
Cytokines in Cancer Immunotherapy
Menghan Liu
1,*,†
, Yuanyuan Pang
2,†
and Xinyang Wan
3,†
1
Wellington College International Tianjin, Tianjin 300120, China
2
Department of Chemistry, University of Washington, Seattle 98195, U.S.A.
3
Macleans College, Auckland 2012, New Zealand
Keywords:
Cancer, Cytokines, Immunotherapy.
Abstract:
Cytokine immunotherapy is a widely concerned field in cancer treatment. As proteins of the immune system,
cytokines can regulate the immune response of the host to tumor cells and directly induce tumor cell death.
However, there are some limitations in the treatment of cytokines. On the one hand, low dose single drug
therapy of cytokines has no significant therapeutic effect; on the other hand, high dose therapy may cause a
variety of side effects due to the pleiotropic effect of cytokines. The involvement of cytokines in pro-tumor
and anti-tumor immune responses remains an urgent issue. This article mainly introduces the application of
cytokines in cancer treatment. Although the efficacy of early stage cytokines in cancer treatment is modest,
advances in molecular biology and genomics are expected to optimize and enhance cytokine therapy in
clinical practice in the future.
1 INTRODUCTION
Cancer immunotherapy is one of the most effective
ways to help the immune system recognize and fight
cancer cells (Conlon, K. C. et al. 2019). Cytokines
promote the function of the immune system in cell
signaling.
Autocrine is where cytokines act on the mother
cell that secretes them, while paracrine is where
cytokines may act on nearby cells. Endocrinology is
caused by the action of cytokines on distant cells. The
types of cytokines identified include chemokines,
interferon (INF), interleukins (ILs), colony
stimulating factors (CSFs), tumor necrosis factors
(TNFs), transforming growth factors (TGFs),
lymphocytokines, and single cytokines. There are
also artificially produced interleukins that can be used
to treat alder interleukin cancer. These cytokines can
be roughly divided into three categories according to
their roles and functions. Lymphokines and single
cytokines are produced by immune system cells and
are involved in various aspects of immune function.
The second and third groups include growth factors
and colony-stimulating factors, which control tissue
growth and blood cell proliferation. Chemokines are
produced by chemotactic activity and are involved in
†
These authors contributed equally
the regulation of the immune system.
Cytokines act
through cell surface receptors and modulate the
balance between humoral and cell-mediated
immunity. They are produced by a range of cells,
including B lymphocytes, T lymphocytes,
macrophages, mast cells, endothelial cells, fibroblasts
and stromal cells. The cytokines have effects of
pleiotropism, which means that one cytokine that
binds to different targets can produce different
effects. For example, the activated T helper cell can
produce IL-4, which can bind to B cells, thymocytes
and mast cells. The IL-4 can induce activation,
proliferation, and differentiation on B cells. However,
it can only induce proliferation on thymocytes and
mast cells. Another property of some cytokines is
redundancy. Multiple cytokines may have the same
effects on the same target. As an example, the
activated T helper cell can produce IL-2, IL-4, IL-5 at
the same time. If they all bind to the receptor on B
lymphocytes, they may induce the same function of
proliferation. In addition, some cytokines can
influence the activity of other cytokines in different
ways, and they can also act synergistically or
antagonistically (Zhang, J. M., & An, J. 2007).
In this review, it mainly focused on 5 concepts,
including the discovery of cytokine immunotherapy,
Liu, M., Pang, Y. and Wan, X.
Cytokines in Cancer Immunotherapy.
DOI: 10.5220/0012020900003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 343-351
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
343
the mechanism of cytokines, interleukins and
interferons and their targeted cancer, the current
progress and issues of cytokine immunotherapy, and
the predicted improvements.
2 THE DISCOVERY OF
CYTOKINES
Cytokines play an important role in the diagnosis,
prognosis and treatment of human diseases
(Dinarello, C. A. 2007). IL-2 was the first cytokine
found to have therapeutic effects, significantly
stimulating the growth of T cells and natural killer
cells (Robert Gallo, M. D. et al. 1976).
IL-2 successfully treated patients with advanced
metastatic renal cell carcinoma and melanoma.
Currently, researchers are focusing on whether IL-2
is effective in combination with other cytokines in
these cancer patients (Jiang, T., Zhou, C., & Ren, S.
2016). IL-7 is a major regulator of T cell homeostasis.
The cytokine driven regeneration of T cells was
demonstrated in the first human clinical trial using IL-
7, and IL-7-based therapies may also restore immune
function in other immunocompromised individuals,
such as those living with HIV and the elderly, and
may enhance the efficacy of vaccines and other
cancer immunotherapies
(Fry, T. J., & Mackall, C. L.
2002). Similar to IL-2, IL-15 triggers the production
of immune cells that attack and kill cancer cells.
Results from the first human clinical trial showed that
IL-15 significantly increased T and NK cell growth
and activity. IL-15 is currently being investigated for
its potential to enhance the effectiveness of vaccines
against viruses that cause cancer and autoimmune
diseases (Mackall, C. L. et al. 2011).
