
A Categorical Guide to Basic Terminologies, Principles, and
Disconnections in Retrosynthesis
Zixuan Lin
1,*
and Jiaqiu He
2
1
Shenzhen Middle School, Shenzhen, Guangdong 518024, China
2
WLSA Shanghai Academy, Shanghai 201900, China
Keywords:
Organic Chemistry, Retrosynthesis, Disconnection, Synthon, Functional Group.
Abstract:
Retrosynthesis is a powerful tool for the synthetic analysis of organic compounds. Although it is a relatively
newly proposed idea, it has now been put into wide use. All compounds can have several possible
retrosynthetic paths, but the feasibility and practicality of a path is determined by certain concepts and
principles. This paper is an introduction to retrosynthesis, starting with basic concepts, reactions, and guiding
principles, and proceeding to a detailed guide to basic disconnections categorized with regard to the class of
target molecules. This work attempts to provide introductory-level students of organic chemistry with a
handbook allowing them to quickly learn to recognize and design basic retrosynthetic strategies.
1 INTRODUCTION
Retrosynthetic analysis was proposed by American
organic chemist E. J. Corey of Harvard University,
who later won the Nobel prize in Chemistry in 1990
for the proposal (Shampo, 2012). The period between
1960 and 1990 witnessed the evolution of
retrosynthesis, and the concept developed into a
mature subject that now deserves a separate space in
university courses. Early development focused on the
idea of antagonistic methods and perfected the
disconnections (Rao, 2020).
The importance of retrosynthetic analysis lies in
its wide applicability. For example, natural products
including alkaloids, rubber, as well as dyes and
fragrances. Chemists soon starts to separate, purify,
analyze and determine the structure of these
compounds. These substances can be applied in
various fields, such as medicine, plastics, electronics,
etc. (Divakaran, 2008). Retrosynthesis provides a
means for the large-scale production of compounds,
and makes them available for research and use in a
low-cost method for the benefit of mankind.
2 WHAT IS RETROSYNTHESIS?
Retrosynthesis is the process of recursively
