
Biodiesel Production from Used Cooking Oil Using Coal Low Rating
as Environmentally Friendly Heterogeneous Catalyst
Mustafa
1,3
, Pramila Tumanaidu
2,3
and Muh. Irwan
1
1
Department of Chemical Engineering, Politeknik Negeri Samarinda, Jalan Dr. Cipto Mangunkusumo,
Kampus Gunung Lipan Samarinda, 75131, Kalimantan Timur Province, Indonesia
2
Malaysia-Japan International Institute of Technology (MJIIT), Universiti Teknologi Malaysia,
Jalan Sultan Yahya Petra, 54100 UTM Kuala Lumpur, Malaysia
3
Razak Faculty of Technology and Informatics, Universiti Teknologi Malaysia, Jalan Sultan Yahya Petra,
54100 UTM Kuala Lumpur, Malaysia
Keywords: Biodiesel, Catalyst, Low Rank Coal, Used Cooking Oil.
Abstract: The increasing need for fossil fuels encourages efforts to meet these needs, by developing alternative fuels
made from renewable raw materials, such as cooking oil from palm oil. Cooking oil is an important
commodity at this time which is quite widespread in Indonesia. The repeated use of cooking oil can produce
used cooking oil that cannot be re-consumed and has a negative impact on the environment. The purpose of
this study was to identify the characteristics of low rank coal with thermal assistance as a heterogeneous
catalyst to produce biodiesel and to study the effect of biodiesel product quality parameters on the
esterification and transesterification processes. Therefore, in this study the production of biodiesel from used
cooking oil was carried out using a low rank coal catalyst, to determine the effect of the amount of catalyst
on the yield and quality of the biodiesel produced. Biodiesel is obtained by reacting oil and methanol, then
low rank coal catalyst is added with various mass additions of 3, 5, 7, 9, and 11 grams. Based on this study,
the best results were obtained with the addition of 7 grams of catalyst, with biodiesel yield reaching 82.20%,
density 864.43 kg/m3, kinematic viscosity 3.60 cSt, water content 0.20%. , the acid number is 0.99 mgKOH/g,
and the methyl ester content is 97.48%. In general, the biodiesel produced has met the requirements of SNI
7182:2015, except for the parameters of water content and acid number.
1 INTRODUCTION
Indonesia is a country with various natural
resources, one of which is oil palm. Based on
statistics from the Directorate General of Indonesian
Plantations, the Volume and Value of Palm Oil
Exports (CPO) 2015-2017 showed a decline from
26,467,564 tons in 2015 to 24,150,232 tons in 2016.
It is estimated that this is due to the large
consumption of domestic palm oil. from the
excessive use of palm oil is the production of waste
palm oil or what is known as used cooking oil or
used cooking oil. To overcome this problem, efforts
are needed to convert used cooking oil into more
viable products, such as biodiesel (Efendi et al,
2018).
Used cooking oil is used cooking oil for frying
which is used repeatedly, with the production and
consumption of cooking oil, the availability of
cooking oil is abundant. Cooking oil is a waste and
when viewed from its chemical composition, used
cooking oil contains carcinogenic compounds that
occur during the frying process. The use of cooking
oil in a sustainable manner can damage human
health, cause cancer, and further reduce the
intelligence of the next generation (Siswani et al,
2012).
For this reason, proper handling is needed so that
this used cooking oil waste can be useful and not
cause harm from aspects of human health and the
environment through a conversion from used
cooking oil into biodiesel (Darmawan, 2013).
Biodiesel is a fuel made from vegetable oil or
animal fat. Biodiesel is a fuel consisting of a mixture
of mono-alkyl esters derived from long-chain fatty
acids, which are renewable sources from nature.
Biodiesel is also known as an environmentally
friendly fuel because it produces relatively cleaner
1072
Mustafa, ., Tumanaidu, P. and Irwan, M.
Biodiesel Production from Used Cooking Oil Using Coal Low Rating as Environmentally Friendly Heterogeneous Catalyst.
DOI: 10.5220/0012064500003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 1072-1078
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

exhaust emissions than diesel. In addition, the use of
biodiesel is generally easy, because there is no need
to modify the diesel engine (Aziz et al, 2012).
Biodiesel has advantages over petroleum diesel.
Biodiesel fuel is renewable. In addition, it can also
strengthen the country's economy and create jobs.
Biodiesel is an ideal fuel for the transportation
industry because it can be used in various diesel
engines, including agricultural machinery.
