
Design of Targeted and Release Controlled Liposome for Paclitaxel
and Doxorubicin Combination in Breast Cancer Therapy
Chengpei Ouyang
1,*
, Qi Zhu
2
, Yifan Liu
3
and Xiangqi Meng
4
1
College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China
2
JSerra Catholic High School, San Juan Capistrano, California 92675, U.S.A.
3
Beijing No.101 High School, Beijing 101407, China
4
Beijing City International School, Beijing 100022, China
Keywords:
Combination Therapy, Paclitaxel, Doxorubicin, Bi-Layer Liposome, Co-Delivery.
Abstract: Paclitaxel and doxorubicin are commonly used in chemotherapy of breast cancer. Now we have developed a
kind of nanocarrier liposome that can deliver the combination of Dox and PTX to alleviate the pain brought
by side effects and decrease the resistance. In this paper, we expected the result data of the drug loading
capacity and the drug loading efficiency, the release of PTX and Dox in different pH environment, and the
absorption of drugs which is estimated by the amount of free drug remains in the cells. The combination of
Dox and PTX in liposome was modified with folic acid for tumor targeting, and achieved pH responsive drug
release in tumor cell by introduction of N-(4-carboxybenzyl)-N, N-dimethyl-2,3-bis (oleoyloxy) propan-1-
aminium (DOBAQ), a kind of pH sensitive lipid. The double layers were loaded with hydrophobic drug PTX,
and the hydrophilic drug DOX is loaded in the aqueous core of the vesicle. We expected the results to reduce
side effects and improve specificity when treating the cancer.
1 INTRODUCTION
Breast cancer is the disease in which breast epithelial
cells proliferate out of control under the action of a
variety of carcinogens. The early stage of the disease
often manifests as breast lumps, nipple discharge,
axillary lymphadenopathy, and other symptoms (Yin,
2010). In the late stage, cancer cells may metastasize
to a distance, and multiple organ diseases may appear,
which directly threaten the life of the patient. Breast
cancer is often called the "pink killer", and its
incidence ranks first among female malignant tumors.
Male breast cancer is relatively rare. With the
improvement of medical treatment, breast cancer has
become one of the solid tumors with the best curative
effect. Risk factors for breast cancer include genetic
factors, hormonal changes, mental and psychological
factors, and history of past breast diseases. According
to the latest data from the International Agency for
Research on Cancer (IARC) in 2018, the incidence of
breast cancer in female cancers worldwide is 24.2%,
ranking first among female cancers, of which 52.9%
occur in developing countries (Feng, 2021).
Breast cancer treatment is personalized. All
patients need to be formulated by authoritative
experts based on their own conditions. The treatment
plan depends on many factors, including tumor
subtypes, patient’s age, general health, menopause
and eating habits, stage of the tumor, hormone
receptor status (ER, PR) and HER2 status, genetic
information (such as BRCA1 or BRCA2), and genetic
testing results, such as full genetic testing and breast
cancer 21 gene testing (Oncotype DX™) (Reddy,
2011). At present, the conventional methods of
treatment of breast cancer mainly include surgery,
radiotherapy, chemotherapy, hormone therapy,
targeted therapy, immunotherapy, etc. Radiation
therapy, chemotherapy, targeted therapy and/or
hormone therapy can be used to assist the treatment
(Bodei, 2007).
1.1 Surgery
For ductal carcinoma in situ and early invasive breast
cancer, doctors usually recommend surgery to remove
the tumor (Trayes, 2021). Most patients with invasive
breast cancer will undergo sentinel lymph node
biopsy or axillary lymph node dissection. Most
important thing in the treatment of early- stage breast
cancer is to reduce the risk of recurrence by removing
Ouyang, C., Zhu, Q., Liu, Y. and Meng, X.
Design of Targeted and Release Controlled Liposome for Paclitaxel and Doxorubicin Combination in Breast Cancer Therapy.
DOI: 10.5220/0012032000003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 425-434
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
425

all remaining cancer cells. These cancer cells are
undetectable, but they can cause cancer to recur
because they grow over time.
1.2 Radiation Therapy
Radiation therapy uses high-energy X-rays or proton
rays to destroy cancer cells. Radiation therapy helps
reduce the risk of breast recurrence. Through surgery
and radiotherapy, within 10 years of treatment, the
recurrence rate of breast cancer is now less than 5 %
(McGale, 2014). In the past, traditional radiotherapy
increased the long-term risk of heart disease in
women with left breast cancer. Now, proton therapy
can protect the heart from radiation damage.
Therefore, more breast cancer patients can reasonably
obtain a longer survival period and better quality of
life through modern medical methods.
1.3 Medication
Systemic therapy using medication can kill cancer
cells comprehensively. Types of systemic therapy for
breast cancer include chemotherapy, hormone
therapy, targeted therapy, and immunotherapy
(Bodei, 2007).
Chemotherapy began in the 1940s and 1950s, and
its application has greatly improved the efficiency of
anti-tumor. Although surgery can remove tumor
tissue, it cannot be completely removing all cancer
cells, especially for circulating cancer cells in the
blood. At this time, chemotherapy is needed to kill the
remaining cancer cells and reduce the risk of
recurrence and distant metastasis. Therefore,
chemotherapy is of great significance to tumor
treatment (Hassan, 2010). In the classification and
treatment of breast cancer today, chemotherapy also
plays a very important role. For early breast cancer
patients, the risk of recurrence and metastasis can be
reduced; for advanced patients, it can alleviate the
condition, prolong survival, and improve the quality
of life.
After decades of development, the commonly
used chemo-drugs for breast cancer currently include
anthracyclines (such as DOX), taxanes (such as
PTX), and antimetabolites (such as gemcitabine).
DOX is an anti-tumor drug commonly used in clinic.