3 MECHANISM OF CYTOKINES
Cytokines help the immune system do its job.
Immune cells, cytokines and organs must
communicate with each other to prevent pathogens or
harmful invaders from entering the body. The first
immune cell that notices the pathogen creates and
sends out messages in the form of cytokines to the rest
of the organs or cells in the body, responding directly.
Different types of cytokines will be released into the
blood or directly into tissues, and then, locating the
immune cells that are designed to target and bind to
the cell receptors.
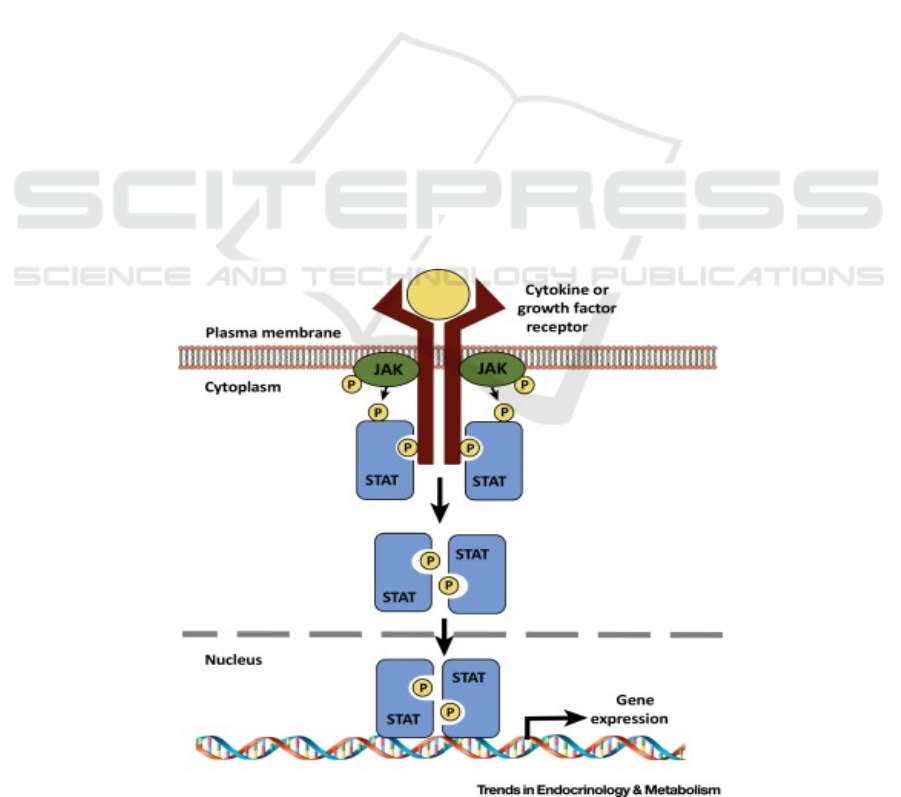
3.1 Jak-STAT Pathway
Cytokines use multiple signaling pathways. One of
the pathways is the Jak-STAT pathway which is
shown in figure 1.
Figure 1: Cytokine signaling through JAK–STAT pathway (Dodington, D. W. et al. 2018).
ICBB 2022 - International Conference on Biotechnology and Biomedicine
344
The Jak-STAT pathway is a very rapid cytosol-to-
nuclear signaling pathway, and it involves both Janus
kinase protein (JAK) and signaling transducer and
activator protein (STAT). Cytokine molecules first
bind to the receptors and stabilize heterodimer and
bring the JAKs together (Leonard, W. J., & Lin, J. X.
2000). JAK phosphorylates each other which can
increase the activity of the tyrosine kinase domain.
Activation of JAK kinase in turn phosphorylates the
relevant receptor tyrosine residues.
These phosphotyrosines act as docking sites for
STAT proteins
(Greenlund, A. C. et al. 1995). All
STAT share basic features (Darnell, J. E. et al. 1994;
Darnell, J. E. 1997), including an N-terminal domain
(important for dimer formation), a coiled-coil domain
(crucial for dimerization tag and nuclear localization
signal), a DNA binding domain (binding to a specific
DNA sequence), a linker domain, a SH2 domain
(docking STAT to phosphorylated tyrosine residue),
and a C-terminal region that contains a critical
tyrosine residue and a transactivation domain
(Mitchell, T. J., & John, S. 2005). They are then
separated from the receptor to form homo-dimer or
hetero-dimer after they are recruited (Horvath, C. M.,
& Darnell, J. E. 1997). The STAT protein is then
transferred to the nucleus, where it binds the DNA to
the regulatory region of the target gene and regulates
gene expression.
3.2 Inflammation
Cytokines mainly occur when the body is invaded by
pathogens and cause inflammatory responses by
changing the porosity of the blood vessel cell wall and
reducing cell contact area. Blood then leaks into
surrounding tissue, allowing immune cells to enter
the damaged area and begin the healing process (Bio-
Rad. n.d.). The inflammatory response, along with
physical tissue damage, directs brain cells to release
chemicals. Cytokines are essential for a healthy
immune response, but the concentrations need to be
just right. Too high levels can overwhelm the body,
creating a phenomenon known as "cytokine storm."