According to the Indonesian Palm Oil
Association (Gapki), domestic palm oil
consumption in 2020 was 17.35 million tons or grew
3.6% compared to 2019 of 16.75 million tons. The
development of biodiesel based on used cooking oil
has the opportunity to be marketed, both
domestically and for export. Throughout 2020,
domestic biodiesel consumption increased by about
24% from 5.83 million tons in 2019 to 7.23 million
tons.
In addition, the use of biodiesel as a fuel has
many advantages, including being renewable and
environmentally friendly (reducing vehicle
emissions), being able to lubricate the engine as well
as being a fuel so as to increase the life of the
vehicle, it is safe to store and transport because this
fuel does not toxic and biodegradable and can
reduce dependence on fossil fuels (Balat et al, 2010).
The use of biodiesel as a renewable and
environmentally friendly fuel (reducing vehicle
emissions) is a treatment in the biodiesel
manufacturing process by adding a catalyst and with
ultrasonic assistance in order to increase the
conversion and yield produced.
To make it as a catalyst, thermal assistance is
needed so that the binding mechanism becomes a
simpler hydrocarbon. In line with that, in improving
the quality of biodiesel by using a catalyst, it is also
possible to break the bonds in this case using an
ultrasonic device. Ultrasonic waves can increase the
rate of transesterification of used cooking oil into
biodiesel so that the conversion of used cooking oil
into biodiesel with the use of ultrasonic waves is
higher than other uses.
In the research of Pasae et al., (2019), biodiesel
was produced from used cooking oil using a
heterogeneous catalyst in the form of shells, with
variations in temperature in the calcination and
transesterification processes. The amount of oil and
methanol used was 200 mL of oil and 600 mL of
methanol (1:3 v/v), with the addition of 2 grams of
clam shells as a catalyst. The best results in this
study were transesterification for 3 hours, with a
yield of 66.09%, a density of 853 kg/m
2
, a kinematic
viscosity of 2.77 mm
2
/s, an acid number of 0.56
mgKOH/g, and a saponification rate of 201
mgKOH/g. These results have met SNI Biodiesel
7182: 2015 except for the number of acids produced.
In the research of Saputri et al., (2016), biodiesel
methyl esters were made from used cooking oil and
methanol using a heterogeneous catalyst in the form
of rubbing ash with variations of 2, 4, 6, 8, and 10
grams. The best results in this study were achieved
using 10 grams of rubbing ash as a catalyst, with an
acid number of 0.71 mg KOH/g, a total glycerol
content of 0.01%, a density of 0.88 g/cm3, a
viscosity of 47.94 cSt, an iodine number. 66.83 g
I
2
/100, water content 0.06%, flash point 249.33ºC,
saponification number 154.84 mg KOH/g, and
methyl ester content 99.5%. The results obtained
have met the biodiesel standard SNI 7182: 2015,
except for the viscosity and water content of the
biodiesel produced.
In the research of Khoiruummah et al., (2020),
activated carbon from acacia wood was impregnated
using KOH and NaOH, then used as a heterogeneous
catalyst in the manufacture of biodiesel from used
cooking oil. This study used variations in the amount
of KOH catalyst/activated carbon, namely 1,3, and
5% w/w oil, as well as variations in temperature,
namely 45, 55, 65, and 75ºC, with an oil-methanol
molar ratio of 1:6. In addition, this study also used
variations in the amount of NaOH catalyst/activated
carbon, namely 3, 4, and 5% w/w oil, with variations
in transesterification temperature of 50, 55, 60, and
65ºC.
The best results on the use of KOH/activated
carbon catalyst is the catalyst variation of 3% w/w
with a transesterification temperature of 65ºC,
which produces a yield of 87.51%. The use of KOH
catalyst/activated carbon produces biodiesel with a
density of 0.7724-0.8585 g/mL, viscosity 4.5485-
5.3672 cSt, acid number 1.1222-2.2444 mgKOH/g,
water content 0.034-0.246% , and a flash point of
150-170ºC.