Its main function is to insert the flat n-loop to the
middle of the base strand of DNA, which prevents the
transfer and replication of DNA and +RNA, thus
avoiding cells’ proliferation and metabolism. Breast
cancer is a major indication of DOX, which is often
used in combination with cyclophosphamide, in
addition, it can be combined with PTX and docetaxel,
which can increase the efficacy (Darya Alizadeh,
2014). In addition, DOX can be used to treat bladder
cancer, head and neck malignancies, testicular
malignancies, liver cancer, and stomach cancer.
PTX is a natural secondary metabolite extracted
from the bark of Taxus chinensis, which has good
antitumor effect. It is often used in the treatment of
ovarian cancer, uterine cancer, and breast cancer. PTX
is a natural secondary metabolite extracted from the
bark of Taxus chinensis; it allows tubulin and tubulin
dimers that make up microtubules out of dynamic
equilibrium, which leads to them death of cells (Sun,
2008). DOX and PTX can be administered separately,
but the therapeutic effect is not good. A single drug is
easy to cause drug resistance. Once drug resistance
occurs, the therapeutic effect of drugs will be
significantly reduced, even large amounts of doses
will not achieve the original effect. And improved
drug dosage will lead to the relatively large toxicity
and side effects. Therefore, we decided to use a Co-
administration Combination therapy, refers to the use
of two or more drugs for treatment, which can
improve the efficacy and reduce adverse reactions.
The combination of more drugs can reduce the dose
of individual drugs, thus reducing the toxicity and
side effects (Moussa, 2018). The combination of the
two drugs can reduce the side effects and resistance
significantly. PTX has the function of preventing the
cell division form beginning of cell division cycle.
DOX functions in cell DNA damage. It causes the
cleavage of DNA and thus indirectly causes the
generation of hydroperoxide through the oxidative
reaction of NAD(P)H. Eventually, it leads to the
apoptosis of cells (Hideki Mizutani, 2005). When
combining the two drugs, the result suggested that it
had become more effective on tumor regression. It
can also reduce the side effects and the resistance
(Gill, 2019). In order to improve the therapeutic effect
of combined drug delivery and reduce its side effects,
we decided to load these two drugs into liposome
with. modifications of targeting and sustained release
on liposomes.
Folate acid is applied for tumor targeting in our
liposome system, although folate receptors are widely
distributed in normal tissues and tumor tissues, the
density and activity of folate receptors in most tumor
cells are much higher than those in normal cells.
Thus, it can achieve drug accumulation to the surface
of breast cancer cells and increase the concentration
of the drug in the tumor area, which can improve the
treatment effect and reduce side effects. PH
responsive drug release in tumor cell is achieved by
DOBAQ, a kind of pH sensitive Cationic lipid, which
exhibits pH dependent ionization and promote
ICBB 2022 - International Conference on Biotechnology and Biomedicine
426
liposome disruption in pH 5.5 endosome in tumor cell
(Su, 2019). Because liposomes will dissociate and
change with each other under different pH conditions,
then control the drug release. Thus, we developed this
way of delivering PTX and DOX. Using both drugs
can limit the side effects. Additionally, in order to
ease the cardiotoxin of those two drugs, we used pH-
sensitive liposome to deliver the drug and only
targeting on the breast cancer cells that has large
amount of folate receptors (Sharma, 2006). The
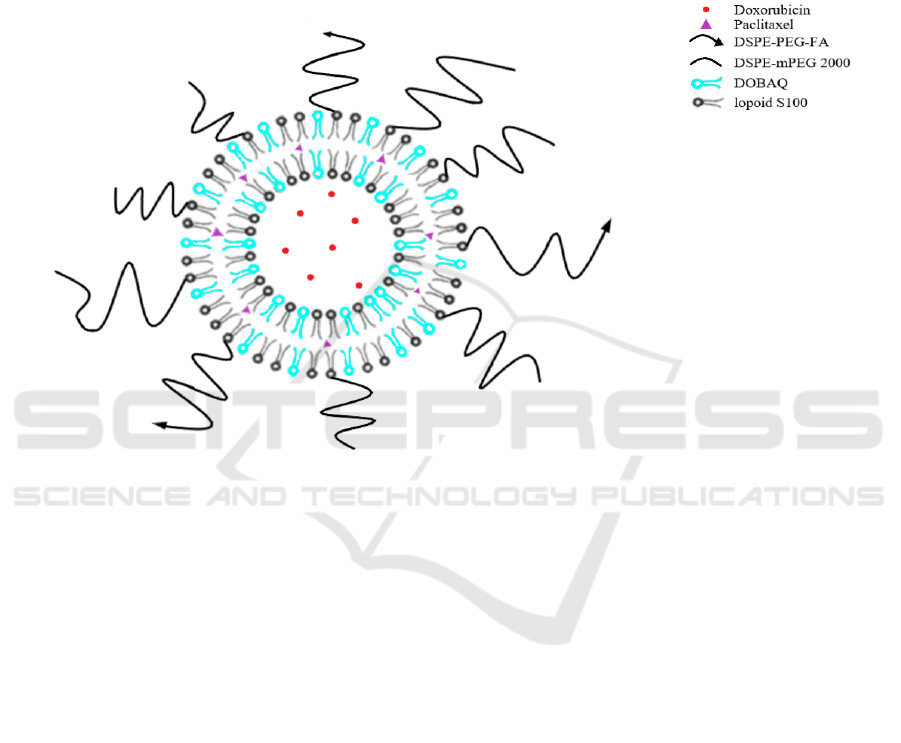
structure of liposome is shown in Figure 1. We hoped
the bilayer liposome can delivering those two drugs
effectively and reach our propose of reducing the side
effects and lower the simple drug resistance.
Therefore, we will find a more efficient way that can
bring patient a better life quality and overall improve
the survival rate.
Figure 1. Schematic representation of the co-delivery of hydrophobic drug paclitaxel (purple) and hydrophilic drug
doxorubicin (red) through bilayer liposome (Liu, 2014).