decomposing a target molecule into simpler and
available starting materials (Ghosh, 2020). For
instance, a hexagon can be made by first imagining
its constituent pieces, as shown in Figure 1.
Figure 1: Decomposing a hexagon.
Chemical molecules can be made in a similar way,
except that instead of breaking down shapes at
random as done with geometric shapes, molecules
*
Correspondence author
can be deconstructed by disconnecting bonds, as
shown in Figure 2.
Lin, Z. and He, J.
A Categorical Guide to Basic Terminologies, Principles, and Disconnections in Retrosynthesis.
DOI: 10.5220/0012020200003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 301-309
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
301
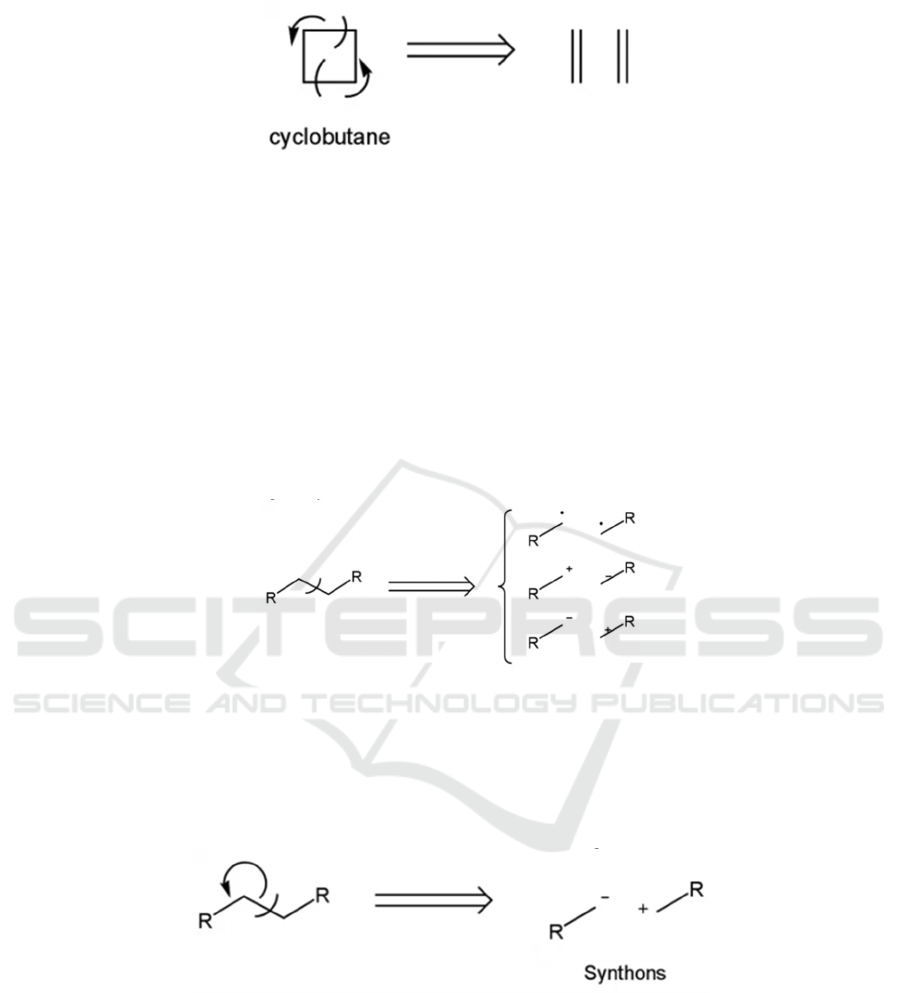
Figure 2: Disconnecting a cyclobutene.
In retrosynthesis, the symbol “⟹”represents the
reverse of a synthetic reaction, the symbol “(”
indicates the disconnection of bonds, and “ ↝ ”
indicates electron transfer.
3 CONCEPTS
3.1 Target Molecule (T.M.)
The target molecule is the desired molecule, and the
molecule whose synthesis is being analyzed.
3.2 Disconnection
A disconnection is an analytical operation which
involves the imaginary cleavage of a bond that
deconstructs a molecule into simpler pieces; the
reverse of a synthetic reaction.
Disconnections are in essence the transfer of
electrons. There are two possibilities: after the
disconnection of a single bond, the electrons are
either retained by respective atoms, or they are both
transferred to a single atom (see Figure 3). However,
the latter is more common in retrosynthesis.
Figure 3: Three possibilities of a disconnection
3.3 Synthon
A synthon is a fragment resulting from a
disconnection, usually a cation or an anion (Ghosh,
2020). (It may sometimes be used interchangeably
with “synthetic equivalent”.) There are two kinds of
synthons, namely the negatively charged
nucleophiles and the positively charged nucleophiles,
as shown in Figure 4.
Figure 4: Synthons.
3.4 Synthetic Equivalent
A synthetic equivalent carries out the functions of a
synthon which cannot be used itself, usually because
it is too unstable (Warren, 2002). The concept will be