Meanwhile, the best results on the use of
NaOH/activated carbon catalyst are catalyst
variations of 3% w/w with a transesterification
temperature of 60ºC. In this variation, a yield of
88.35% was obtained. Overall, variations in the
amount of NaOH catalyst/activated carbon produced
biodiesel with a density of 0.89282-0.90722 g/mL,
kinematic viscosity 2.3439-4.1601 cSt, acid number
0.84165-2.2444 mgKOH/g, content water
0.000592-0.071963%, and a flash point of 128-
153ºC. In general, the biodiesel produced has
complied with SNI 7182:2015 on the parameters of
viscosity and flash point. As for the water content
and density parameters, some do not meet SNI
Biodiesel Production from Used Cooking Oil Using Coal Low Rating as Environmentally Friendly Heterogeneous Catalyst
1073

7182:2015. While for the acid number parameter, all
of them do not meet SNI 7182:2015.
Meanwhile, in the research of Oko et al., (2021)
the process of making biodiesel from used cooking
oil was carried out through two reaction stages,
namely esterification and transesterification
reactions. The esterification reaction was carried out
for 1 hour with 100 grams of used cooking oil as raw
material, then 52 grams of methanol and 1 gram of
H
2
SO
4
catalyst were added. Then, in the
transesterification reaction, the mass ratio of CaO
and C was varied in the NaOH/CaO/C catalyst. The
best result of this research is the mass ratio of CaO/C
1:1 with a catalyst of 3% (w/w), with a yield of
83.45% (w/w), kinematic viscosity 2.3 cSt, density
0.8612 g/ mL, water content 0.0273% (w/w), and
acid number 0.2516 mgNaOH/g. All of these results
have met the biodiesel standard of SNI 7182:2015.
In this research, the process of making biodiesel
is carried out through two reaction stages, namely
the esterification reaction which refers to the
research of Oko et al., (2021), and the
transesterification reaction, with a fairly large
oil:methanol ratio of 1:3 (v/v). referring to the
research of Pasae et al., (2019). The addition of large
amounts of methanol is expected to increase the
yield of biodiesel and prevent the kinematic
viscosity from being too high in the final product. In
addition, in this study a different heterogeneous
catalyst was used, namely activated carbon catalyst
from low rank coal impregnated with NaOH. By
using this catalyst, it is expected that the biodiesel
produced is able to meet several criteria in SNI
7182:2015.
2 METHODOLOGY
There are two stages in this research, namely the
production of catalysts from low rank coal and the
production of biodiesel.
For the production of catalysts from low
rank coal, first prepare low rank coal, then
analyze coal samples with analysis of
proximate, total sulfur, and calorific value,
then carry out the carbonization process in a
furnace at a temperature of 600 º C for 2
hours. After that the carbon is cleaned and
then cooled in a desiccator. Then do physical
activation on the catalyst with a temperature
of 950ºC for 2 hours. Then cool the catalyst
in a desiccator. Then puree the catalyst using
a blender, then filter the catalyst with a size
of -100 +120 mesh. After that, a proximate
analysis was carried out on the low rank coal
catalyst.
Biodiesel production is the first collected
used cooking oil. Then analyzed the used
cooking oil which includes water content,
density at 40 º C, kinematic viscosity at
40ºC, FFA content, and acid number. If the
FFA level exceeds 1%, then an esterification
reaction is carried out (Hadrah et al., 2018),
which is to react 100 grams of used cooking
oil with 52 g of methanol and 1 g of H2SO4
(Oko et al., 2021). The reaction was
maintained at a temperature of 50-60ºC for
1 hour (Arifin et al., 2016). Then separate the
esterification product in a separatory funnel
for 1 hour, then take the bottom layer for use
in the transesterification reaction (Oko et al.,
2021).
Then carry out a transesterification reaction by
reacting the esterified oil with a catalyst-methanol
mixture at a temperature of 60-70ºC for 1 hour 30
minutes, where the variations of the added catalyst are
3, 5, 7, 9, and 11 grams (Saputri et al., 2016) . The
ratio of oil:methanol used was 1:3 v/v (Pasae et al.,
2019). Then filtering the catalyst while separating the
transesterification results in a separating funnel for 12
hours (Pasae et al., 2019). Then take the top layer and
heat it on a hot plate at a temperature of 60-70ºC for
± 1 hour to remove the remaining methanol
(Khoiruummah et al., 2020). Then wash the biodiesel
produced using aquadest at a temperature of 70-80ºC
until the water becomes clear (Khoiruummah et al.,
2020). After that, the biodiesel is put in the oven for
± 3 hours to reduce the water content (Khoiruummah
et al., 2020). Then save the obtained biodiesel.
