Cell-Free Immunotherapies-Effective Approaches Against COVID-19
Yiran Liu
Leonard N. Stern School of Business, New York University, U.S.A.
Keywords:
Interferons, IVIg (Intravenous Immunoglobulin), Antibodies, SARS-CoV-2.
Abstract:
In 2019, an infectious coronavirus disease, known as COVID-19, was discovered to be caused by severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2). The highly contagious nature of the virus led the
scientific community to urgently develop therapeutic approaches for fighting against SARS-CoV-2. The
mechanisms of COVID-19 are lung damage and dysregulated immune response. This article attempted to
systematically review the available literature on cell-free immunotherapeutic strategies against COVID-19,
shedding light on our understanding of COVID-19. Cell-free immunotherapy methods still have room for
improvement, and understanding the immunology of COVID-19 is crucial for developing therapeutic
strategies. As a result, cell-free immunotherapy could be used more appropriately, which may help scientists
to determine the direction of future research.
1 INTRODUCTION
In 2019, the contagious disease COVID-19 rapidly
became a global pandemic and has to this date caused
approximately 260 million infections and 5 million
deaths around the world. SARS-CoV-2 is recognized
as a novel beta coronavirus (β CoV), which is a
single-stranded RNA virus. The mechanisms of
COVID-19 are cytopathological damage of the cell
and dysregulated immune response. Despite the fact
that many people infected with the coronavirus might
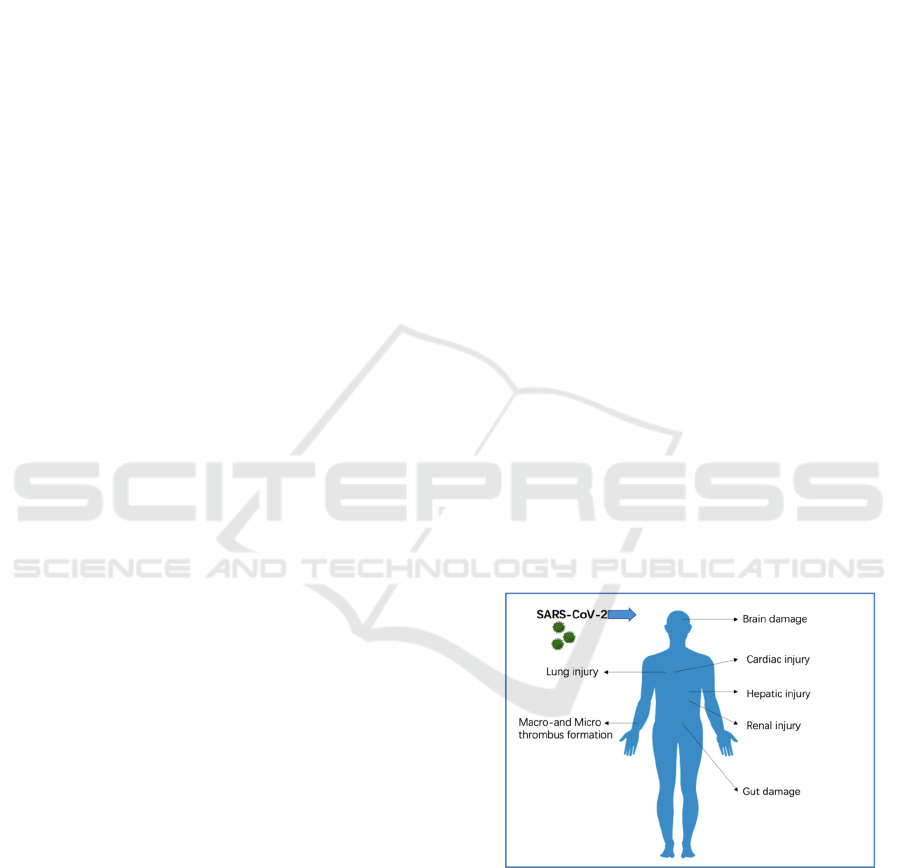
experience organ dysfunction (Figure 1), they could
recover without hospitalization. By contrast, the
elderly and immunocompromised are more likely to
develop serious illnesses or face a higher risk of
mortality. Therefore, the highly contagious nature of
SARS-CoV-2 led the scientific community to
urgently develop therapeutic treatments for
combating SARS-CoV-2. Immunotherapies are
effective methods for combating viral infections by
inducing, enhancing, or suppressing the immune
response. Immunotherapies include cell-based
therapies and cell-free therapies. Cell-based therapies
aim to inject a donor's healthy cells into a patient's
body to fight against disease. Cell-free therapies have
advantages in overcoming the risks associated with
cell-based therapies, such as macro thrombosis and
micro thrombosis. Therefore, Cell-free therapies are
safer, cheaper, and more beneficial to humans.