Cytokine storms usually occur when pathogens enter
the body at the same time, or when the body mis-
produces cytokines early in the immune response.
Every organ has cytokine receptors, and in this case,
oversignaling helps the immune system precisely
clear pathogens. In addition, cytokine storms can
have negative effects on the body. Patients with
bacterial infections often experience cytokine storms,
symptoms of some diseases such as COVID-19.
Cytokine storms participate in an uncontrolled
immune response that causes a decrease in oxygen in
the blood (Ragab, D. et al. 2020). Fluid builds up in
the lungs causing breathing difficulties and nervous
system problems. The brain is naturally protected
from harmful chemicals because of the blood-brain
barrier, but the cytokines are so small that they easily
cross the brain's protective membrane (Manoylov, M.
K. 2020, November 6).
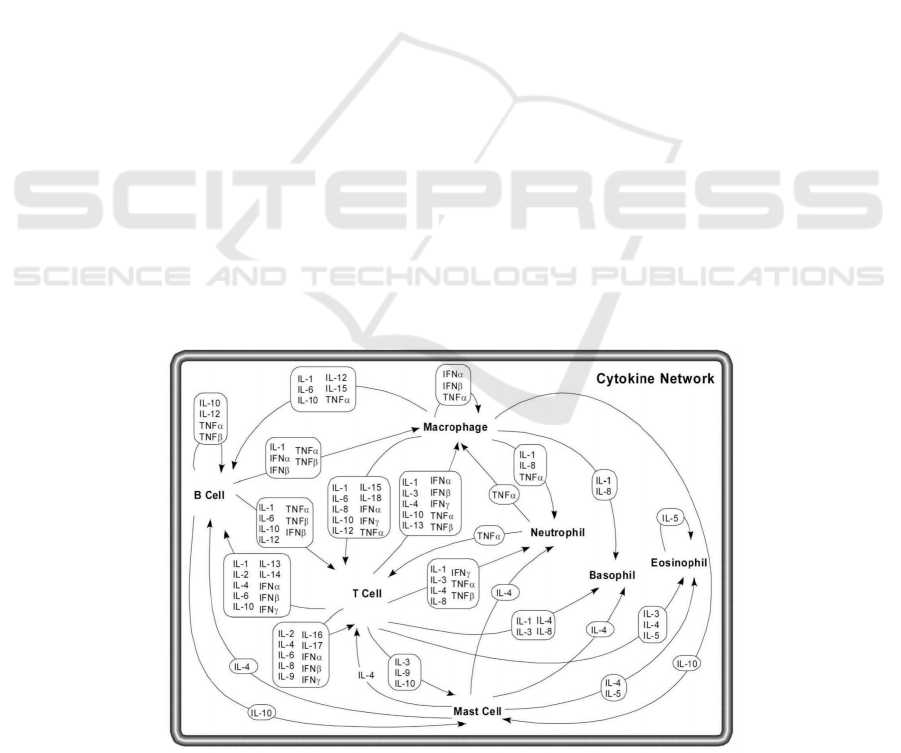
3.3 Network of Pleiotropic Cytokines
The work of different cytokines on different cells can
be concluded in figure 2.
Figure 2: Network of cytokines (Zhang, J.-M., & An, J. 2007).
Cytokines in Cancer Immunotherapy
345

It shows the paracrine signaling network of
different cytokines. Cytokines are pleiotropic, which
means that different cells may secrete the same
cytokines, and a single cytokine may act on different
cells. Cytokines include B cells, T cells,
macrophages, mast cells, neutrophils, basophils, and
eosinophils. Macrophages engulf foreign cells and
present pathogens, use cytokines to stimulate specific
immune responses of B cells and T cells, and activate
non-specific immune responses produced by other
cells (such as natural killer cells). T cells secrete a
variety of factors, interferon and interleukin, which
participate in the immune response of specific
antigens. Proliferation and activation of eosinophils,
neutrophils, and basophils also play a role in
cytokines. They fight cancer by interfering with
cancer cell growth and reproduction, stimulating the
immune system, encouraging killer T cells and other
cells to attack cancer cells, and encouraging cancer
cells to produce chemicals that attract immune cells.
4 INTERLEUKINS AND
INTERFERONS
4.1 Interleukins (ILs)
Interleukins are cytokines involved in the regulation
of immune response, inflammation and
hematopoiesis (Sims, J. E. et al. 1988). A majority of
them are produced by macrophages, CD4+ T cells,
monocytes and endothelial cells, and bind to their
target receptors. They have different effects when
they target different target cells.