The procedure of the process can be described as
shown in the figure below, namely in Figure 1 and
Figure 2.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
1074
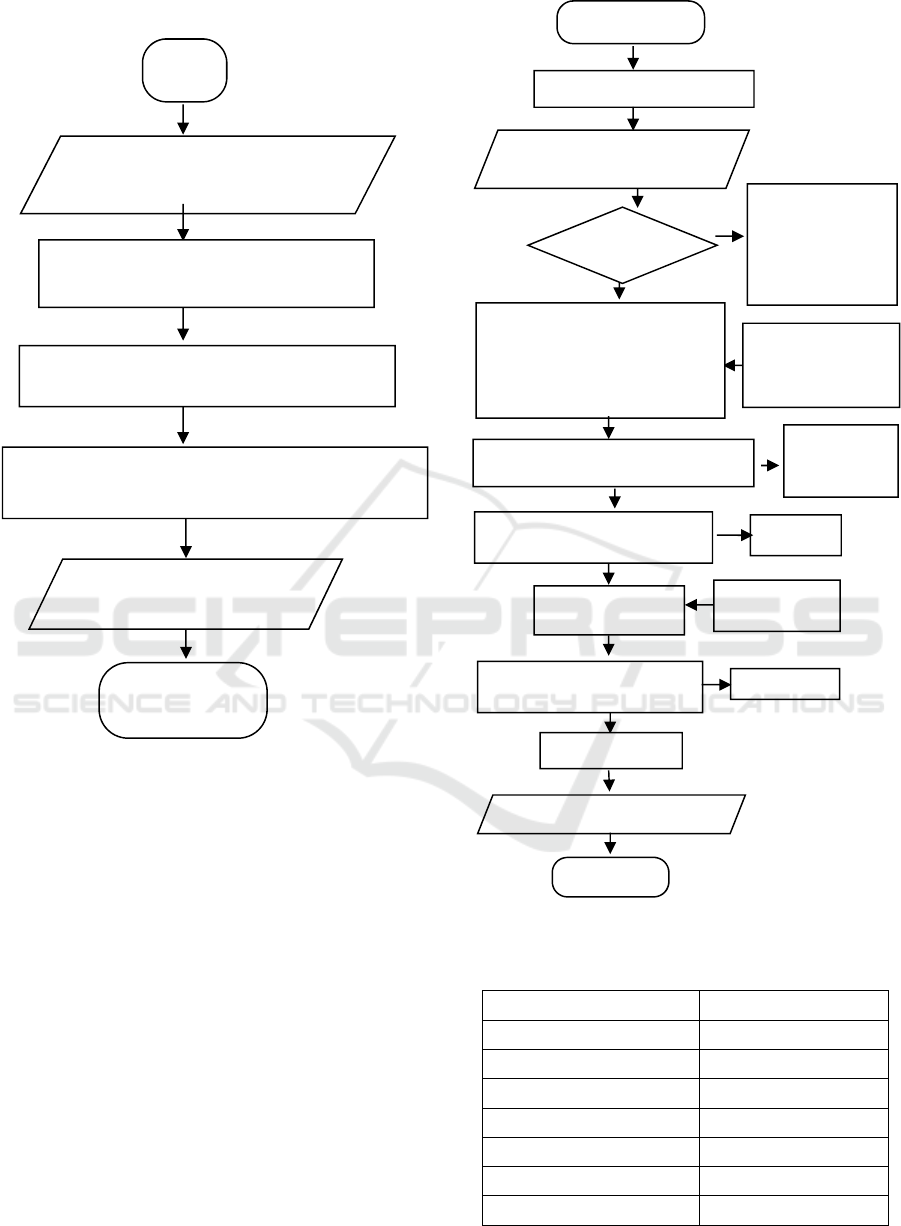
Research Procedure
Figure 1: Catalyst Production from Low Rank Coal.
3 RESULT AND DISCUSSION
3.1 Analysis Results
In this study, biodiesel was made from used cooking
oil using a low rank coal activated carbon catalyst,
with variations in the addition of a catalyst of 3, 5, 7,
9, and 11 grams which aims to determine the effect of
the catalyst on the yield of biodiesel produced.
The initial stage of making biodiesel is collecting
used cooking oil, then analyzing the oil with the
parameters of free fatty acid content, color, density at
40ºC, kinematic viscosity at 40ºC, and acid number.
This analysis aims to determine the condition of used
cooking oil before it is used as raw material, as well
as to determine the reaction stage used in the
manufacture of biodiesel.
Figure 2: Biodiesel Production.
