
Potential Drug Induced Liver Injury (Dili) Event During Remdesivir
Treatment in Covid-19 Patients
Ulvi Nur Rista
1,2
, Desi Setyowati
2
and Julaeha
1
1
Faculty of Pharmacy, Universitas 17 Agustus 1945 Jakarta, Jakarta, Indonesia
2
Mitra Keluarga Kenjeran Hospital, Surabaya, Indonesia
Keywords: Liver injury, Drug Induced Liver Injury (DILI), Remdesivir, COVID-19, Adverse Drug Reaction.
Abstract: The global pandemic of COVID-19 has already caused about 1.4 million deaths, and liver injury was one of
the foremost problems due to antiviral treatment besides mortality. Remdesivir was one of the antivirals
approved for COVID-19 treatment. Nevertheless, some studies reported that remdesivir caused liver injury.
This study aimed to identify the pattern and severity of liver toxicity during remdesivir treatment. The study
adopted a retrospective cohort design conducted in Mitra Keluarga Kenjeran Hospital Indonesia. This study
involved patients with COVID-19 on remdesivir treatment between June and August 2021. Patients with liver
impairment or abnormal alanine aminotransferase (ALT) were excluded. We described the severity of liver
toxicity based on the grading system and analyzed the comparison of baseline ALT and end-point ALT using
the t-test/Wilcoxon test. The 83 patients were included in this study. Our study showed ALT elevation was
observed in 21 patients (25.3%), including grade 1 in 14 patients (66.7%), grade 2 in 6 patients (28.6%), and
grade 3 in 1 patient (4.7%). Median end-point ALT value was significantly higher than median baseline ALT
(p-value < 0.001). The finding of this study suggests that monitoring of liver function tests must be considered
before the start of remdesivir treatment.
1 INTRODUCTION
Since COVID-19 was declared a global pandemic by
the World Health Organization (WHO) on March 11,
2020, various studies have been conducted to
improve understanding of the characteristics of the
Severe Acute Respiratory Syndrome Coronavirus 2
(SARS-Cov-2). COVID-19 is a disease that
predominantly attacks the respiratory tract, but some
hypotheses suggest that COVID-19 disease can also
attack the kidneys and liver (Sanders et al., 2020).
Some mechanisms that can explain the pathogenesis
of the occurrence of COVID-19-induced liver injury
are direct cytotoxicity of liver cells due to viral
replication, inflammatory responses mediated by the
immune system, responses from hypoxia and
ischemic due to severe sepsis, toxicity from COVID-
19 therapeutic drugs (Aleem et al., 2021).
Drugs used to treat various symptoms are also
increasingly being developed, one of which is
remdesivir. Some research shows that remdesivir has
the potential to cause Drug Induced Liver Injury
(DILI) (Aleem et al., 2021). Remdesivir structurally
belongs to the class of nucleoside drugs.
It is well
known that nucleoside analogues are an important
class of antiviral drugs, which can cause liver
disturbance through a variety of mechanisms. The
most typical mechanism is a mitochondrial type of
liver disturbance, which may be due to the
incorporation of nucleoside analogues into or
blocking mitochondrial DNA synthesis by
mitochondrial gamma polymerase, leading to
mitochondrial depletion (Zhai et al., 2021). This is in
accordance with the cross-sectional study in
Bangladesh in severe COVID-19 patients, the group
who received supportive therapy with remdesivir
showed a significant increase in AST (aspartate
aminotransferase) and ALT (alanine
aminotransferase) compared to the group who
received supportive therapy without remdesivir
(Ghosh et al., 2020). However, in the meta-analysis
study, different results were obtained, the risk of
increasing ALT and AST was significantly lower in
the remdesivir group than placebo/control group
(Angamo et al., 2022). There are differences in the
results of existing studies, so we aimed to conduct a
study to identify the pattern and severity of liver
372
Rista, U., Setyowati, D. and Julaeha, .