2 METHODS AND MATERIALS
2.1 Materials
DSPE-PEG (2000)-Folate and Doxorubicin
hydrochloride (DOX) was purchased from Sigma-
Aldrich (Shanghai) Trading Co.Ltd. (Shanghai,
China). DOBAQ, DSPE-mPEG2000 and DSPE-
PEG2000-FITC were purchased from Avanti Polar
Lipids (Alabaster, USA). Lipoid S100 were
purchased from Lipoid GmbH (Würzburg, German).
Paclitaxel (PTX) were purchased from Macklin Inc.
(Shanghai, China). Gibco™ PBS were purchased
from Thermo Fisher Scientific (USA). Sephadex G-
50 columns were purchased from Pfizer Inc. (USA).
Amicon Ultra 0.5 mL centrifugal filters were
purchased from Sigma-Aldrich (Shanghai) Trading
Co.Ltd. (Shanghai, China). Pierce™ Protein
Concentrator PES, 3K MWCO cut-off tubes, Attune
NxT flow cytometry and Nunc™ multi-well cell
culture plates were purchased from Thermo Fisher
Scientific™. Leica SP2 CLSM Confocal laser
scanning microscopy was from Leica Microsystems
(Wetzlar, Germany).
2.2 Preparation of liposome
Liposomes were prepared by the lipid hydration
method (Schiffelers, 2003). Briefly, different masses
of lipid substances, were dissolved in round-bottomed
vials using an organic solvent mixture (chloroform:
methanol=2:1). Then, the round bottom vials are
placed on the rotary evaporator. By using the
principle of lowering the boiling point of liquids
under reduced pressure, volatile solvents are removed
by heating continuous distillation. After completion
of the rotary evaporation operation, the resulting film
in the vial was transferred to vacuum and dried for
more than 6 hours to completely remove the residual
organic solvent. The obtained films were placed in 2
mL of phosphate-buffered solution (PBS, pH 7.4) at
37°C for 30 min of hydration to obtain liposomes with
Design of Targeted and Release Controlled Liposome for Paclitaxel and Doxorubicin Combination in Breast Cancer Therapy
427

a final lipid concentration of 20 mg mL-1, followed
by 60 seconds of sonication. Then it was further
intermittently sonicated by a probe sonicator in ice-
bath at 80 W for 75 s. The free (NH4)2SO4 was
removed by passing through a Sephadex G-50
column in PBS (pH 7.4) solution. The obtained
liposomes were purified by 3 K Amicon centrifugal
filters and washed twice with fresh PBS solution (10
mM, pH 7.4).
For the preparation of liposomes containing DOX
(D-LPs), we set up four concentration gradient groups
(10 µg/ml, 1 µg/ml, 0.1 µg/ml, 0.01 µg/ml), and
added gradient concentrations of DOX, stirred, and
then incubated at 45℃ for 20 min. The PTX-loaded
liposomes (P-LPs) were made at the same
concentration and by the same method. By using gel
filtration, the large molecule liposomes are eluted
first due to their weak retention ability, while the
small molecule compounds are retained strongly and
exit the column last, thereby removing the free
PTX/DOX. The liposomes were stored at 4℃ for
later use.
For the preparation of DOX and PTX-loaded
liposomes (DP-LPs), as shown in the Figure 1, the
PBS was replaced by 1 ml 300 Mm (pH 4)
(NH4)2SO4 solution. As mentioned above, the PBS
solution (pH, 7.4) was added to the SephadexG-50 gel
filtration column to equilibrate the pH, and then PTX-
LPs were used to replace the outer phase consisting
of (NH4)2SO4 solution. Doxorubicin was then
remotely loaded using the (NH4)2SO4 gradient
method. (Bolotin E M, 1994). Similarly, we set up
four concentration gradient groups (10µg/ml, 1µg/ml,
0.1µg/ml, 0.01µg/ml) and the molar ratio of PTX to
DOX was 1:1. Briefly, PTX-LPs were preheated at
50°C, and the appropriate amount of DOX solution
was added and incubated with liposomes at 50°C for
20 min with gentle stirring to load DOX into the
liposomal internal phase. Free DOX and PTX were
then removed by 3 K Amicon centrifugal filters.
Liposomes were stored at 4℃ and used within 24 h
of preparation (Qiu, 2016). The Tables 1-3 list the
lipid ratios of the liposomes used in the following
experiments (Walsh, 2012; Moghimipour E, 2018;
Campbell R B, 2001; Bernsdorff C, 1999; Sampedro
F, 1994; Sharma A, 1994).
Table 1: Liposome formulation of FA-LPs.
Corresponding compositions (%)
DSPE-PEG2000-Folate DSPE-mPEG2000 DOBAQ lipoid S100
Molar Ratio (%) 1 4 50 45
Table 2: Liposome formulation of FA-FITC-LPs for cell uptake study
Corresponding compositions (%)
DSPE-
PEG2000-Folate
DSPE-
mPEG2000
DOBAQ lipoid S100
DSPE-
PEG2000-FITC
Molar
Ratio (%)
1 4 49 45 1
Table 3: Liposome formulation of FITC-LPs for cell uptake study
Corresponding compositions (%)
DSPE-PEG2000-FITC DSPE-mPEG2000 DOBAQ lipoid S100
Molar Ratio (%) 1 4 50 45
ICBB 2022 - International Conference on Biotechnology and Biomedicine
428

2.3 Physicochemical Characterization
of Liposomes
For encapsulation efficiency measurement (Shew R
L, 1985), 0.1 ml of the liposome suspension was
passed through a Sephadex G-50 gel filtration column
and the PTX and DOX contents were measured by
high performance liquid chromatography (HPLC) at
227 nm and at Ex of 470 nm, Em of 590 nm,
respectively. Drug entrapment efficiency (DEE, wt%)
and drug loading capacity (DLC, wt%) were
calculated according to the following formulas (Tang,
2014):
DEE = (amount of loaded drug/amount of drug
added) × 100%
DLC = (amount of loaded drug/amount of loaded
drug + amount of drug carrier) ×100%
The column used for high performance liquid
chromatography (HPLC) is Acclaim™ 300 C18
column, flow rate is 1 mL/min, the liquid phase is of
paclitaxel was methanol-water (65:35, v/v) with the
detection wavelength of 227 nm and the liquid phase
of adriamycin was methanol-water (70:30).