IL-2 are secreted by T cells (Th1-cells), and target
on activated cells, macrophages on their receptors of
CD25/IL2RA, CD122/IL2RB, CD132/IL2RG,
resulting in a growth and differentiation (Ymer, S. et
al. 1985). The source cell of IL-2 includes activated
Th cells, mast cells, NK cells, endothelium and
eosinophils. The target cells, including hematopoietic
stem cells and mast cells, have receptors of
CD123/IL3RA, CD131/IL3RB. They will induce the
differentiation and proliferation of myeloid
progenitor cells, and growth and histamine release of
the mast cells (Dorssers, L. et al. 1987).
Other interleukins have different functions by
acting on other leukocytes. As one of the basic
cytokines with multipotency for the resistant
framework, the natural capacity of IL-2 is intervened
by IL-2 receptor, which has a place with type I
cytokine receptor (Lippitz, B. E. 2013).
IL-2 receptor
is a trimer complex made of three subunits α, β, γ.
Binding of IL-2 to its receptor can induce multiple
signaling pathways (STAT, PI3K-Akt and MAPK,
three downstream signaling pathways). Activation of
IL-2 can promote the growth of immune cells and
enhance the activity of immune cells, which can
attack and kill cancer cells (Spolski, R. et al. 2018).
In the 1990s, high doses of IL-2 were approved for
several patients with metastatic melanoma and
metastatic renal cell carcinoma and showed long-
lasting complete responses. In immunotherapy
clinical studies, patients with advanced melanoma
and neurocytoma who have responded to high-dose
IL-2 therapy have been reported to have prolonged
survival of 3-5 years in patients with melanoma and
neurocytoma who have responded to high-dose
interleukin-2 therapy (Chow, S. et al. 2016).
At the
same time, low doses of IL-2 can also treat some
autoimmune diseases. Low doses of IL-2 may be
targeted at the underlying role of Treg cells, leading
to the re-control of autoimmune diseases and
inflammation (Orozco Valencia, A. et al. 2020).
The
FDA first approved recombinant IL-2 as effective
tumor immunotherapy for patients with cancer.
However, severe adverse reactions and priority
amplification of immunosuppressed Treg cells limit
recombinant IL-2 in cancer therapy. In order to
reduce toxicity and prevent the targeting effect of
Treg cells, some newly developed fusion proteins can
improve the efficacy and lower toxicity of tumor
therapy. These include NKTR-214, which has been
shown to mask the region of IL-2Rα interaction with
its six releasable PEG chains, thereby mediating the
preferentially activated effector cells and showing
good tolerability and significant clinical activity in
patients with advanced melanoma. It is reported that
high-dose IL-2 therapy for renal cell carcinoma and
metastatic melanoma showed that about 16% of
patients reacted emphatically to treatment and the
reaction kept going longer in patients with metastatic
melanoma (Clark, J. I. et al. 2021).
The use of high
doses of IL-2 has been associated with severe side
effects, such as encephalitis and meningitis. The
combination of IL-2 agents with other anticancer
immunotherapy agents (such as cell metastasis,
antigen-specific vaccines, and cytotoxic T-cell
associated antigen 4 can further develop therapy
proficiency while lessening IL-2 dosages and
diminishing antagonistic occasions (Dhupkar, P., &
Gordon, N. 2017).
The utilization of IL-2 in
malignant growth, intense leukemia, immune system
illnesses, human immunodeficiency (HIV) and
different infections, among which the therapy of
foundational lupus erythematosus (SLE) has gained
momentous headway (Orozco Valencia, A. et al.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
346

2020).
At present, IL-2 is an effective and safe
treatment. However, the toxicity of IL-2 is expected
and controllable in clinical medicine. The treatment
of IL-2 without treatment-related death can also be
applied to inflammatory diseases or autoimmune
diseases to improve the therapeutic effect of IL-2-
based cytokine. IL-2 therapy can amplify and activate
effector T cells and NK cells, providing alternative
immunotherapy options for patients following
conventional cancer treatment.
4.2 Interferons (IFNs)
There are two main types of interferons: type I
interferons (IFN-alpha and IFN-beta), type II
interferons (IFN-gamma). Type I interferons are a
group of antiviral cytokines. They are induced during
viral infections by viral replication products (Eg.
double stranded RNA). IFN-alpha is produced by
white blood cells except lymphocytes, whereas IFN-
beta is produced by fibroblasts. These two kinds of
type I interferons are both produced when cells have
been infected by pathogens, as a warning signal to the
body’s immune system. Their main function is to
trigger immune cells (including natural killer cells
and cytotoxic T lymphocytes) to release type II
interferons, also known as IFN-gamma, to fight the
germs. The type II IFNs are produced by increasing
phagocytosis by macrophages. One common function
of these interferons is that they all inhibit viral
replication (Katze, M. et al. 2002). IFN-alpha can also
be used to treat cancers of hairy cell leukemia, chronic
myelogenous leukemia (CML), kidney cancer,
melanoma and so on (American Cancer Society n.d.).