Potential Drug Induced Liver Injury (Dili) Event During Remdesivir Treatment in Covid-19 Patients.
DOI: 10.5220/0012025400003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 372-375
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
toxicity during remdesivir treatment in COVID-19
patients.
2 METHODS
The study was a cohort retrospective design with
purposively sampled. Ethical approval was obtained
from the Health Research Ethics Committee of 17
August 1945 University, Jakarta with number
30/KEPK-UTA45JKT/EC/EXE/03/ 2022. This study
was conducted at Mitra Keluarga Kenjeran Hospital.
This study involved Inpatients with COVID-19
diagnosed who getting a minimum one dose of
remdesivir in Mitra Keluarga Kenjeran Hospital
between June and August 2021. Patients with liver
impairment or abnormal alanine aminotransferase
(ALT) and getting hepatoprotection treatment
(glycyrrhizin and silymarin) before/during remdesivir
treatment were excluded. All of the patient’s data
were collected from the electronic medical records,
such as patient characteristics, medication treatment
during hospitalized, duration of remdesivir treatment,
baseline and end-point (during/after remdesivir
therapy) ALT value, the clinical manifestation of
COVID-19, co-existing condition, and other COVID-
19 treatment. ALT value was used as a liver injury
marker, because it is more specific for liver damage
than aspartate aminotransferase (AST) (Kim et al.,
2008). Descriptive analysis was performed for patient
characteristics, medication treatment, and percentage
of cases of ALT elevation based on the DILI grading
system developed by the Acquired Immune
Deficiency Syndrome (AIDS) Clinical Trials Group
(CTG). The grading system is used to assess the
severity of liver test abnormality, with the value
expressed as multiples of the upper limit of the
normal range (ULN). The following grades are Grade
1 (1.25-2.5) indicating mild, Grade 2 (> 2.5-5)
moderate, Grade 3 (> 5-10) severe, and Grade 4 (>
10) life-threatening (LiverTox, 2012). Furthermore,
the IBM SPSS for windows version 24.0 was used for
non-parametric analysis. Wilcoxon signed-rank test
was performed to find out differences in ALT value
between baseline and end-point measurement, p-
value < 0.05 means statistical significantly.
3 RESULTS AND DISCUSSION
3.1 Results
In total, 159 patients received remdesivir treatment
from June until August 2021. 76 of these patients
were excluded because of missing end-point ALT, the
elevation of ALT on pre-remdesivir, getting