discussed in more detail in a later section.
3.5 “Fine tuning”
“Fine tunings” are operations on functional groups
that facilitate disconnections. The first and most
common type of fine tuning is functional group
interconversion, or FGI (see Figure 5). It is the
operation of converting a functional group into
another, usually through oxidation. FGI can also be
used for protection of certain functional groups from
ICBB 2022 - International Conference on Biotechnology and Biomedicine
302
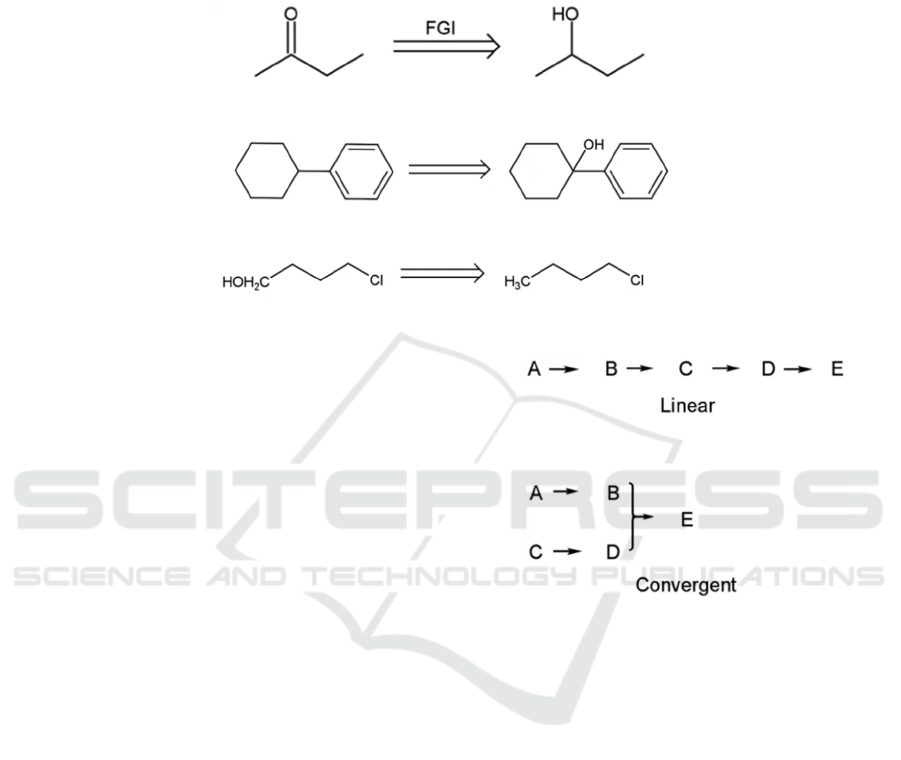
unwanted reactions. FGI is the most frequently used
fine tuning method, and most retrosynthesis involve a
sequence of disconnections and functional group
interconversions. The second is functional group
addition, or FGA (see Figure 6), often used to guide
disconnections, or to enable the installation of other
functional groups (Singh, 2013). The third is
functional group removal, or FGR, which is the
operation of removing a functional group (see Figure
7).
Figure 5: Functional group interconversion.
Figure 6: Functional group addition.
Figure 7: Functional group removal.
3.6 Guiding Principles
A molecule can have several possible retrosynthetic
routes. While all of them may be correct, certain
strategies are better than others. The following
guiding principles will help you recognize and design
strategic disconnections. The first rule is greatest
simplification. Resulting compounds after a
disconnection should be easier to make than the target
molecule. A disconnection should also have a
reasonable mechanism: a strategic disconnection
should give stable and recognizable synthons, as will
be demonstrated in subsequent examples. The third
principle is minimal fine tuning. While fine tuning
does facilitate disconnections to a certain extent, they
are not simplifying, and frequent use of fine tuning
may lead to redundancy. Lastly, good strategies
should have maximum convergency. Since most
chemical reactions do not have full efficiency, a
synthesis with too many steps will have a low yield.
(Warren, 2002) To avoid the problem, convergent
instead of linear strategies should be employed, as