In the late 1980s, cytokine-based immunotherapy
became the primary treatment for locally advanced or
metastatic renal cell carcinoma (RCC) (Koneru, R., &
Hotte, S. J. 2009). The antitumor effect is regulated
by different mechanisms such as immunomodulation,
antiproliferative activity, regulation of gene
differentiation, etc. In the randomized controlled
trials, patients treated with the IFN-alpha subtype
were studied with patients treated with non-IFN-
alpha subtypes as a control group. The result
demonstrated that the IFN-alpha subtype is related to
a more significant remission, indicating a better
efficacy than the control group. The IFN-alpha group
also had lower 1-year mortality. From the research,
there was no difference between the IFN-alpha 2a and
2b subgroups (Coppin, C. et al. 2005). The dose-
response effect regarding the IFN-alpha group
remained unclear. However, with the increased dose,
the toxicity level would be expected to be higher. The
dose might be individualized based on the severity
and the progress of the disease.
IFN-alpha was also an antitumoral agent common
in leukemia; the direct effects include cell growth
inhibition, apoptosis induction, enhancement of acute
myeloid leukemia (AML). The binding of IFN-alpha
regulates these effects to their receptors which can be
expressed on the leukemic cell surfaces. The binding
can activate specific cell signal pathways, including
Jak-STAT pathway activators. Even though people
have understood specific pathways were directly
related to the leukemia treatment, the precise role of
these pathways in AML remained to be researched
(Anguille, S. et al. 2011). AML patients can have
impaired immune function, and some of their immune
responses might not be as intense. Therefore, other
indirect effects of IFN involved activating dendritic
cells, T cells, and natural killer cells, which played a
massive role in antileukemic immune responses. As
for other types of leukemia, such as chronic myeloid
leukemia (CML), IFN was once the best treatment for
CML. However, when tyrosine kinase inhibitors were
invented and used, they became the first-line
treatment, and interferon is rarely used for CML.
The last class of cancer usually treated IFN-alpha
as a single agent was lymphoma. One of the review
articles published in 2003 indicated the IFN as a
single treatment of cutaneous T-cell lymphoma was
efficacious. The IFN was filtered by the glomeruli,
and they can undergo fast proteolytic degradations
during tubular reabsorption (Olsen, E. A. 2003).
One
of the problems with the interferon usage in
lymphoma patients was the development of the
neutralizing antibodies, which might be influenced by
the underlying disease, the dosing regimen of
cytokine-based immunotherapy, or the duration of the
treatment. The presence of the antibody can
essentially decrease the efficacy of the medication in
lymphoma patients. One of the possible reasons for
the development of antibodies can involve the
overactive immune response. Therefore, people
started to look for other solutions. In 2015, one
randomized controlled trial was done to compare the
concurrent use of IFN-alpha with low doses of
methotrexate or retinoids, which can suppress the
immune system. It was found that the use of IFN with
retinoids or a low dose of the cytotoxic drug can be
preferred in patients with refractory T-cell
lymphoma, and the toxicity was minimal for this
combination (Aviles, A. et al. 2015).
So far, only these three cancer types were using
cytokine-targeted medications for treatment as
monotherapy agents, and people have found other
solutions with the development of the newer
Cytokines in Cancer Immunotherapy
347

medication. As for other cancers such as brain tumors
and phase 1 and 2 clinical studies of IFN were
conducted on patients with malignant brain tumors.
People did find some of the patients have shown
improved disease states. However, the effect
remained in the local area, and it did not show the
systemic effect, so it failed to move onto the next
stage (Nagai, M., & Arai, T. 1984). Due to the lack of
response to monotherapy, people, in general, do not
use them. Still, since they are immunomodulators and
they can influence the immune response in general,
IFN combination with radiotherapy or chemotherapy
brought people's interest. People have done an
investigation on combination therapy.
5 THE CURRENT PROGRESS
AND ISSUE OF CYTOKINES
At the laboratory level, experiments on mice
demonstrated that the cytokines had a preclinical
effect. Interferon can treat hair cell leukemia and
interleukin can treat advanced melanoma and
metastatic kidney cancer. IL-12, IL-15, IL-21 and
granulocyte macrophage colony stimulating factors
have also been used in clinical trials. Despite this,
cytokine as a monotherapy did not fulfill its early
promise, because parenteral administration of
cytokine did not reach sufficient concentrations in
tumors. Increased cytokine concentration may
indicate an uncontrolled immune response, which is
called cytokine release syndrome or cytokine storm.
This can lead to severe inflammation, shock,
respiratory failure, organ failure, and in some cases
even death (Waldmann T. A. 2018).
As previously discussed, cytokine-based therapy
indicated antitumor characteristics; people further
found the effect was achieved by inhibiting
proliferative effects on cells, so tumor cells were not
growing as fast. Meanwhile, they also indirectly
stimulated our immune system, which can kill tumor
cells. Therefore, the mechanism included two
perspectives. The first is to decrease cancer cells'
growth and increase the immune system to get rid of
them. There are multiple cytokines found that have
therapeutic effects. However, more specifically for
cancer, only IL- 2 and IFN-alpha demonstrated
antitumor effects and the FDA approved them for the
treatment of cancers. The other cytokines, such as IL-
17, can be used for the treatment of autoimmune
diseases like psoriasis. Even though the antitumor
effects were not as desired due to the low efficacy,
they showed better outcomes when using other
anticancer agents. The combination therapy showed a
more promising effect than the monotherapy.