hepatoprotection treatment before/after the start of
remdesivir, and getting double antivirals. Of the 83
remaining patients included in this study.
3.1.1 Baseline Characteristics of Patients
Table 1 shows the demographic and clinical
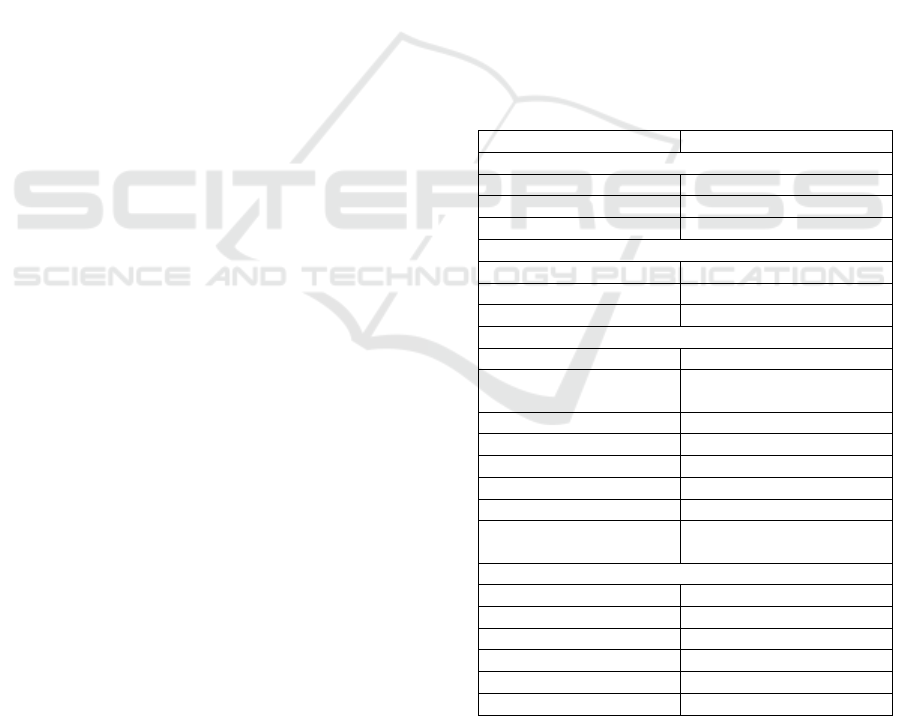
characteristics of 83 patients in this study. A total of
43 patients (52%) were women, the mean age was 56
± 16.35 years. The mean duration of remdesivir
treatment was 6.5 ± 1.95 days. Most patients (58
patients (69.9%)) had moderate symptoms on
admission. Of 3 patients (3.6%) had mild symptoms
on admission, but they got remdesivir because of
worsening symptoms during the in-hospital. The
most of patients having co-existing conditions were
hypertension (33 patients (39.8%)) and type 2
diabetes mellitus (25 patients (30.1%). More than
50% of patients got other drugs, which are
levofloxacin and dexamethasone.
Table 1: Demographic and Clinical Characteristics of the
Patients (N=83).
Patient characteristic N
(
%
)
Sex
Men 40
(
48%
)
Women 43(52%)
Age (years)
56 ± 16.35
Clinical manifestation of COVID-19
Mil
d
3.6%
Moderate 69.9%
Severe 26.5%
Co-existin
g
condition
H
yp
ertension 39.8%
Type 2 Diabetes
Mellitus
30.1%
Chronic Kidne
y
Disease 4.8%
Coronary Heart Disease 9.6%
Dyslipidemia 6.0%
Stroke 1.2%
Asthma 2.4%
Duration of remdesivir
treatment (days)
6.5 ± 1.95
Othe
r
medication*
Levofloxacin 68.67%
Azithrom
y
cin 4.82%
Moxifloxacin 12.05%
Dexamethasone 57.83%
Hydrocortisone 3.61%
Tocilizumab 26.51%
*Other medication belongs to standard of care of COVID-
19 in Indonesia
Potential Drug Induced Liver Injury (Dili) Event During Remdesivir Treatment in Covid-19 Patients
373

3.1.2 An ALT Elevation during Remdesivir
Treatment
Based on classification of DILI by AIDS CTG, ALT
elevation was observed in 21 patients (25.3%),
including grade 1 elevation in 14 patients, grade 2
elevation in 6 patients, and grade 3 in 1 patient. The
majority of patients who having ALT elevation were
men, having moderate symptoms and the mean
duration of remdesivir treatment was approximately
5-9 days (shown in Table 2)
Table 2: The Characteristic of Patients Who Having ALT
Elevation (N=21).
Characteristics Grade 1
(
N=14
)
Grade 2
(
N=6
)
Grade 3
(
N=1
)
Age (years)
44.8 ± 10.8 43 ± 20
24
Sex
Men 7 4 1
Women 7 2 0
Clinical Manifestation of COVID-19
Mil
d
1 1 0
Moderate 8 3 0
Severe 5 2 1
Duration of
Remdesivir
Treatment
(days)
6.9 ± 1.8 6.5 ± 2.0
5
Table 3: Statistic Result of The Difference in ALT Level.