Hydrodynamic diameter, polydispersity index
(PDI) and zeta potential of liposomes were measured
by NS-90Z Nanoparticle size and potential analyzer
from Omec (Zhuhai, China), using dynamic light
scattering (DLS) and electrophoretic light scattering
technique. The liposome was diluted with Milli Q
water before measurement.
Transmission electron microscopy (TEM)
samples were prepared by diluting the liposome
solution to a concentration of 0.1 mg/mL and adding
dropwise 5 μL of liposome dissolved into a 200-mesh
formvar-coated copper grid (TABB Laboratories
Equipment, UK). After five minutes, the solution was
aspirated through filter paper and 2 μL of uranyl
acetate solution (2%, w/v) was added. After five
minutes, the solution was blotted off with filter paper.
The TEM sample was air-dried and then assayed.
2.4 In Vitro Release Kinetic of
Liposomes
Here, we used the dialysis method to investigate the
release kinetics of DOX and PTX from liposomes in
phosphate-buffered saline (PBS), a method that has
been used several times in previous liposome delivery
studies (Campbell R B, 2001; Lv, 2014; Wang, 2016)
The pH of the PBS release medium was set to 7.4 and
5.5, respectively. Briefly, the liposomes loaded with
PTX and Dox were suspended in 5 ml of PBS release
medium and transfer to a dialysis bag (MWCO 3500
Da). The release experiments were started by placing
the dialysis bag into 45 mL of release medium with
continuous shaking at 100 rpm at 37℃. At the
scheduled point in time (1,2,4,612,24,48,72,108 h), 4
mL of the incubation solution was extracted and
replaced with an equal volume of fresh PBS. DOX
release was determined using the HPLC method
mentioned above. The concentration of paclitaxel in
1 mL of solution will be detected at 227 nm (flow rate
1 mL/min) on a C18 column. At the end of 108 h, the
dialysis bag was opened and 2.0 ml of 10% TritonX-
100 was mixed completely with the release medium.
The concentrations of paclitaxel and Adriamycin
were then determined by HPLC and UV-Vis
spectrometer as mentioned above, and the maximum
drug release was calculated.
After calculating the free drug concentration, the
percentage of drug release can then be calculated with
the following formula: percentage of drug release =
amount of drug released / amount of drug contained
in the package × 100%. The drug co-delivery release
profiles of Dox and PTX have demonstrated that
liposomes have slow linear sustained release kinetics
and efficient drug loading yields. Drug release
profiles at pH = 7.4 and 5.5 have also been clearly
studied (Lv, 2014; Zhu, 2015). Based on previous
studies, we predicted the release profiles of bilayer
liposomes loaded with Dox and/PTX drugs
administered in combination or alone at 1, 2, 4, 6, 12,
24, 48, 72, and 108 hours and compared them.
2.5 Stability Studies in Human Plasma
At 37°C, 10 mg of liposomes were added to 1 mL of
human plasma and stirred at low speed (200 rpm)
with a magnetic stirrer. After every period (1, 2, 4, 6,
12, 24h), the particle size of the liposomes was
determined using dynamic light scattering. The
liposomes were observed for the appearance of
agglomerated precipitation.
2.6 Breast Cancer Cell and Liposome
Interaction Studies
2.6.1 Cell Culture
Two types of human breast cancer lines, including
MDA-MB-231 and MCF-7 were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) with
high glucose containing 10% fetal bovine serum
(FBS), supplemented with 100 mg/ml streptomycin
and 100 U/ml penicillin. The cells were maintained at
37℃ in a humidified incubator under 5% CO2 (Qiu,
2016).
Design of Targeted and Release Controlled Liposome for Paclitaxel and Doxorubicin Combination in Breast Cancer Therapy
429

2.6.2 Confocal Laser Scanning Microscopy
(CLSM) Observation of Liposome
Uptake by Cells
The uptake of liposomes by both MDA-MB-231 and
MCF-7 cells was determined by observing the
fluorescence characteristics of DOX released from
FITC-labeled liposomes (FITC-LPs) by confocal
laser scanning microscopy (CLSM) at an emission
wave of 480 nm and an excitation wave of 590 nm.
Firstly, the cells were seeded on the coverslips in 24-
well cell culture plates at a density of 1 ×105
cells/well in 2 mL of DMEM. Then the cells were
incubated for 24 h to 50% confluence and the original
medium was replaced with 200 ml of 3 mmol/ml
DOX-PTX-FA-FITC-LPs (DP-FA-FITC-LPs) and
DOX-PTX-FITC-LPs (DP-FITC-LPs). After 3 or 6 h
incubation, then the supernatant was removed by
centrifugation to obtain a cell mass. The cell masses
were washed three times with cold PBS, fixed in 4%
paraformaldehyde at room temperature for 30 min,
followed by cell nuclei staining with DAPI for 5 min
before washed three times with PBS for confocal
microscopy analysis. Later, the coverslips were
detected on CLSM. Fluorescence images of cells
were analyzed using ImageJ or FIJI software (Lv,
2014).
2.6.3 Qualitative Analysis of Cellular
Uptake Using Flow Cytometry
1.0 ml of MDA-MB-231 and MCF-7 cells (1 × 10
5
cells/well) were inoculated into 24-well tissue culture
plates and cultured at 37°C, 5% CO
2
for 24 h until the
cells grew almost confluently. The medium was then
replaced with 1.0 ml of 0.5 mg/ml DP-FITC-LPs and
DP-FA-FITC-LPs diluted with DMEM medium,
respectively, and the plates were incubated at 37°C
and 5% CO
2
.After 4 h, the medium was removed, and
cell monolayer was suspended by brief treatment with
trypsin and then washed three times with cold PBS.