Current cell-free immunotherapies include
corticosteroids, interferons, monoclonal &
polyclonal antibodies, and other cell-free
immunotherapies (Figure 2). This article reviewed
these cell-free immunotherapy techniques against
SARS-CoV-2 and discussed current peer-reviewed
cell-free immunotherapeutic strategies against
COVID-19.
.
Figure 1: Organ damages caused by the SARS-CoV-2 can
be solved by cell-free immunotherapies: cell-free
immunotherapies might mitigate cardiac, kidney, liver,
nervous system, and lung injury; decrease macro- and
micro-thrombus formation and endothelial inflammation;
and repair lung epithelial and endothelial cells.
476
Liu, Y.
Cell-Free Immunotherapies-Effective Approaches Against COVID-19.
DOI: 10.5220/0012032700003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 476-482
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
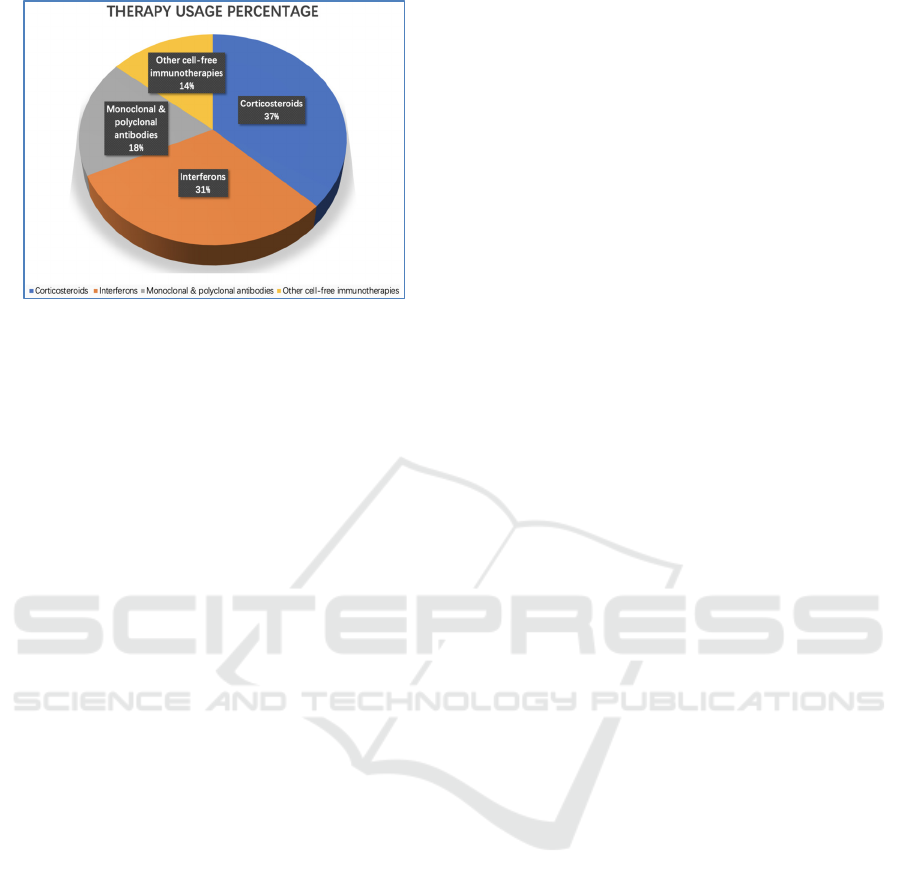
Figure 2: Graphical representation of usage percentage of
cell-free immunotherapies for COVID-19, which data were
from surveys in Chinese hospitals.
2 CORTICOSTEROIDS FOR
COVID-19
Patients infected with coronavirus disease have a
severe immune response leading to acute lung injury
and acute respiratory distress syndrome (ARDS)
(Erika, 2021). Among the cell-free drugs that target
the immune system, corticosteroids are usually used
to mitigate inflammation by suppressing the immune
system in severe cases of coronavirus disease
including SARS, MERS and COVID-19.