Table 1: Results of Low Rank Coal Analysis.
Parameter Analysis Results
Total Moisture, % 37.40
Inherent Moisture, % 14.02
Ash Content, % 2.86
Volatile Matter, % 45.61
Fixed Carbon, % 32.51
Total Sulphur, % 0.98
Calorific Value, kcal/kg 4714
Finish
Catalyst proximate analysis
Proximate analysis of low rank
coal samples
Carbonization (T = 600ºC; t = 2 hours)
Refining and sieving catalyst up to -100 +120 mesh
Physical activation (T = 950ºC; t = 2 hours)
Start
Start
Esterification
T=50-60ºC
t = 1 hour
oil = 100 g
mmethanol = 52 g
m H
2
SO
4
= 1 g
No
FFA < 1%
Yes
Transesterification
T = 60-70ºC
t = 1 hour 30 minutes
Ratio of oil: methanol = 1:3 (v/v)
Variation of catalyst = 3, 5, 7, 9, 11
grams
Separation of
esterification results
(t = 1 hour)
Catalyst and
glycerol
Separation of transesterification results
(
t = 12 hours
)
Methanol
Analysis of the quality of used
cooking
oil
Collecting used cooking oil
Reduction of residual methanol
(T = 60-70ºC; t = ±1 hour)
Aquadest
(T = 70-80ºC)
Biodiesel washing
(T=70-80ºC)
Water va
p
o
r
Reduction of water content
(T = 105-110ºC; t = ±3 hours)
Biodiesel Yiel
d
Biodiesel characteristic test
Finish
Biodiesel Production from Used Cooking Oil Using Coal Low Rating as Environmentally Friendly Heterogeneous Catalyst
1075
Table 2: Results of Low Rank Coal Analysis (Physical
Activation).
Parameter Analysis Results
Inherent Moisture, % 3.13
Ash Content, % 38.23
Volatile Matter, % 6.64
Fixed Carbon, % 55.13
Table 3: Results of Analysis of Used Cooking Oil
Characteristics.
Parameter Analysis Results
Free fatty acid content, % 4.34
Warna Color
Abnormal (clear
brown)
Density, 40ºC, kg/m3 896.04
Kinematic viscosity, 40ºC, cSt 35.39
Acid number, mgKOH/g 12.57
Table 3 above shows that with the use of 7 grams of
catalyst, the highest yield was obtained at 82.20%.
The quality of the biodiesel produced is as follows:
density: 864.43 kg/m3, kinematic viscosity: 3.60 cSt,
water content: 0.20%, and acid number: 0.99
mgKOH/g.
Table 4: Results of Biodiesel Analysis.
Parameter
Analysis Results
SNI
7182:2015
Catalyst
3 grams
Catalyst
5 grams
Catalyst
7 grams
Catalyst
9 grams
Catalyst
11 grams
Density,
40ºC,
kg/m3
865.18 865.91 864.43 859.42 858.07 850-890
Kinematic
viscosity,
40ºC, cSt
3.59 3.64 3.60 3.58 3.34 2.3-6,0
Moisture
content, %
(v/v)
0.94 0.04 0.20 0.18 0.17 0.05
Acid
number,
mgKOH/g
1.22 1.23 0.99 1.00 0.89 0.5
Yield, % 21.97 35.22 82.20 61.46 69.13 -
4 DISCUSSION
• Effect of Catalyst Amount on Biodiesel Yield
Variations in the addition of catalysts as much as 3, 5,
7, 9 and 11 grams in the transesterification reaction
have a significant effect on the amount of
biodiesel produced, as shown in the figure below:
Figure 2: Graph of the Effect of Catalyst Amount on
Biodiesel Yield.
Based on Figure 2 above, the addition of catalyst
in the range of 3 to 7 grams showed a significant
increase in yield, where the highest yield was
obtained at the addition of 7 grams of catalyst, which
was 82.04%. The addition of a catalyst can reduce the
activation energy, where the more catalyst is added,
the activation energy decreases, so the reaction rate
will increase. With the increase in the reaction rate,
the conversion of used cooking oil into biodiesel is
also getting bigger (Prihanto & Irawan, 2018).
Meanwhile, the addition of 9 and 11 grams of
catalyst actually reduced the amount of biodiesel
produced. This is caused by the formation of soap that
occurs through a saponification reaction, where the
formation of soap can hinder the conversion of oil
into methyl esters. In addition, with the addition of 9
grams of catalyst, the yield of biodiesel produced is
actually lower than that of 11 grams of catalyst.