There are a few barriers related to these
medications, and some of them should be further
developed. One of the most significant issues with
these medications was the low efficacy while having
high toxicities. One of the guesses related to the high
kidney response and low liver response might relate
to the pharmacokinetics of this class of medication.
They are mainly renally cleared and the liver
clearance was minimal. The major toxicities involved
the loss of appetite, high infection risk, flu-like
symptoms such as fever, chills, and fatigue. All of
these could be very common, and people might see
them in patients on cytokine-based immunotherapy.
The possible further research can focus on improving
pharmacokinetics and pharmacodynamics, improving
local reaction instead of a systemic reaction, and
optimizing the combination therapy to achieve better
outcomes. In general, cancer cells can develop
multiple different mechanisms to fight against drugs
and survive, including the inhibition of apoptosis,
drug expulsion, and increased proliferation. Recent
studies have found unregulated cytokine expressions
are highly involved in the drug resistance mechanism
(Jones, V. S. et al. 2016).
Even though IFN-alpha and IL-2 do not provide
very effective clinical outcomes, they are excellent
candidates for combination therapy with other agents.
In the past clinical trial, it was studied in the patients
with hepatocellular carcinoma, which was not one of
the diseases treated with IFN-alpha. In this trial, 106
patients with hepatocellular carcinoma received 5
million units of IFN-alpha on day 1,3,5 and each
week of the treatment; meanwhile, they also received
5-fluorouracil (5-FU) 500mg on day 1-5 during the
first two weeks of the 4-week cycle. The 5-FU is a
standard medication used in hepatocellular
carcinoma, so in the trial, people compared the
combination with IFN-alpha with the standard
therapy alone from the historical data. It turned out,
about 20% of patients in the treatment group showed
complete response and 36% of patients showed
partial response. The efficacy was improved
significantly compared to the standard therapy alone.
The survival rate at 1-year and 2-year time points
were 34% and 18%, while historically, with patients
treated with standard therapy, the survival rate was
15% and 5%. Therefore, the survival rate largely
improved with the combination of IFN-alpha, and the
combination therapy was safe and more clinically
effective (Obi, S. et al. 2006). From this example, the
future of this class of medication should be
ICBB 2022 - International Conference on Biotechnology and Biomedicine
348

emphasized on the combination therapy with other
agents due to its unique mechanism of action.
6 PREDICTED IMPROVEMENTS
OF CYTOKINES
The biological basis and rationale of the use of
cytokines are powerful, however, the clinical use of
this technology faces a range of issues. There are
some possible improvements in cytokine technology
which researchers are going to do next. Firstly,
improving pharmacokinetics and pharmacodynamics.
Clinical pharmacology is the study of the interaction
between the human body and drugs.
Pharmacokinetics and pharmacodynamics are the two
main branches of clinical pharmacology.
Pharmacokinetic describes the absorption,
distribution, metabolism, and excretion of drugs
(ADME), while pharmacodynamic describes how
biological processes in the body respond to or are
affected by drugs (Otagiri, M., Imai, T., & Fukuhara,
A. 1999). Pharmacokinetics and pharmacodynamics
were vital in determining the safety and effectiveness
of drugs. Secondly, improving local injection. Some
cytokines can be produced in the laboratory and used
to treat cancer. Some are used to help prevent or
control the side effects of chemotherapy. They are
injected under the skin, into muscles or veins. The
two most common types are interleukins and
interferons (Zhang, Z. & An, J. 2007). IFN-beta can
be injected in areas of the body with a layer of fat
between the skin and muscle, such as the thigh.
However, interferon beta-1a can control the
symptoms, but it cannot cure it. IL-2 and Interferon-
alpha have been combined based on data from
preclinical studies and have shown a synergistic
effect. A review of available phase I and II trials with
more than 1,400 patients indicated a response rate of
approximately 20%, 3% to 5% of patients completely
regressed. In order to prove that this combination
improves the overall response rate, randomized trials
were needed. The result of a phase III study displayed
that, compared with patients who received either
cytokine alone, patients treated with continuous
infusion recombinant (r) IL-2 and subcutaneous IFN-
alpha had a significant improved response rate 18.6%
and 1-year event-free survival 20.9%. The clinical
results are consistent with the improved response rate
in patients receiving rIL-2 and IFN-alpha. Continued
research on novel and new treatment methods
remains a priority (Bukowski R. M. 2000).
Since the 1950s, a variety of cytokines have been
used in the study of preclinical disease models, and
with the development of recombinant protein
technology in the 1980s, some of these cytokines
have become successful biopharmaceutical products.
However, due to cellular pleiotropy, the clinical
translation of these innate immune signaling
molecules is limited and they play primarily local
roles in tissues. In view of the clinical potential and
clinical trials in cancer immunotherapy, a range of
molecular and formulation engineering strategies are
being applied to reduce therapeutic toxicity while
maintaining or enhancing therapeutic efficacy.