Then the cell samples were examined by flow
cytometry using a Attune NxT (Thermo Fisher). the
level of cells that have taken up liposomes (positive
event, %) and the level of liposomes that have been
taken up (mean fluorescence) are measured. 10,000
sensor events collected and analyzed using FCS
Express software (Qiu, 2016).
2.7 In Vitro Cytotoxic Studies
The cells were cultured as shown above. MDA-MB-
231 and MCF-7 cells were plated at a density of 5 ×
10
3
cells/well in 10% FBS-containing medium in 96-
well plates and grown for 24 h. The cells were then
exposed to liposomes (single drug/drug
combinations; FA/without FA) at different
concentrations of combined drugs or free drugs
(10µg/ml, 1µg/ml, 0.1µg/ml, 0.01µg/ml), for 48 h. To
determine the cytotoxicity of empty liposomes, 100
ml of 6 mmol/ml liposomes were added to each well
of a 96-well plate and incubated with the cells
described above. The cell viability was assessed using
the CellTiter from Promega according to the
manufacturer’s instructions. The principle of
detecting cell viability is that the fluorometric signal
of CellTiter is proportional to the ATP content of the
cells, which is proportional to the number of cells.
Toxicity of each drug concentration was subsequently
determined for each well. The data was analyzed by
nonlinear regression to get the IC50 value. The
combination index (CI) values were calculated by the
equation:
CI =
,
,
+
,
,
(1)
CA,x and CB,x are the concentrations of drug A and
drug B used in combination to achieve x% drug
effect. ICx,A and ICx,B are the concentrations for
single agents to achieve the same effect. A CI of less
than, equal to, and more than 1 indicates synergy,
additivity, and antagonism, respectively (Zhao,
2004). Using this analysis method, a CI = 0.9−1.1
reflects additive activity, and a CI >1.1 indicates
antagonism, while a CI < 0.9 suggests synergy (Liu,
2014).
3 EXPECTED RESULTS AND
CONCLUSIONS
3.1 Characteristics of Co-Delivery via
pH Release FA Targeted Liposomes
Firstly, we characterized the physical properties of the
dual- versus single-drug-loaded liposomes to
determine whether the drug combination could alter
the physical properties of the liposomal formulation.
Dynamic light scattering (DLS) measurements
showed that the resulting dual-loaded liposomes had
similar mean hydrodynamic diameters as the single-
loaded liposomes. Therefore, the effect of drugs on
liposome particle formation is negligible (Qiu, 2016).
Next, we determined the encapsulation efficiency
or loading yield of the liposomes. Particle size and
PDI are important characteristics of drug-laden
liposomes that determine the release kinetics of the
drug, as shown in the table 4. Single- and dual-loaded
ICBB 2022 - International Conference on Biotechnology and Biomedicine