ALT level Median
(interquartile
ran
g
e
)
p value*
Baseline 22 (14) < 0.001
En
d
-
p
oint 29 (33)
*Wilcoxon test, the ALT level decreased in 29 patients,
increased in 49 patients, and steady in 5 patients
Table 3 showed there was significantly
differences (p<0.001) ALT value before and
during/after (end-point) remdesivir treatment. There
are several drugs of standard therapy in COVID-19
other than remdesivir, were used in this study, which
may be caused liver toxicity according to the
likelihood score of DILI LiverTox. These drugs have
shown in Table 4, including the percentage of patients
who received concomitant medications.
Table 4: Percentage of Patients with Concomitant
Medications (N=83).
Medication ALT
elevation
Grou
p
(
N=21
)
ALT non-
elevation
Grou
p
(
N=62
)
Levofloxacin 15
(
71.43%
)
42
(
67.74%
)
Azithromycin 2 (9.52%) 2 (3.23%)
Moxifloxacin 2
(
9.52%
)
8
(
1.9%
)
Dexamethasone 20 (95.2%) 28 (45.16%)
Hydrocortisone 1 (4.76%) 2 (3.23%)
Tocilizumab 8
(
38.10%
)
14
(
22.58%
)
3.2 Discussion
Liver injury associated COVID-19 has occurred in
14-53% of patients. Various mechanisms have been
hypothesized to explain that pathogenesis, such as the
the worsening of COVID-19 illness and drug toxicity.
Several drugs in the management of COVID-19 are
potential hepatotoxic (Aleem et al., 2021). Liver
injury during remdesivir treatment was reported and
registered in WHO vigibase pharmacovigilance.
Increased liver transaminase (88%) was the most
frequent adverse drug reaction of remdesivir
(Montastruc et al., 2020). These reports are consistent
with our result that ALT value increased significantly
during/after remdesivir treatment (p<0.001).
According to the AIDS CTG grading system, ALT
elevation occurred in 21 patients (25.3%). Our result
is contrary to a meta-analysis study which showed
that treatment with remdesivir was associated with a
lower risk of ALT elevation (p=0.006) (Angamo et
al., 2022). However, the cut-off value of ALT to
define ALT elevation in that study was unclear.
We also found that most of our patients had mild-
moderate liver injury (grade 1-2), and only one had a
severe liver injury (grade 3). All of our patients didn’t
require to stop remdesivir, some patients got
hepatoprotection treatment. This finding was also
reported by Ghosh, et al, that remdesivir caused
frequent grade-1 (1.25 to 3-fold) and grade-2 (3 to 5-
fold) elevation of ALT which didn’t require drug
discontinuation (Ghosh et al., 2020). In contrast to
Wang et al., two patients with severe COVID-19 in
the remdesivir group had increased ALT events (any
grade) leading to drug discontinuation (Wang et al.,
2020).
We observed liver injury in our study possibly
occurred in patients with moderate COVID-19
illness. In most of the previous studies, liver injury
following remdesivir treatment occurred in patients
with severe-critical COVID-19 illness, due to
subjects who included in that studies were only
severe/critical illness (Ghosh et al., 2020; Grein et al.,
2020; Wang et al., 2020).
According to the standard of therapy in the
national guideline, our patients got other drugs than
remdesivir such as dexamethasone, levofloxacin, and
tocilizumab. All three were the most used
concomitant with remdesivir. Based on the likelihood
score of DILI LiverTox, dexamethasone and
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
374

levofloxacin are well-established causes of liver
injury (Category A), and tocilizumab is a probable
rare cause of liver injury (Category C) (LiverTox,
2012). Therefore, the possibility of another cause-
induced liver injury could not be sorted.
A caution interpretation of the findings of this
study is required due to several methodological
limitations. Firstly, the sample size of the study might
not reflect and represent overall liver injury condition
due to remdesivir in Indonesia. Further study with
multiple sites and large sample size is necessary.
Secondly, this study did not observe a long-term
period of the patient’s clinical condition. Therefore, a
longitudinal study is strongly recommended to
observe the effect of remdesivir on liver injury.
Despite several limitations, our study has superiority
in milestone contribution on evidence-based
providing of remdesivir safety and efficacy in
COVID-19 treatment.