shown in Figure 8.
Figure 8: Linear and convergent strategies.
Other concepts and principles will be introduced
in application in later sections.
4 DISCONNECTING
STRATEGIES
4.1 One-Group Disconnections
4.1.1 Alcohols
As mentioned previously, disconnections are the
transfer of electrons. Lone pair electrons can serve as
a guide. For instance, the disconnection of alcohols
can start with the transfer of a lone pair electron on
the oxygen atom, as shown in Figure 9.
A Categorical Guide to Basic Terminologies, Principles, and Disconnections in Retrosynthesis
303
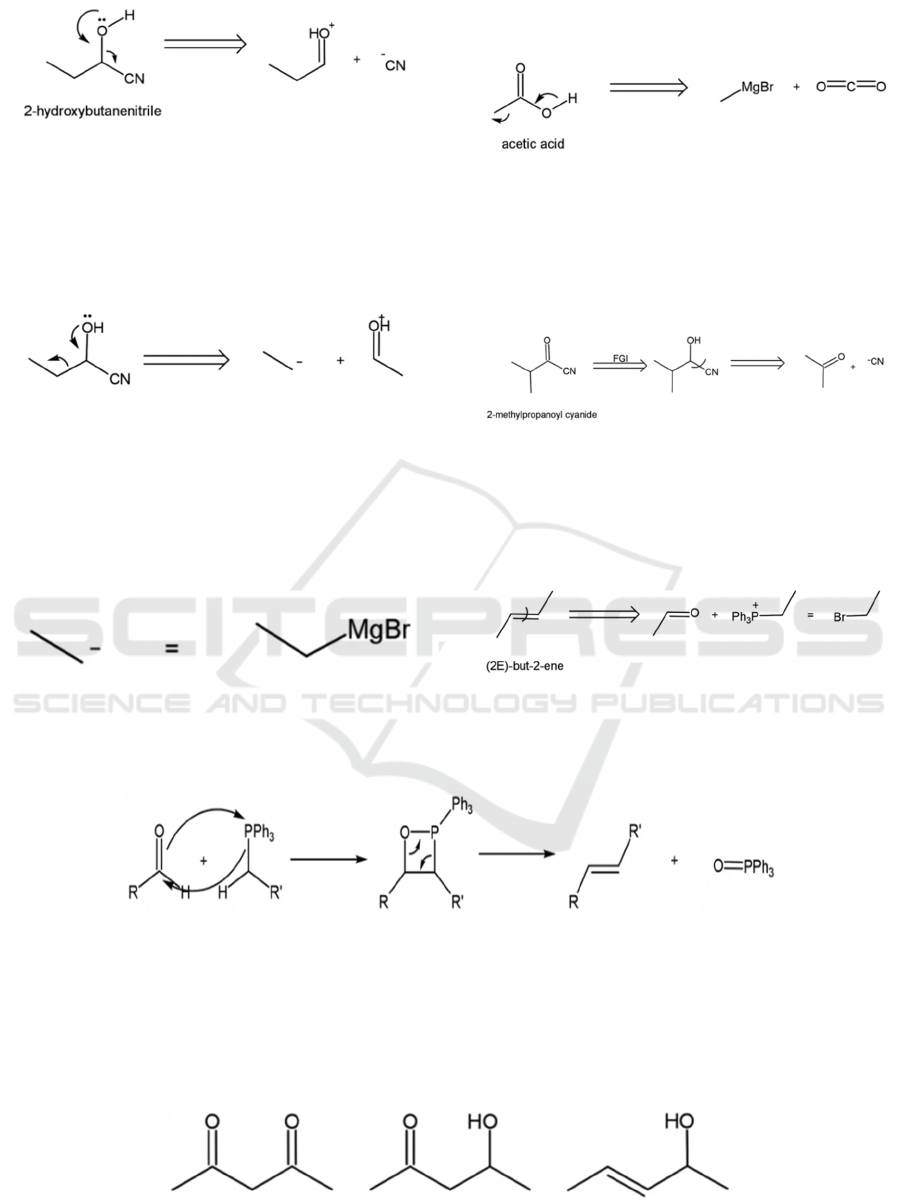
Figure 9: Disconnection of an alcohol.
Both ions are stable, so this is considered a
strategic disconnection. Figure 10 shows an
alternative route, where the electron goes the other
way. But the resulting anion is clearly unstable, so
this is an undesirable strategy.
Figure 10: Undesired disconnection for an alcohol.
However, most of the times, there may not be a
disconnection that gives stable synthons. The
problem can be solved by using stable synthetic
equivalents. For instance, Grignard reagents (see
Figure 11) are good synthetic equivalents for unstable
carbanions, while NaBH
4
and LiAlH
4
are equivalent
to the hydrogen anion H
-
. (Warren, 2002)
Figure 11: Grignard reagent as synthetic equivalent
4.1.2 Acids
The disconnection of acids can also start from the
oxygen, as shown in Figure 12.
Figure 12: Disconnection of an acid.
4.1.3 Ketones
A simple retrosynthesis for ketones is to first convert
them to alcohols, and then make the disconnection
(see Figure 13).
Figure 13: Disconnection of a ketone.
4.1.4 Olefins
Olefins can be made by simply disconnecting the
double bond (see Figure 14).
Figure 14: disconnection of an olefin
This is the Wittig reaction, with its mechanism
presented in Figure 15.
Figure 15: mechanism of the Wittig reaction
4.2 Two-Group Disconnections
When a compound contains two oxygenation groups,
they can be used together to guide disconnections.
4.2.1 1, 3-dioxygenated Compounds
Figure 16 shows a few of the variations of the 1,3-
dioxygenation pattern. Note that a double bond is
equivalent to a hydroxy group.