Cytokine technology is promised to become more
effective and widespread in the use of clinical
treatment.
7 CONCLUSION
Cytokine therapy to activate the immune system of
cancer patients is an important treatment method. The
generation of a specific, effective cytokine-based
immunotherapy requires a variety of cytokines and
their receptors to combine with each other and give
an optimum effect. Understanding the molecular
signaling pathways of cytokine receptors is critical to
the development of cytokine based cancer therapies.
The most common cancers using cytokine-based
therapy are kidney cancer, leukemia and lymphoma.
These are the only three cancer types that use
cytokine-targeted medications for treatment as
monotherapy agents. This paper is introducing and
comparing three subtypes of interferon in the
treatment of kidney cancer, as well as the role of
antitumor agent IFN-alpha in the treatment of
leukemia and lymphoma. This paper derives from in-
depth research on cytokine-based immunotherapy
and its current barriers to indicate the future
medication development focus using the study of
hepatocellular carcinoma, and the combination
therapy of IFN-alpha and the standard medication 5-
FU on the treatment of hepatocellular carcinoma,
which resulted a higher survival rate. The extensive
pleiotropy and redundancy of cytokine signaling
pathways suggest that cytokine therapy may use
combination regimenes to amplify antitumor
responses, inhibit regulatory pathways, and minimize
toxicity. In general, cytokine based immunotherapy
has shown its great potential, and cytokine therapy
will have a broader prospect in the future.
Cytokines in Cancer Immunotherapy
349

REFERENCES
Anguille, S., Lion, E., Willemen, Y., Van Tendeloo, V. F.
I., Berneman, Z. N., & Smits, E. L. J. M. (2011).
Interferon-α in acute myeloid leukemia: an old drug
revisited. Leukemia, 25(5), 739–748.
Aviles, A., Neri, N., Fernandez-Diez, J., Silva, L., &
Nambo, M.-J. (2015). Interferon and low doses of
methotrexate versus interferon and retinoids in the
treatment of refractory/relapsed cutaneous T-cell
lymphoma. Hematology (Amsterdam, Netherlands),
20(9), 538–542.
Bio-Rad. (n.d.). Inflammation. Bio-Rad.
Bukowski R. M. (2000). Cytokine combinations:
therapeutic use in patients with advanced renal cell
carcinoma. Seminars in oncology, 27(2), 204–212.
Characterization of a human multilineage-colony-
stimulating factor cDNA clone identified by a
conserved noncoding sequence in mouse interleukin-
3—ScienceDirect. (n.d.).
Chow, S., Galvis, V., Pillai, M., Leach, R., Keene, E.,
Spencer-Shaw, A., Shablak, A., Shanks, J., Liptrot, T.,
Thistlethwaite, F., & Hawkins, R. E. (2016). High-dose
interleukin2 – a 10-year single-site experience in the
treatment of metastatic renal cell carcinoma: careful
selection of patients gives an excellent outcome.
Journal for ImmunoTherapy of Cancer, 4(1).
Clark, J. I., Curti, B., Davis, E. J., Kaufman, H., Amin, A.,
Alva, A., Logan, T. F., Hauke, R., Miletello, G. P.,
Vaishampayan, U., Johnson, D. B., White, R. L.,
Wiernik, P. H., & Dutcher, J. P. (2021). Long-term
progression-free survival of patients with metastatic
melanoma or renal cell carcinoma following high-dose
interleukin-2. Journal of Investigative Medicine, 69(4),
888–892.
Cytokines and Their Side Effects. (n.d.).
Coppin, C., Porzsolt, F., Awa, A., Kumpf, J., Coldman, A.,
& Wilt, T. (2005). Immunotherapy for advanced renal
cell cancer. The Cochrane Database of Systematic
Reviews, 1, CD001425.
Constitutive synthesis of interleukin-3 by leukaemia cell
line WEHI-3B is due to retroviral insertion near the
gene | Nature. (n.d.).
Conlon, K. C., Miljkovic, M. D., & Waldmann, T. A.
(2019). Cytokines in the Treatment of Cancer. Journal
of Interferon & Cytokine Research: The Official
Journal of the International Society for Interferon and
Cytokine Research, 39(1), 6–21.
Darnell, J. E., Kerr, I. M., & Stark, G. R. (1994). Jak-STAT
pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science
(New York, N.Y.), 264(5164), 1415–1421.
Darnell, J. E. (1997). STATs and gene regulation. Science
(New York, N.Y.), 277(5332), 1630–1635.
Dhupkar, P., & Gordon, N. (2017). Interleukin-2: Old and
New Approaches to Enhance Immune-Therapeutic
Efficacy. Advances in Experimental Medicine and
Biology, 995(995), 33–51.
Dinarello, C. A. (2007). Historical Review of Cytokines.
European Journal of Immunology, 37(Suppl 1), S34–
S45.
Discovery of interleukin-2 (IL-2) | NIH Intramural
Research Program. (n.d.).
Dodington, D. W., Desai, H. R., & Woo, M. (2018).
