya, N., and White, G. (2016).
Real-time task discrimination for myoelectric con-
trol employing task-specific muscle synergies. IEEE
Transactions on Neural Systems and Rehabilitation
Engineering, 24:98–108.
Rodrigues, C., Correia, M., Abrantes, J., Rodrigues, M.
A. B., and Nadal, J. (2022). Muscle synergies es-
timation with pca from lower limb semg at differ-
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
34

ent stretch-shortening cycles. In IFMBE Proceedings.
SPRINGER.
Roh J., R. W. and Bee, R. F. (2015). Evidence for altered up-
per extremity muscle synergies in chronic stroke sur-
vivors with mild and moderate impairment. Frontiers
in Human Neuroscience, 9:6:1–14.
Yesilyaprak, S. S. (2020). Kinesiology of the shoulder com-
plex, volume 4493. Elsevier, Rio de Janeir0.
Zhao, K., Wen, H., Zhang, Z., Atzori, M., Muller, H., Xie,
Z., and Scano, A. (2022). Evaluation of methods for
the extraction of spatial muscle synergies. NUST Jour-
nal of Engineering Sciences, 16.
Constrained ALS for Estimation of Human Upper Limb Synergies
35