This is due to imperfect filtering of the catalyst, so
that the catalyst is carried into the product and
increases the amount of impurities. This increase in
the amount of impurities makes the washing process
more difficult, so that a lot of biodiesel is wasted
during the washing process.
According to Bintang et al., (2015), the washing
process and water separation after washing can
reduce the amount of biodiesel produced. The
purification process needs to be carried out properly
because the remaining impurities can affect the
quality of biodiesel, especially on the density
parameter (Faizal et al., 2013)
• Biodiesel Quality
The quality of biodiesel produced can be seen in table
4 with analytical parameters including: density at
40ºC, kinematic viscosity at 40ºC, water content, and
acid number. For the variation of the catalyst with the
highest yield (7 grams), additional analysis was
carried out in the form of methyl ester content to
determine the purity level of the biodiesel produced.
10
30
50
70
90
357911
Biodiesel Yield (%)
Amount of Catalyst (grams)
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
1076
Based on table 4, the obtained biodiesel density
ranges from 858.07-865.91 kg/m3. These results have
met the density standard of SNI 7182: 2015 which is
between 850-890 kg/m3.
According to Hadrah et al., (2018), biodiesel with
a density according to SNI standards is able to
produce perfect combustion. In addition to density,
the kinematic viscosity parameter also meets SNI
7182:2015, where the results obtained are in the range
of 3.55-3.87 cSt. These results are within the range
allowed by SNI, namely 2.3-6.0 cSt. The water
content parameters in general do not meet SNI
7182:2015, except for the 5 gram catalyst variation.
High water content can be caused by an incomplete
evaporation process (Kusumaningtyas & Bachtiar,
2012).
Meanwhile, the acid number parameter in general
does not meet SNI 7182:2015. The high acid number
in biodiesel indicates the presence of free fatty acids
in biodiesel produced by the hydrolysis reaction
between oil and water (Zamhari et al., 2021). In
addition, the acid number is also closely related to the
pH of biodiesel (Faizal et al., 2013).
Therefore, to reduce the acid number, the
evaporation process of the water content can be
carried out to prevent the hydrolysis reaction, as well
as carry out neutralization until the biodiesel pH is
close to 7. The parameters of the methyl ester content
were only reviewed for biodiesel with the highest
yield of 82.20% (7 grams of catalyst variation).
Methyl ester analysis was carried out using an FT-
IR instrument, with the resulting IR spectrum as
follows:
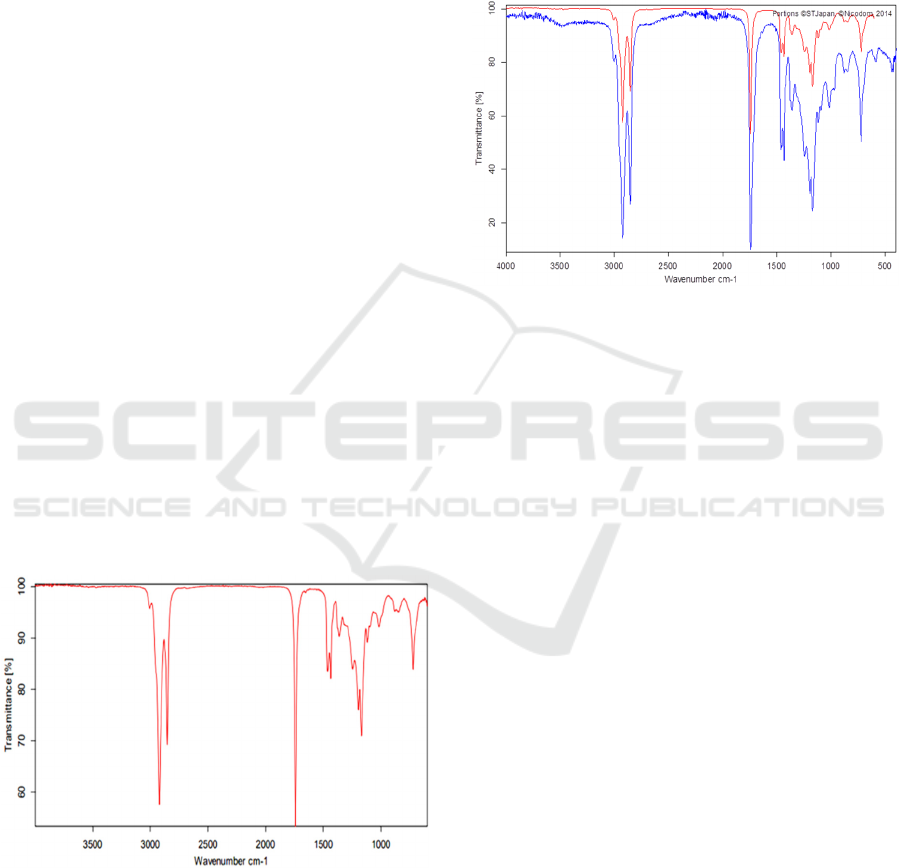
Figure 3: IR Spectrum on Biodiesel.