Corticosteroids are artificial drugs that are known as
steroids, which are naturally produced in the adrenal
cortex of vertebrates. They could be used in the
management and treatment of almost all areas of
medicine.
On the one hand, WHO claimed that systemic
corticosteroids are powerful immunomodulators,
which are generally low-cost, easy to administer and
accessible in healthcare systems pressured by the
COVID-19 global pandemic (WHO, 2020). Some
studies reported signs of beneficial effects of
corticosteroids through evidence comparing systemic
corticosteroid use to usual care in COVID-19. The
recovery trial demonstrated a lower 28-day mortality
in patients who received corticosteroids and were
either receiving oxygen alone or receiving invasive
mechanical ventilation, compared to usual care
(WHO, 2020). It was shown that the superior potency
of corticosteroids against COVID-19, because they
could lower the fatality rate and reduce the need for
receiving invasive mechanical ventilation.
On the other hand, systemic use of corticosteroids
has potential harms, such as hyperglycaemia
(especially in diabetics), hypernatremia,
gastrointestinal bleeding, neuropsychiatric effects,
neuromuscular weakness, superinfection,
immunocompromise, stroke or myocardial infarction
(WHO, 2020). Additionally, there is reason to
suspect that systemic corticosteroids may also delay
viral clearance and increase possibilities of secondary
infections, even though there is no firm data and
conclusion that could be drawn from studies and
research. As a result, this method is not
recommended for all patients, especially those who
have mild/non-severe/moderate symptoms
associated with COVID-19, because the adverse
effects of mass use of corticosteroids may have more
disadvantages than advantages.
On September 2, 2020, the WHO made a strong
recommendation for systemic corticosteroids in
severe and critical COVID-19 based on the moderate
certainty of evidence which showed a reduction in
mortality of 3.4% in patients with COVID-19 who
were critically or severely ill (WHO, 2020). In
addition, WHO made a conditional recommendation
not to use corticosteroids in the treatment of patients
with non-severe COVID-19. The reasons were low
certainty evidence which suggested an increased 28-
day mortality in patients with non-severe COVID-19;
that systemic corticosteroid use has potential harms
(e.g. hyperglycaemia, neuromuscular weakness,
superinfection); that indiscriminate use of the therapy
for COVID-19 may potentially rapidly deplete global
resources, and deprive patients who may benefit from
it most (WHO, 2020). Ultimately, the impact of
systemic corticosteroids in COVID-19 still remains
unknown and controversial, which means that further
investigations and trials on exploring the roles of the
corticosteroid therapy in the management of patients
are necessary. The clinical use of corticosteroids
should be more conscious about inconclusive adverse
events from corticosteroid administration in severe
COVID-19 cases.
3 INTERFERONS
Interferons (IFNs) are proteins generated by a
plethora of cells through the inflammatory response
to viral infections. In a typical scenario, virus-
infected cells release interferons, which kick off
fundamental cellular defense mechanisms in nearby
cells, such as heightening their antiviral defenses.
There are three types of IFNs: alpha, beta and
gamma. IFN-alpha is from leukocytes infected with
the virus, IFN-beta is produced in fibroblasts infected
with the virus, and IFN-gamma is induced by the
stimulation of lymphocytes.
Cell-Free Immunotherapies-Effective Approaches Against COVID-19
477

Type I IFNs have potentially positive effects for
fighting against coronaviruses. Type I IFNs are
famous for their antiviral and immunomodulatory
properties, and they are usually used for limiting the
spread of infectious agents, enhancing antigen
presentation, and triggering the adaptive immune
response. Studies showed the effectiveness of IFNβ
against viral infections compared to IFNα. For
example, a recent study published by Scientific
Reports assigned patients randomly in a 1:1:1 ratio to
IFNβ1a, IFNβ1b, or the control group (Ilad, 2021).