JAK/STAT – Emerging Players in Metabolism. Trends
in Endocrinology & Metabolism, 29(1), 55–65.
Fry, T. J., & Mackall, C. L. (2002). Interleukin-7: From
bench to clinic. Blood, 99(11), 3892–3904.
Frontiers | The COVID-19 Cytokine Storm; What We
Know So Far | Immunology. (n.d.).
Greenlund, A. C., Morales, M. O., Viviano, B. L., Yan, H.,
Krolewski, J., & Schreiber, R. D. (1995). Stat
recruitment by tyrosine-phosphorylated cytokine
receptors: An ordered reversible affinity-driven
process. Immunity, 2(6), 677–687.
Jiang, T., Zhou, C., & Ren, S. (2016). Role of IL-2 in cancer
immunotherapy. Oncoimmunology, 5(6), e1163462.
Jones, V. S., Huang, R.-Y., Chen, L.-P., Chen, Z.-S., Fu, L.,
& Huang, R.-P. (2016). Cytokines in cancer drug
resistance: Cues to new therapeutic strategies.
Biochimica et Biophysica Acta (BBA) - Reviews on
Cancer, 1865(2), 255–265.
Jun-Ming Zhang, Jianxiong An Int Anesthesiol Clin.
Author manuscript; available in PMC 2009 Nov 30.
Published in final edited form as: Int Anesthesiol Clin.
2007 Spring; 45(2): 27–37.
Katze, M., He, Y. & Gale, M. Viruses and interferon: a fight
for supremacy. Nat Rev Immunol 2, 675–687 (2002).
Koneru, R., & Hotte, S. J. (2009). Role of cytokine therapy
for renal cell carcinoma in the era of targeted agents.
Current Oncology (Toronto, Ont.), 16 Suppl 1(Suppl 1),
S40–S44.
Leonard, W. J., & Lin, J. X. (2000). Cytokine receptor
signaling pathways. The Journal of Allergy and Clinical
Immunology, 105(5), 877–888.
Lippitz, B. E. (2013). Cytokine patterns in patients with
cancer: a systematic review. The Lancet Oncology,
14(6), e218–e228.
Mackall, C. L., Fry, T. J., & Gress, R. E. (2011). Harnessing
the biology of IL-7 for therapeutic application. Nature
Reviews. Immunology, 11(5), 330–342.
Manoylov, M. K. (2020, November 6). What are cytokines?
Livescience.Com.
Mitchell, T. J., & John, S. (2005). Signal transducer and
activator of transcription (STAT) signaling and T-cell
lymphomas. Immunology, 114(3), 301–312.
Nagai, M., & Arai, T. (1984). Clinical effect of interferon
in malignant brain tumors. Neurosurgical Review, 7(1),
55–64.
Obi, S., Yoshida, H., Toune, R., Unuma, T., Kanda, M.,
Sato, S., Tateishi, R., Teratani, T., Shiina, S., & Omata,
M. (2006). Combination therapy of intraarterial 5-
fluorouracil and systemic interferon-alpha for advanced
hepatocellular carcinoma with portal venous invasion.
Cancer, 106(9), 1990–1997.
Orozco Valencia, A., Camargo Knirsch, M., Suavinho
Ferro, E., & Antonio Stephano, M. (2020). Interleukin-
ICBB 2022 - International Conference on Biotechnology and Biomedicine
350

2 as immunotherapeutic in autoimmune diseases.
International Immunopharmacology, 81(81), 106296.
Olsen, E. A. (2003). Interferon in the treatment of cutaneous
T-cell lymphoma. Dermatologic Therapy, 16(4), 311–
321.
Otagiri, M., Imai, T., & Fukuhara, A. (1999). Improving the
pharmacokinetic and pharmacodynamic properties of a
drug by chemical conversion to a chimera drug. Journal
of controlled release : official journal of the Controlled
Release Society, 62(1-2), 223–229.
Sims, J. E., March, C. J., Cosman, D., Widmer, M. B.,
MacDonald, H. R., McMahan, C. J., Grubin, C. E.,
Wignall, J. M., Jackson, J. L., Call, S. M., Friend, D.,
Alpert, A. R., Gillis, S., Urdal, D. L., & Dower, S. K.
(1988). CDNA Expression Cloning of the IL-1
Receptor, a Member of the Immunoglobulin
Superfamily. Science, 241(4865), 585–589.
Spolski, R., Li, P., & Leonard, W. J. (2018). Biology and
regulation of IL-2: from molecular mechanisms to
human therapy. Nature Reviews Immunology, 18(10),
648–659.
The state of the STATs: Recent developments in the study
of signal transduction to the nucleus—PubMed. (n.d.).
Waldmann T. A. (2018). Cytokines in Cancer
Immunotherapy. Cold Spring Harbor perspectives in
biology, 10(12), a028472.
Zhang, J.-M., & An, J. (2007). Cytokines, Inflammation
and Pain. International Anesthesiology Clinics, 45(2),
27–37.
Cytokines in Cancer Immunotherapy
351