4 CONCLUSIONS
This study highlighted that ALT elevation is probably
due to remdesivir. Mostly, this event occurred in
moderate COVID-19 patients. However, there were
other causes of induced liver injury that could not be
sorted.
ACKNOWLEDGEMENTS
The authors appreciated Mirta Keluarga Kenjeran
Hospital, Surabaya to support this study by providing
and facilitating data collection.
REFERENCES
Aleem, A., Mahadevaiah, G., Shariff, N., & Kothadia, J. P.
(2021). Hepatic manifestations of COVID-19 and effect
of remdesivir on liver function in patients with COVID-
19 illness. Baylor University Medical Center
Proceedings, 34(4), 473–477.
https://doi.org/10.1080/08998280.2021.1885289
Angamo, M. T., Mohammed, M. A., & Peterson, G. M.
(2022). Efficacy and safety of remdesivir in
hospitalised COVID-19 patients: A systematic review
and meta-analysis. Infection, 50(1), 27–41.
https://doi.org/10.1007/s15010-021-01671-0
Categorization Of The Likelihood Of Drug Induced Liver
Injury. (2012). In LiverTox: Clinical and Research
Information on Drug-Induced Liver Injury. National
Institute of Diabetes and Digestive and Kidney
Diseases.
http://www.ncbi.nlm.nih.gov/books/NBK548392/
Ghosh, C., Hasan, S. M., & Dey, S. (2020). Remdesivir
Induced Liver Injury and Severe COVID-19 Infection.
American Journal of Internal Medicine, 8, 285–288.
https://doi.org/10.11648/j.ajim.20200806.18
Grein, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E.,
Castagna, A., Feldt, T., Green, G., Green, M. L.,
Lescure, F.-X., Nicastri, E., Oda, R., Yo, K., Quiros-
Roldan, E., Studemeister, A., Redinski, J., Ahmed, S.,
Bernett, J., Chelliah, D., … Flanigan, T. (2020).
Compassionate Use of Remdesivir for Patients with
Severe Covid-19. New England Journal of Medicine,
382(24), 2327–2336.
https://doi.org/10.1056/NEJMoa2007016
Kim, W. R., Flamm, S. L., Di Bisceglie, A. M., &
Bodenheimer, H. C. (2008). Serum activity of alanine
aminotransferase (ALT) as an indicator of health and
disease. Hepatology, 47(4), 1363–1370.
https://doi.org/10.1002/hep.22109
Montastruc, F., Thuriot, S., & Durrieu, G. (2020). Hepatic
Disorders With the Use of Remdesivir for Coronavirus
2019. Clinical Gastroenterology and Hepatology,
18(12), 2835–2836.
https://doi.org/10.1016/j.cgh.2020.07.050
Sanders, J. M., Monogue, M. L., Jodlowski, T. Z., &
Cutrell, J. B. (2020). Pharmacologic Treatments for
Coronavirus Disease 2019 (COVID-19): A Review.
JAMA, 323(18), 1824–1836.
https://doi.org/10.1001/jama.2020.6019
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., Fu,
S., Gao, L., Cheng, Z., Lu, Q., Hu, Y., Luo, G., Wang,
K., Lu, Y., Li, H., Wang, S., Ruan, S., Yang, C., Mei,
C., … Wang, C. (2020). Remdesivir in adults with
severe COVID-19: A randomised, double-blind,
placebo-controlled, multicentre trial. The Lancet,
395(10236), 1569–1578.
https://doi.org/10.1016/S0140-6736(20)31022-9
Zhai, G., Li, M., Wang, Y., & Wu, J. (2021). Drug-Induced
Liver Disturbance During the Treatment of COVID-19.
Frontiers in Pharmacology, 12.
https://www.frontiersin.org/articles/10.3389/fphar.202
1.719308
Potential Drug Induced Liver Injury (Dili) Event During Remdesivir Treatment in Covid-19 Patients
375