Through comparing IFNβ1a and IFNβ1b against each
other and a control group, IFNβ1a was associated
with a significant difference against the control group
while the IFNβ1b indicated no significant difference
compared with the control group (Ilad, 2021). In
general, mortality was indeed lower in both of the
intervention groups: 20% lower mortality in the
IFNβ1a group, 30% lower mortality in the IFNβ1b
group and 45% lower mortality in the control group
(Ilad, 2021). Also, these three groups did not show
significant differences regarding adverse effects
(Ilad, 2021). Given the limits of this study, further
confirmation in larger studies was required. In
addition, Sheahan et al found potent inhibition of
MERS-CoV with IFNb (Timothy, 2020). Some other
researchers conducted a preliminary study on 22
SARS patients. Comparing patients treated with
corticosteroids alone, this study indicated the
potential effect of interferon alfacon-1 combined
with corticosteroids (Mona, 2004). Therefore, the
studies on different types of viruses inferred that
interferon could be able to be protective in treating
patients with SARS-CoV-2.
However, such research on interferon therapy in
SARS-CoV is far from enough, especially given that
the virus is highly mutable and keeps changing.
Interferon might not contribute beneficial results for
hospitalized patients with COVID-19 (National
Institutes of Health, 2021). Nevertheless, these
findings still had clinical importance. For example,
the combination of interferon and other therapies may
be helpful for patients fighting COVID-19.
4 MONOCLONAL &
POLYCLONAL ANTIBODIES
Through different ways in which antibodies are
created from lymphocytes when an infection occurs,
antibodies are classified into two main types:
monoclonal and polyclonal. Both play important
roles in the humans’ immune system, diagnostic
exams, and treatments. Polyclonal antibodies (pAbs)
are a heterogeneous mixture induced by different B
cell lineages within the human body, whereas
monoclonal antibodies (mAbs) are secreted by
identical B cells which are clones from a single parent
cell. In addition, unlike polyclonal antibodies which
are from live animals, monoclonal antibodies are
produced in vitro environments using tissue-culture
techniques. The main source of protective pAbs is
recovered patients, whose plasma could be given to
patients infected with COVID-19 as a treatment
practice. In contrast, experimental methods usually
produce antiviral murine, humanized mAbs, or their
fragments. In general, when exposed to a pathogen,
the majority of antibodies produced by the humoral
immune system can target certain antigenic
determinants with sufficient affinity if they are
protective, and some antibodies target domains in the
spike protein can yield protection. This is how
antibodies prevent the reproduction of the virus or its
variants. Emergency use of antibody drugs should be
allowed in order to help patients’ immune systems
fight against viruses.
Scientists expected that pAbs and mAbs could be
useful in reducing viral levels, minimizing damage to
the patients’ lungs, preventing COVID-19 early-
stage infections, and allowing non-hospitalized
patients to heal more rapidly (Michael, 2021). This
treatment has long worked better on individuals who
have higher virus levels. However, the role of this
treatment is still controversial. Scientists are still
researching the population of patients who can
benefit the most from this treatment, and the extent
that this treatment is effective.
5 OTHER CELL-FREE
IMMUNOTHERAPIES FOR
COVID-19
Although there still remain ambiguities in the three
main COVID-19 therapies above, several other
approaches have meaningful results. The following
section discussed other possible treatments for
patients with COVID-19.
5.1 Convalescent Plasma Therapy
Convalescent plasma therapy transfers pathogen-
specific antibodies from recovered patients to help
others recover from the same illness. In response to
SARS-CoV-2 infection, convalescent plasma therapy
was useful when helping hospitalized patients to
ICBB 2022 - International Conference on Biotechnology and Biomedicine
478

recover and helping non-hospitalized patients to
prevent disease. Convalescent plasma therapy is
accessible because requirements for this therapy’s
infrastructure and resources are low. This therapy
only requires the donated plasma from disease
survivors and the standard blood collection
infrastructure. Thus, convalescent plasma therapy
could be readily used in low-resource settings around
the world. In addition, convalescent plasma therapy
could be given to hospitalized patients who have a
weakened immune system and are infected with
SARS-CoV-2. Convalescent plasma therapy could
help them to recover from COVID-19 by lessening
the severity and shortening the time of infection.
Recent studies, which have limited numbers of
patients and do not have control groups, tested the
clinical efficacy of convalescent plasma therapy in
fighting against COVID-19. To be more specific, the
study reported the results of the treatment of five
critically ill patients with COVID-19 with
convalescent plasma in China (Shen, 2020). After
receiving convalescent plasma therapy, four patients’
clinical status improved within 12 days, with
improvements such as enhanced PaO2/FiO2 and
decreased viral loads (Shen, 2020). This study
indicated that using convalescent plasma therapy has
potential to fight against COVID-19, but it must be
noted that this study did not have a control group.