430
liposomes were dissolved in the release medium to
gradually release the encapsulated drug (Dox and/or
PTX). DOX and PTX concentrations were quantified
by fluorophotometer and/or HPLC, respectively. The
results of in vitro drug release assays showed that this
liposome has slow and linear slow-release kinetics for
DOX and PTX, similar to that of single drug
liposomes. These results confirm the ability of the
method to load different hydrophobic drugs into the
same nanoparticles with efficient drug loading rates
and sustained drug release profiles (Wang, 2016; Liu,
2014; Kurbacher C M,1996)
Table 4. Physico-chemical characterization of different liposomes formulations (Wang, 2016).
Sample
Particle size
(nm)
PDI
Zeta
potential
(
mV
)
DLC of
PTX (%)
DLC of
DOX (%)
DEE of
PTX (%)
DEE of
DOX (%)
LP 126.8±3.3 0.14±0.02 +45.3±2.8 NA NA NA NA
PTX LP 125.6±3.5 0.15±0.03 +37.6±2.6 18.6±2.0 NA 82.5±4.3 NA
DOX LP 128.3±3.4 0.12±0.03 +34.0±2.4 NA 10.3±1.6 NA 85.5±4.0
PTX-DOX
LP
129.5±4.4 0.19±0.06 +26.8±3.3 12.5±1.8 9.6±1.2 81.7±4.6 83.6±4.3
* NA means not available.
3.2 In Vitro Release Kinetic of
Liposomes
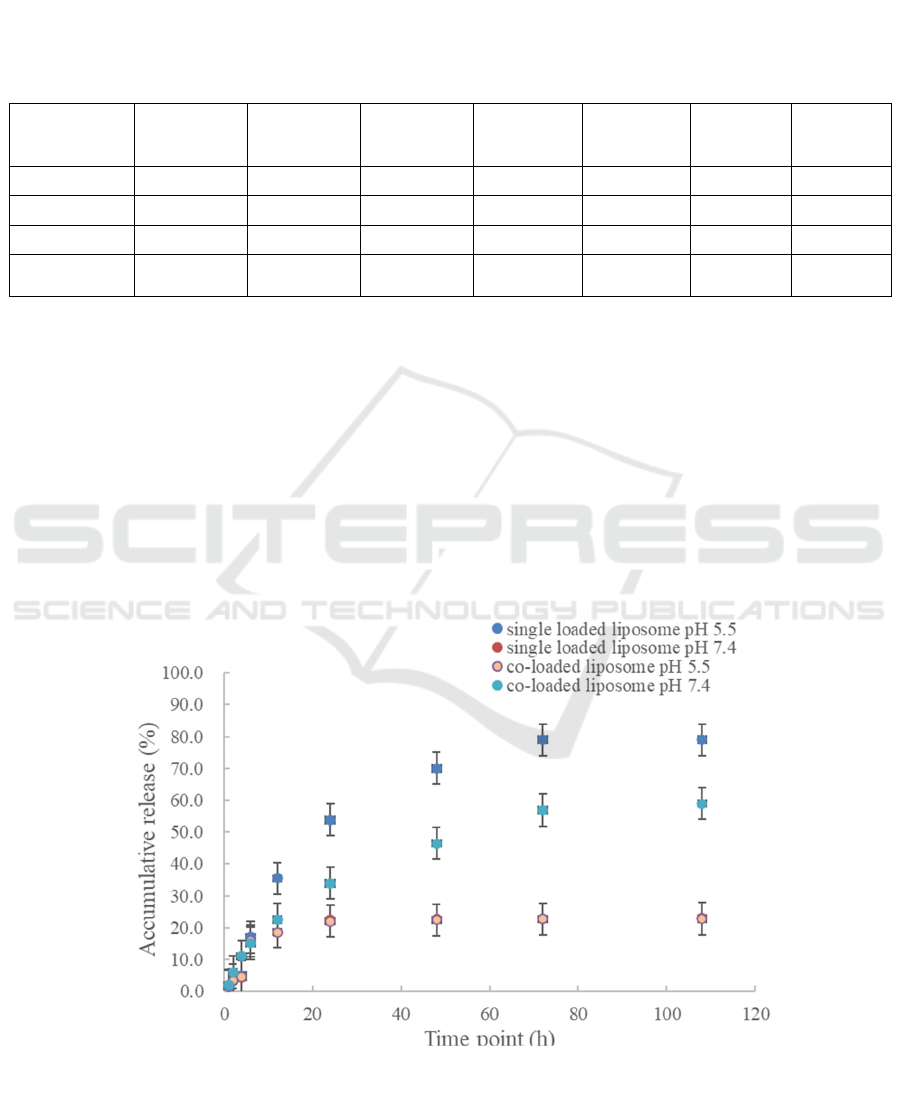
The DOX and PTX release behavior of liposomes
was evaluated by phosphate-buffered saline (PBS)
dialysis at different pH values (7.4 and 5.5). Among
them, the release of DOX was strongly influenced by
the ambient acidity. At pH 5.5, about 79% of DOX
was released; at pH 7.0, less than 23% was released,
as illustrated in Figures 2 and 3. To summarize, we
present a robust combination chemotherapy approach
that encapsulates two different types of antitumor
therapeutics into a specific liposome formulation
through a controlled 1:1 molar ratio. We
demonstrated that liposomes could release Dox and
PTX in vivo at predefined ratios of loaded drugs and
induce synergistic effects in tumor cells. Such
targeted reactive release liposomes have the superior
ability to act as drug carriers, providing a controlled
and sustainable spectrum of Adriamycin drug release
with improved antitumor activity.
Figure 2: In vitro release of DOX in DOX loaded liposome and DOX/PTX co-loaded liposome at pH 7.4 and 5.5 buffer (Lv,
2014; Wang, 2014).
Design of Targeted and Release Controlled Liposome for Paclitaxel and Doxorubicin Combination in Breast Cancer Therapy
431
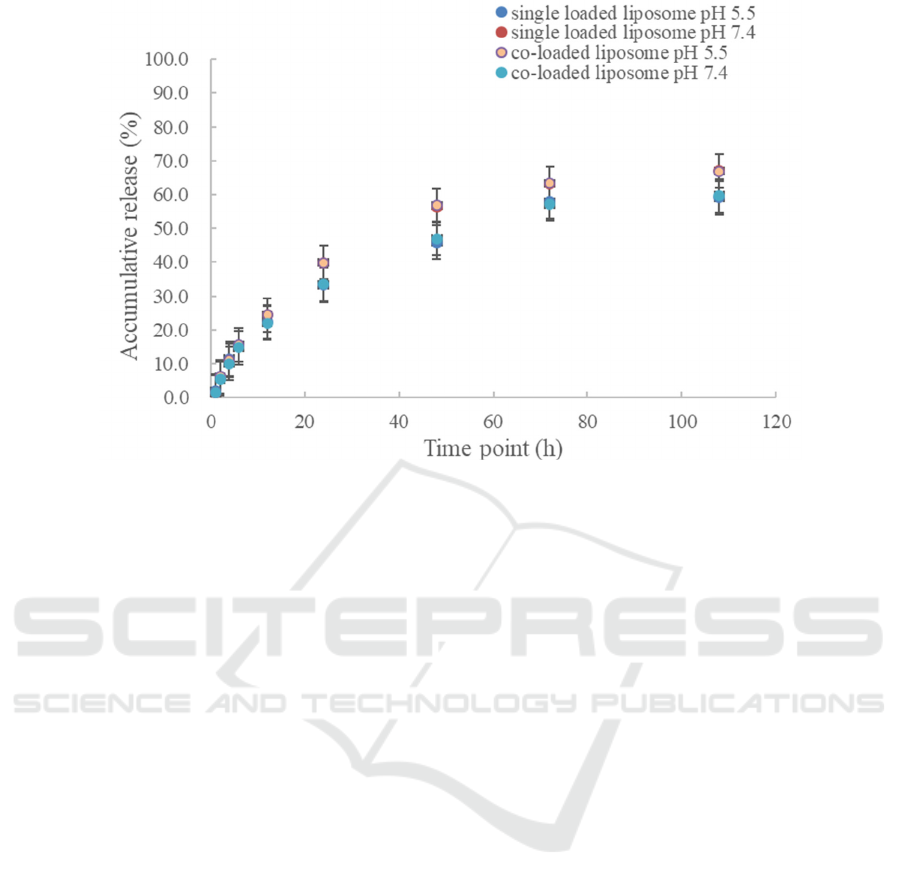
Figure 3: In vitro release of PTX in PTX loaded liposome and DOX/PTX co-loaded liposome at pH 7.4 and 5.5 buffer (Lv,
2014; Wang, 2014).
3.3 Stability Studies in Human Plasma
The particle size of liposomes was determined by
dynamic light scattering at 37℃ for 24h to
characterize liposome stability. No significant
precipitation of liposomes with plasma proteins was
observed, demonstrating good stability of liposomes.