In the spectrum above, it is seen that there is a
sharp absorption at wavelengths 1169.46, 1741.85,
2853.07 and 2922.45 cm-1. Based on the book
Dachriyanus, (2004), the absorption at 1169.46 cm-1
indicates the presence of C-O bonds (1300-1000 cm-
1). Meanwhile, the absorption at 1741.85 cm-1
indicated the presence of an ester group, namely C=O
(1900-1650 cm-1). Then the absorption at 2922.45
cm-1 and 2853.07 cm-1 indicated the presence of C-
H bonds (3000-2700 cm-1). The C=O, C-H and C-O
bonds in biodiesel prove the presence of methyl ester
compounds contained in it. The types of methyl esters
in biodiesel can be identified using the IR spectrum
comparison as follows:
Figure 4: Comparison of the IR Spectrum of Methyl Oleate
(Blue) with Biodiesel (Red).
In Figure 4 above, it can be seen that there is a
comparison between the IR spectrum of biodiesel and
the IR spectrum of methyl oleate under the same
conditions. The IR spectrum of methyl oleate is the
standard spectrum used as a comparison.
According to Siswani et al., (2012), to identify an
unknown compound, a comparison can be made
between the IR spectrum of the compound and the IR
spectrum of a standard compound under the same
conditions. The similarity between the two spectra
being compared indicates that the compound is
identical. The results of the analysis show that
biodiesel has an IR spectrum that is identical to the IR
spectrum of methyl oleate. Therefore, it can be seen
that the methyl ester compound in the biodiesel
produced is predominantly methyl oleate.
Meanwhile, to determine the methyl ester content
in detail, additional analysis was performed using the
FT-IR ATR-PLS-FAME Quantification method,
which refers to the ASTM D7371 and EN 14078
methods. From this analysis, the methyl ester content
was obtained with an estimate of 97.48%. This level
has exceeded the minimum limit determined by SNI
7182:2015 which is 96.5%. Thus, the methyl ester
content in biodiesel has met the SNI 7182:2015
standard.
Biodiesel Production from Used Cooking Oil Using Coal Low Rating as Environmentally Friendly Heterogeneous Catalyst
1077

5 CONCLUSION
1. The more catalyst added to the reaction, the greater
the yield of biodiesel produced, but the yield of
biodiesel will decrease if the addition of catalyst has
reached the optimum condition.
2. The best result of this research is the use of 7 grams
of catalyst, with a yield of 82.20%. The quality of the
biodiesel produced is as follows:
• density: 864.43 kg/m3
• kinematic viscosity: 3.60 cSt
• water content: 0.20%
• acid number: 0.99 mgKOH/g
• methyl ester content: 97.48%.
3. Parameters of density, kinematic viscosity, and
methyl ester content in the biodiesel produced have
complied with SNI 7182:2015. Meanwhile, the
parameters of water content and acid number do not
meet SNI 7182:2015.
ACKNOWLEDGEMENTS
The authors would like to thank the Samarinda State
Polytechnic Research and Community Service Center
(P2M POLNES) which has provided the opportunity
to research, and obtain research funds under the State
Polytechnic Lecturer Research scheme and all parties
who have supported the smooth implementation of
this research. And special thanks to Razak Faculty of
Technology and Informatics, Universiti Teknologi
Malaysia, UTM Kuala Lumpur, Malaysia who helped
push this article.
REFERENCES
Arifin, Z., Rudiyanto, B., & Susmiati, Y. (2015). Production
of Biodiesel from Used Cooking Oil Using
Heterogeneous Catalyst Shell Snail (Achatina Fulica)
with Dry Washing Method”. Journal ROTOR, 100–104,
9(November).