Moreover, it is important to note that the passive
immunotherapy with convalescent plasma therapy
has the most effective therapeutic effects when the
viral load is relatively lower. Furthermore, this study
initiated convalescent plasma therapy from ten to
twenty-two days after admission of patients. Studies
received favorable results showing that convalescent
plasma therapy initiated earlier might have higher
efficacies (Shen, 2020). Furthermore, compared with
therapeutic uses, passive immunotherapies with
convalescent plasma therapy have a better efficiency
when used prophylactically. It is indicated that
scientists should put more effort into investigating
convalescent plasma therapy, especially as vaccines
have already become available around the world.
There are still uncertainties regarding the roles of
convalescent plasma therapy because the study by
Shen et al. was a randomized controlled trial.
Meanwhile, the results were based on limited
evidence so far. Thus, the clinical and therapeutic
impacts of plasma efficacy still need to be confirmed
in the future studies and well-designed clinical trials.
5.2 Intravenous Immunoglobulin (IVIg)
IVIg is the use of a mixture of antibodies from donors
that can be given intravenously. These antibodies are
protective proteins produced by the human immune
system in response to the presence of several
pathogens, such as viruses, bacteria, parasites, and
tumor cells. Donors’ antibodies bind directly with the
abnormal host pathogens, stimulating their removal.
Previous studies on SARS indicated that IVIg has
benefits on SARS patients. A study performed by
Wang et al showed that IVIg therapy improved
leukocyte/platelet counts in patients with severe
leukopenia, thrombocytopenia, and elevated levels of
aminotransferase, lactate dehydrogenase, and
creatine kinase (Wang, 2004). In addition, IgM-
enriched IVIg therapy demonstrated benefits in
patients with COVID-19 who were not cured by
corticosteroid therapy. And a multicenter
retrospective cohort study revealed clinical efficacy
of intravenous immunoglobulin therapy in critical
patients with COVID-19 from analyses on more than
three hundred patients (Shao, 2020). This study
showed that high dose IVIg could be helpful in the
prognosis if administered in the early stage of the
disease (Shao, 2020). A research meta-analysis
retrieved four clinical trials and three cohort studies
including 825 hospitalized patients (Xiang, 2021). In
the critical subgroup, IVIg could reduce the mortality
compared with the control group. However, the
severity of COVID-19 was not related to the efficacy
of IVIg. There was no significant difference in the
severe or non-severe subgroups. In a word, IVIg may
be clinically efficient in patients with COVID-19, but
impacts and roles of IVIg therapy in COVID-19
treatments are still uncertain, and further
effectiveness of it needs to be explored.
Adverse effects associated with IVIg are
commonly muscle pain, blood clots, kidney
problems, anaphylactic reactions and hemolytic
anaemia. However, the role of IVIg in infected
patients remains inconclusive because it is difficult to
isolate its benefits as it has been used in combination
with other drugs. In detail, patients with COVID-19
were benefited by mild dose corticosteroid plus IVIg
therapy (20 g/day), even though they did not benefit
from low dose IVIg therapy (10 g/day) (George,
2020). In addition, researchers found that the
combination of IVIg and methylprednisolone could
reduce respiratory morbidity in COVID-19. Further
studies about therapeutic impacts of IVIg still need to
be conducted, because the specific functions and
effectiveness of IVIg still need to be confirmed
through future research.