3.4 Cellular Uptake Behavior of the
Dual Drug Loaded Liposomes
The cellular uptake behavior of DP-FA-FITC-LPs in
MDA-MB-231 and MCF-7 cells was studied using
confocal laser scanning microscopy (CLSM). Nuclei
were stained with DAPI (blue) and FITC (green)
labeled liposomes were used for subcellular
observation. After 3 h incubation, green fluorescence
was observed in the cells. When the incubation period
was increased to 6 h, the uptake of liposomes by the
cells was enhanced and the green fluorescence was
widely distributed in the cytoplasm, indicating that it
could be successfully internalized by the tumor cells
through endocytosis (Marinello P C, 2019)
3.5 In Vitro Cytotoxic Studies
The semi-inhibitory concentration values (IC50
values) for D-LPs and P-LPs were higher than those
for free DOX and free PTX, respectively, as
demonstrated in Tables 5 and 6. The reasons may be
related to the different cellular uptake pathways of
free drug and drug-loaded nanoparticles, as well as
the different modes of controlled release of drug-
loaded nanoparticles. In cell culture medium, most of
the free drugs can show their effects rapidly after
being transported into the cells by passive diffusion.
In contrast, drug-loaded nanoparticles are mainly
taken up by cells through the endocytic pathway and
exert antitumor activity after the drug molecules are
released from the nanoparticles.CI values below,
equal to or above 1 indicate synergistic, additive or
antagonistic effects, respectively. The calculated
CI50 of free PTX or DOX were greater than one,
indicating that they had no synergistic effect.
Nanoparticles had a significant synergistic effect
with CI50 values less than one, indicating that DOX
and PTX coadministration was significantly better
than free drug coadministration.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
432

Table 5: IC50 of free drug and drug loaded liposomes on MCF-7 cell (Lv, 2014).
Sam
p
les IC50 of PTX IC50 of DOX IC50 of combination
Free PTX 0.151±0.060 NA NA
Free DOX NA 0.132±0.055 NA
PTX LP 0.054±0.010 NA NA
DOX LP NA 0.049±0.012 NA
PTX-DOX LP NA NA 0.017±0.010
* NA means not available
Table 6: IC50 of free drug and drug loaded liposomes on MDA-MB-231 cell (Lv, 2014).
Sam
p
les IC50 of PTX IC50 of DOX IC50 of combination
Free PTX 0.193±0.067 NA NA
Free DOX NA 0.176±0.069 NA
PTX LP 0.062±0.012 NA NA
DOX LP NA 0.059±0.009 NA
PTX-DOX LP NA NA 0.024±0.006
* NA means not available
4 CONCLUSIONS
In brief, we developed a liposome with folate-
targeting and pH-responsive release capabilities for
DOX and PTX co-delivery. It has sufficient structural
stability, efficient delivery capacity, and good
biocompatibility to show its potential to deliver
antitumor drugs by intravenous injection. FITC-
labeled this liposome can be absorb by MDA-MB-
231 and MCF-7 tumor cells and has a synergistic
inhibitory effect on tumor cells. It has high tumor
accumulation, significant tumor suppression
efficiency, and reduced systemic toxicity in vivo.
Thus, our co-delivered liposomes are likely to
achieve excellent results in the treatment of human
breast cancer and also to provide additional design
and value or combination therapy in other diseases. In
the future, when the final dose-dependent response of
the two anticancer drugs, the optimal concentration of
anticancer effects, and the application of this strategy
in the treatment of different tumors s are clearly
investigated, clinics will be able to use lower
concentrations of conventional drugs to provide
better therapeutics and reduce patient suffering.
REFERENCES
Bernsdorff C, Reszka R, Winter R. Interaction of the
anticancer agent Taxol™(paclitaxel) with phospholipid
bilayers [J]. Journal of Biomedical Materials Research:
An Official Journal of The Society for Biomaterials,
The Japanese Society for Biomaterials, and The
Australian Society for Biomaterials, 1999, 46(2): 141-
149.
Bolotin E M, Cohen R, Bar L K, et al. Ammonium sulfate
gradients for efficient and stable remote loading of
amphipathic weak bases into liposomes and
ligandoliposomes [J]. Journal of liposome research,
1994, 4(1): 455-479.
Campbell R B, Balasubramanian S V, Straubinger R M.
Influence of cationic lipids on the stability and
membrane properties of paclitaxel‐containing
liposomes [J]. Journal of pharmaceutical sciences,
2001, 90(8): 1091-1105.
cOun, R., Moussa, Y. E., & Wheate, N. J. (2018). The Side
Effects of Platinum-Based Chemotherapy Drugs: A
Review for Chemists. Dalton Transactions, 47, 6645-
6653.
Darya Alizadeh, Malika Trad, Neale T. Hanke, Claire B.
Larmonier, Nona Janikashvili, BernardBonnotte,
Emmanuel Katsanis and Nicolas Larmonier (2014).
Doxorubicin Eliminates Myeloid-Derived Suppressor
Cells and Enhances the Efficacy of Adoptive T-Cell
Transfer in Breast Cancer. Cancer Res, 74 (1) 104-118.
Feng, Y., Ci, H., & Wu, Q. (2021). Expression of
mammalian sterile 20-like kinase 1 and 2 and Yes-
associated protein 1 proteins in triple-negative breast
cancer and the clinicopathological significance.
Medicine, 100(34), e27032.
Gill, J. H., Rockley, K. L., Santis, C. D., & Mohamed, A.
K. (2019). Vascular Disrupting Agents in Cancer
Treatment: Cardiovascular Toxicity and Implications
for Co-Administration with Other Cancer
Chemotherapeutics. Pharmacology & therapeutics,
202: 0163-7258.
G. Sharma, S. Anabousi, C. Ehrhardt & M. N. V. Ravi
Kumar (2006). Liposomes as targeted drug delivery
systems in the treatment of breast cancer. Journal of
Drug Targeting, 14:5, 301-310.
Hassan, M. S. U., Ansari, J., Spooner, D., & Hussain, S. A.
(2010). Chemotherapy for Breast Cancer. Oncology
Reports, 24: 1121-1131.