Aziz. I. (2017). Diesel Engine Performance Test Using
Biodiesel From Used Cooking Oil”, Chemistry Study
Program Faculty of Science and Technology UIN Syarif
Hidayatullah Jakarta.
Badan Standardisasi Nasional Indonesia. (2015). SNI
7182:2015. https://akses-sni.bsn.go.id/viewsni/baca/62
24
Balat, M., & Balat, H. J. A. e. (2010), Progress in biodiesel
processing”. 87(6), 1815-1835, 2010
Bintang, M. T. M., Aisyah, A., & Saleh, A. (2015) Synthesis
of Biodiesel from Nyamplung (Callophyllum
innophylum L.) Seed Oil by Ultrasonochemical
Method”., Chimica et Natura Acta, 3(2), 84–89.,
https://doi.org/10.24 198/cna.v3.n2.9199
Dachriyanus, (2004), Spectroscopic Structural Analysis of
Organic Compounds. In Spectroscopic Structural
Analysis of Organic Compounds”, LPTIK Andalas
University, 2004, https://doi.org/10.25077/car.3.1
Darmawan, F. I. J. J. T. M., (2013). The process of producing
biodiesel from used cooking oil using the dry-wash
system washing method.
Efendi, R., Faiz, H. A. N., & Firdaus, E. R., (2018).
Production of used cooking oil biodiesel uses the
esterification-trasesterification method based on the
amount of used cooking oil”, 7182, 402–409.
Faizal, M., Maftuchah, U., & Auriyani, W. A. (2013). Effect
of Methanol Content, Amount of Catalyst, and Reaction
Time on Making Biodiesel from Cow Fat through
Transesterification Process. Chemical Engineering
Department, 19(4), 29–37, http://jtk.unsri.ac.id
Hadrah, H., Kasman, M., & Sari, F. M., (2018). Analysis of
Used Cooking Oil as Biodiesel Fuel with
Transesterification Process. Environmental Cycle Journal,
1(1), 16., https://doi.org/10.33087/daurling. v1i1.4
Khoiruummah, D., Sundari, N., Zamhari, M., & Yuliati, S.
(2020), “Application of Activated Carbon Based Catalyst
from Base Impregnated Acacia Wood (Acacia mangium)
in Biodiesel Synthesis”. 01(01), 20–28.
Kusumaningtyas, R. D., & Bachtiar, A. (2012), “Synthesis of
Biodiesel from Rubber Seed Oil with Variations in
Temperature and Concentration of KOH for
Transesterification Stages”. Journal of Renewable
Natural Materials, 1(2), 9–18.,. https://doi.org/10.15294/
jbat.v4i1.3769
Oko, S., Kurniawan, A., & Rahmatina, J. (2021). Effect of
Mass Comparison of Ca and C on NaOH/CaO/C Catalyst
in Biodiesel Synthesis Using Cooking Oil., Prosiding
The 12th Industrial Research Workshop and National
Seminar, https://jurnal.polban.ac.id
Pasae, Y., Leste, J., Bulo, L., Tandiseno, T., & Tikupadang,
K. (2019), “Biodiesel Production from Waste Cooking
Oil with Catalysts from Clamshell”., 14(3), 596–599.,
http://www.arpnjournals.org
Saputri, M., Restuhadi, F., & Efendi, R. (2016)., “Production
of Biodiesel Methyl Esters from Used Cooking Oil and
Methanol with Scrub Ash Catalyst”. SAGU, Volume 15,
28–37.
Siswani, E. D., Kristianingrum, S., & Suwardi. (2012),
Synthesis and Characterization of Biodiesel from Used
Cooking Oil at Various Times and Temperatures.
Proceedings of the National Research Seminar, 103–
112,. http://staffnew.uny.ac.id
Prihanto, A., & Irawan, T. A. B. (2018), “Effect of
Temperature, Catalyst Concentration and Methanol-Oil
Molar Ratio on Biodiesel Yield from Used Cooking Oil
through Neutralization-Transesterification Process”.
Metana, 13(1), 30., https://doi.org/10.14710/metana.v1
3i1.11340
Zamhari, M., Junaidi, R., Rachmatika, N., & Oktarina, A.
(2021). Production of Activated Carbon Based Catalyst
from Coconut Shell (Cocos Nucifera) Impregnated with
KOH in Biodiesel Synthesis Transesterification
Reaction. Journal of Kinetics, 12(01), 23–31.,
https://jurnal.polsri.ac.id
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
1078