Cell-Free Immunotherapies-Effective Approaches Against COVID-19
479

6 CONCLUSIONS AND
OUTLOOK
On January 30, 2020, the World Health Organization
declared the COVID-19 outbreak as a Public Health
Emergency of International Concern, and later, as a
pandemic on 11 March 2020. From when SARS-
CoV-2 first emerged, it has spread so swiftly that the
need to find effective COVID-19 therapies became
necessary and urgent. In the evaluation of current
COVID-19 therapies, patients’ safety should be the
main consideration. This paper summarized the most
recent research and studies of cell-free
immunotherapy, showing that this therapeutic
intervention is proven to be useful against SARS-
CoV-2. Despite this therapy’s initial success,
research on it still showed ambiguities and room for
improvement. An overview of the advantages and
disadvantages of these novel potential therapies that
need to be formally tested in future clinical trials are
presented in Table1, and the applicable conditions are
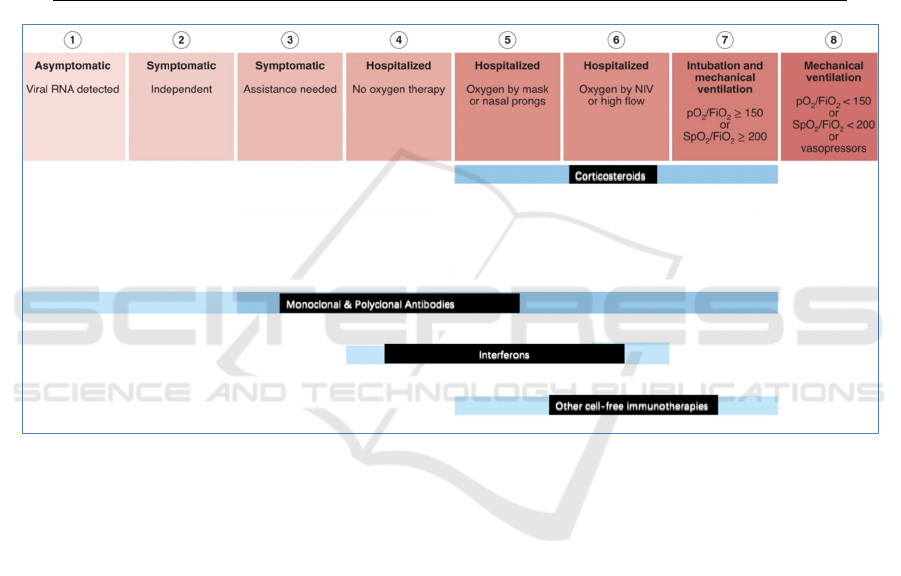
summarized in Figure 3, indicating that among these
methods, monoclonal & polyclonal antibodies are the
most widely applicable, corticosteroids have biggest
most potential, and there are still room for
breakthroughs in interferons in the future.
Moreover, it is vital to note that the virus is
changing. The Delta and Omicron variants appeared
successively in 2021. The Deltacron variants
appeared in 2022. Researchers must adapt their
therapies with the new mutations in mind. Though
there still remain challenges and uncertainty, this
article attempts to provide a systematic overview of
the available literature on cell-free
immunotherapeutic strategies, with the aim to help
scientists who investigate effective controls and
novel treatments for COVID-19. It is significant to
understand the intricacies of the virus-immune
system in order to treat and manage disease properly
in the future.
Table 1: Current cell-free immunotherapies for COVID-19 introductions and their potential beneficial & adverse effects in
COVID-19.
T
yp
e Introduction Advanta
g
es Disadvanta
g
es
Corticosteroid
s
Corticosteroids are
artificial drugs that are
known as steroids, which
are naturally produced in
the adrenal cortex of
vertebrates.
Low cost; easy to
administer and
accessible in
healthcare
systems.
Potential harms, such as
hyperglycaemia (especially in
diabetics), hypernatremia,
gastrointestinal bleeding,
neuropsychiatric effects,
neuromuscular weakness,
superinfection/immunocompromise,
stroke or m
y
ocardial infarction.
Interferons
There are three types of
interferons (IFN): alpha,
beta and gamma. IFN-
alpha is from leukocytes
infected with the virus;
IFN-beta is produced in
fibroblasts infected with
the virus; IFN-gamma is
induced by the stimulation
of lymphocytes.
Limiting the
spread of
infectious agents,
enhancing antigen
presentation, and
triggering the
adaptive immune
response.
Research on interferon therapy in
SARS-CoV is far from enough,
especially given that the virus is
highly mutable and keeps changing.
Interferon might not contribute
beneficial results for hospitalized
patients with COVID-19.
Monoclonal &
Polyclonal
Antibodies
Polyclonal antibodies
(pAbs) are a heterogeneous
mixture induced by
different B cell lineages
within the human body,
whereas monoclonal
antibodies (mAbs) are
secreted by identical B
cells which are clones from
a single parent cell.
Useful in reducing
viral levels,
minimizing
damage to the
patients’ lungs,
preventing
COVID-19 early-
stage infections,
and allowing non-
hospitalized
patients to heal
more rapidly.
The role of this treatment is still
controversial.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
480
Convalescent
Plasma
therapy
Convalescent plasma
therapy transfers pathogen-
specific antibodies from
recovered patients to help
others recover from the
same illness.
Lessening the
severity and
shortening the time
of infection.