Design of Targeted and Release Controlled Liposome for Paclitaxel and Doxorubicin Combination in Breast Cancer Therapy
433

Hideki Mizutani, Saeko Tada-Oikawa, Yusuke Hiraku,
Michio Kojima, Shosuke Kawanishi (2005).
Mechanism of apoptosis induced by doxorubicin
through the generation of hydrogen peroxide. Life
Sciences,76: 1439-1453.
Kurbacher C M, Wagner U, Kolster B, et al. Ascorbic acid
(vitamin C) improves the antineoplastic activity of
doxorubicin, cisplatin, and paclitaxel in human breast
carcinoma cells in vitro[J]. Cancer letters, 1996, 103(2):
183-189.
Liu Y, Fang J, Kim Y J, et al. Codelivery of doxorubicin and
paclitaxel by cross-linked multilamellar liposome
enables synergistic antitumor activity[J]. Molecular
pharmaceutics, 2014, 11(5): 1651-1661.
Liu Y, Fang J, Joo K I, et al. Codelivery of
chemotherapeutics via crosslinked multilamellar
liposomal vesicles to overcome multidrug resistance in
tumor [J]. PLoS One, 2014, 9(10): e110611.
Lv S, Tang Z, Li M, et al. Co-delivery of doxorubicin and
paclitaxel by PEG-polypeptide nanovehicle for the
treatment of non-small cell lung cancer[J].
Biomaterials, 2014, 35(23): 6118-6129.
Marinello P C, Panis C, Silva T N X, et al. Metformin
prevention of doxorubicin resistance in MCF-7 and
MDA-MB-231 involves oxidative stress generation and
modulation of cell adaptation genes[J]. Scientific
reports, 2019, 9(1): 1-11.
Moghimipour E, Rezaei M, Ramezani Z, et al. Folic acid-
modified liposomal drug delivery strategy for tumor
targeting of 5-fluorouracil[J]. European journal of
pharmaceutical sciences, 2018, 114: 166-174.
McGale, P., Correa, C., Cutter, D., Duane, F., Ewertz, M.,
Gray, R., Mannu, G., Pete, R., Whelan, T., Darby, S., &
EBCTCG (Early Breast Cancer Trialists’ Collaborative
Group) (2014). Effect of Radiotherapy after
Mastectomy and Axillary Surgery on 10-Year
Recurrence and 20-Year Breast Cancer Mortality:
Meta-Analysis of Individual Patient Data for 8135
Women in 22 Randomised Trials. The Lancet,
383(9935), 2127-35
Reddy, K. b. (2011). Triple-Negative Breast Cancers: An
Updated Review on Treatment Options. Current
Oncology, 18(4), 173-179.
Sampedro F, Partika J, Santalo P, et al. Liposomes as
carriers of different new lipophilic antitumour drugs: a
preliminary report [J]. Journal of microencapsulation,
1994, 11(3): 309-318.
Sharma A, Straubinger R M. Novel taxol formulations:
preparation and characterization of taxol-containing
liposomes [J]. Pharmaceutical research, 1994, 11(6):
889-896.
Shew R L, Deamer D W. A novel method for encapsulation
of macromolecules in liposomes [J]. Biochimica et
Biophysica Acta (BBA)-Biomembranes, 1985, 816(1):
1-
Sun, J., Duan, J., Dai, S., Ren, J., Guo, L., Jiang, W., & Li,
Y. (2008). Preparation and Anti-Tumor Efficiency
Evaluation of Doxorubicin-Loaded Bacterial
Magnetosomes: Magnetic Nanoparticles as Drug
Carriers Isolated from Magnetospirillum
Gryphiswaldense. Biotechnology and Bioengineering,
101: 1313-1320.
Su, Z., Resend-ochir, T., Ganbold, T., & Baigude, H.
(2019). Design of Curdlan-Based PH-Sensitive
Polymers with Endosome Buffering Functionality for
SiRNA Delivery. Biological Macromolecules, 146:
0141-8130.
Schiffelers R M, Koning G A, ten Hagen T L M, et al. Anti-
tumor efficacy of tumor vasculature-targeted liposomal
doxorubicin[J]. Journal of Controlled Release, 2003,
91(1-2): 115-122.
Trayes, K. P., & Cokenakes, S. E. h. (2021). Breast Cancer
Treatment. Am Fam Physician, 104(2):171-178.
Walsh C L. Design, Synthesis, and Characterization of
Novel Zwitterionic Lipids for Drug and siRNA
Delivery Applications[M]. University of California,
San Francisco, 2012.
Wang Y, Zhang H, Hao J, et al. Lung cancer combination
therapy: co-delivery of paclitaxel and doxorubicin by
nanostructured lipid carriers for synergistic effect[J].
Drug delivery, 2016, 23(4): 1398-1403.
W. j. g., Bodei, L., Giammarile, F., Maecke, H. r., Tennvall,
J., Luster, M., & Brans, B. (2007). Targeted Therapy in
Nuclear Medicine—Current Status and Future
Prospects. Annals of Oncology, 18: 1782-1729.
Yin, W., Di, G., Liu, G., Wu, J., Lu, J., Han, Q., Shen, Z.,
Shao, Z. (2010). Clinicopathological features of breast
cancer patients with nipple discharge. Molecular
Medicine Reports 3.5: 863-868.
Yuan M, Qiu Y, Zhang L, et al. Targeted delivery of
transferrin and TAT co-modified liposomes
encapsulating both paclitaxel and doxorubicin for
melanoma [J]. Drug delivery, 2016, 23(4): 1171-1183.
Zhu Y, Wang M, Zhang J, et al. Improved oral
bioavailability of capsaicin via liposomal
nanoformulation: preparation, in vitro drug release and
pharmacokinetics in rats[J]. Archives of pharmacal
Research, 2015, 38(4): 512-521.
Zhao L, Wientjes M G, Au J L S. Evaluation of combination
chemotherapy: integration of nonlinear regression,
curve shift, isobologram, and combination index
analyses [J]. Clinical cancer research, 2004, 10(23):
7994-8004.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
434