There are still uncertainties
regarding the roles of convalescent
plasma therapy.
IVIg
(Intravenous
Immunoglobu
lin)
IVIg is the use of a mixture
of antibodies that can be
given intravenously. IVIg
gives antibodies that the
human body is not making
on its own but can fight
infections.
IVIg may be
clinically efficient
in patients with
COVID-19.
Clinical and laboratory data on
severe acute respiratory syndrome
(SARS) are limited. Adverse effects
associated with IVIg are commonly
muscle pain, blood clots, kidney
problems, anaphylactic reactions
and hemol
y
tic anaemia.
Figure 3: An overview of the options for cell-free immunotherapies in patients with COVID-19 depending on the stage of the
disease, according to WHO Clinical Progression Score (Frank, 2022). The treatments based on high-quality randomized trials
are presented in dark blue, while the more speculative treatments based on observational or small case-series studies are
presented in light blue.
REFERENCES
Chenguang Shen, Zhaoqin Wang, Fang Zhao, et al. 2020.
Treatment of 5 Critically Ill Patients With COVID-19
With Convalescent Plasma. The Journal of the
American Medical Association, 323 (16): 1582–1589.
Erika Vainieri. Dexamethasone in Hospitalized Patients
with Covid-19. 2021. The New England Journal of
Medicine, 384: 693-704.
Frank L. van de Veerdonk, Evangelos Giamarellos-
Bourboulis, Peter Pickkers, et al. 2022. A guide to
immunotherapy for COVID-19. Nature Medicine, 28:
39–50.
George Sakoulas, Matthew Geriak, Ravina Kullar, et al.
2020. Intravenous Immunoglobulin Plus
Methylprednisolone Mitigate Respiratory Morbidity in
Coronavirus Disease 2019. Critical Care Explorations,
2(11): e0280.
Huai-rong Xiang, Xuan Cheng, Yun Li, Wen-wen Luo, Qi-
zhi Zhang, and Wen-xing Peng. 2021. Efficacy of IVIG
(intravenous immunoglobulin) for coronavirus disease
2019 (COVID-19): A meta-analysis. International
Immunopharmacology, 96: 107732.
Ilad A. Darazam, Mohamad A. Pourhoseingholi,Shervin
Shokouhi, et al. 2021. Role of interferon therapy in
severe COVID-19: the COVIFERON randomized
controlled trial. Scientific Reports, 11(1): 8059.
Jann-Tay Wang, Wang-Huei Sheng, Chi-Tai Fang, et al.
2004. Clinical manifestations, laboratory findings, and
treatment outcomes of SARS patients. Emerging
infectious diseases, 10(5): 818-824.
Mona R Loutfy, Lawrence M Blatt, Katharine A
Siminovitch, et al. 2004. Interferon Alfacon-1 Plus
Cell-Free Immunotherapies-Effective Approaches Against COVID-19
481

Corticosteroids in Severe Acute Respiratory
Syndrome: A Preliminary Study. The Journal of the
American Medical Association, 290(24): 3222-3228.
Michael Moore. 2021. Monoclonal and Polyclonal
antibodies in COVID-19 treatment. Retrieved
September 27, 2021 from https://www.h-h-
c.com/monoclonal-and-polyclonal-antibodies-in-
covid-19-treatment/
National Institutes of Health (NIH). Interferon does not
improve outcomes for hospitalized adults with
COVID-19. 2021. Retrieved October 18, 2021 from
https://www.nih.gov/news-events/news-
releases/interferon-does-not-improve-outcomes-
hospitalized-adults-covid-19
Timothy P Sheahan, Amy C Sims, Sarah R Leist, et al.
2020. Comparative therapeutic efficacy of remdesivir
and combination lopinavir, ritonavir, and interferon
beta against MERS-CoV. Nature Communications,
11(1): 222.
WHO. 2020. Corticosteroids for COVID-19. Retrieved
September 2, 2020 from
https://www.who.int/publications/i/item/WHO-2019-
nCoV-Corticosteroids-2020.1
Ziyun Shao, Yongwen Feng, Li Zhong L, et al. 2020.
Clinical Efficacy of Intravenous Immunoglobulin
Therapy in Critical Patients with COVID-19: A
Multicenter Retrospective Cohort Study. SSRN
Electronic Journal.
http://dx.doi.org/10.2139/ssrn.3576827.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
482