Figure 16: 1, 3-dioxygenated compounds
ICBB 2022 - International Conference on Biotechnology and Biomedicine
304
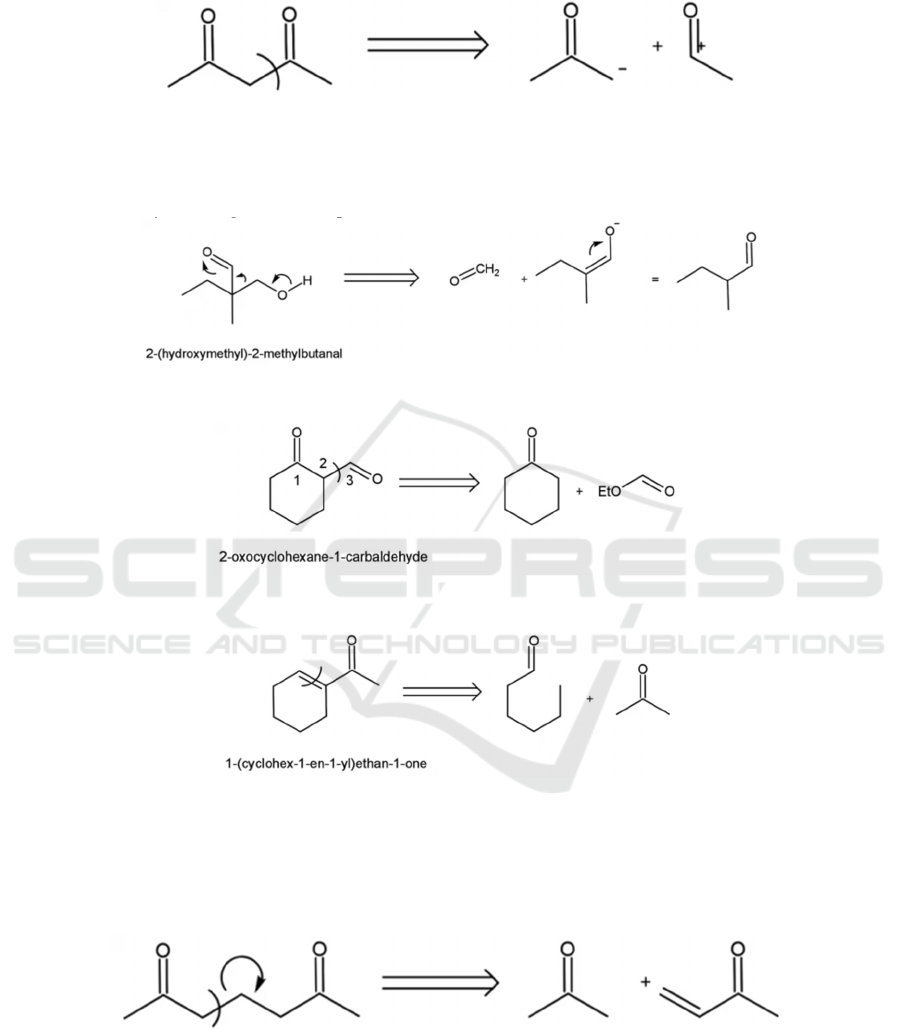
1, 3-dioxygenated compounds can generally be
disconnected at the 𝛼, 𝛽 bond (see Figure 17).
Figure 17: General disconnection strategy for 1,3-dioxygenated compounds.
For instance, the 𝛽-hydroxy carbonyl (see Figure
18), 1,3-dicarbonyl (see Figure 19), and 𝛼 , 𝛽 -
unsaturated carbonyl (see Figure 20) compounds can
all be disconnected following the pattern shown in
Figure 17.
Figure 18: Disconnection of a β-hydroxy carbonyl.
Figure 19: Disconnection of a 1,3-dicarbonyl.
Figure 20: Disconnection of an α,β-unsaturated carbonyl.
4.2.2 1,5-dioxygenation
The mechanism of the disconnection of 1,5-
dioxygenation pattern is mostly similar to that of the
1,3-dioxygenation pattern. The compound can be
disconnected at any of its two middle bonds, as
shown in Figure 21.
Figure 21: Disconnection of a 1,5-dioxygenation.
The reverse of this disconnection—the reaction
using 𝛼 , 𝛽 -unsaturated carbonyl compounds as
electrophiles—is called the Michael reaction.
Figure 22 shows a more integrated example, in
which the 𝛼,𝛽-unsaturated ketone has both 1,3- and
1,5-dioxygenation patterns. A good retrosynthetic
path is to first disconnect the C-C double bond, and
A Categorical Guide to Basic Terminologies, Principles, and Disconnections in Retrosynthesis
305
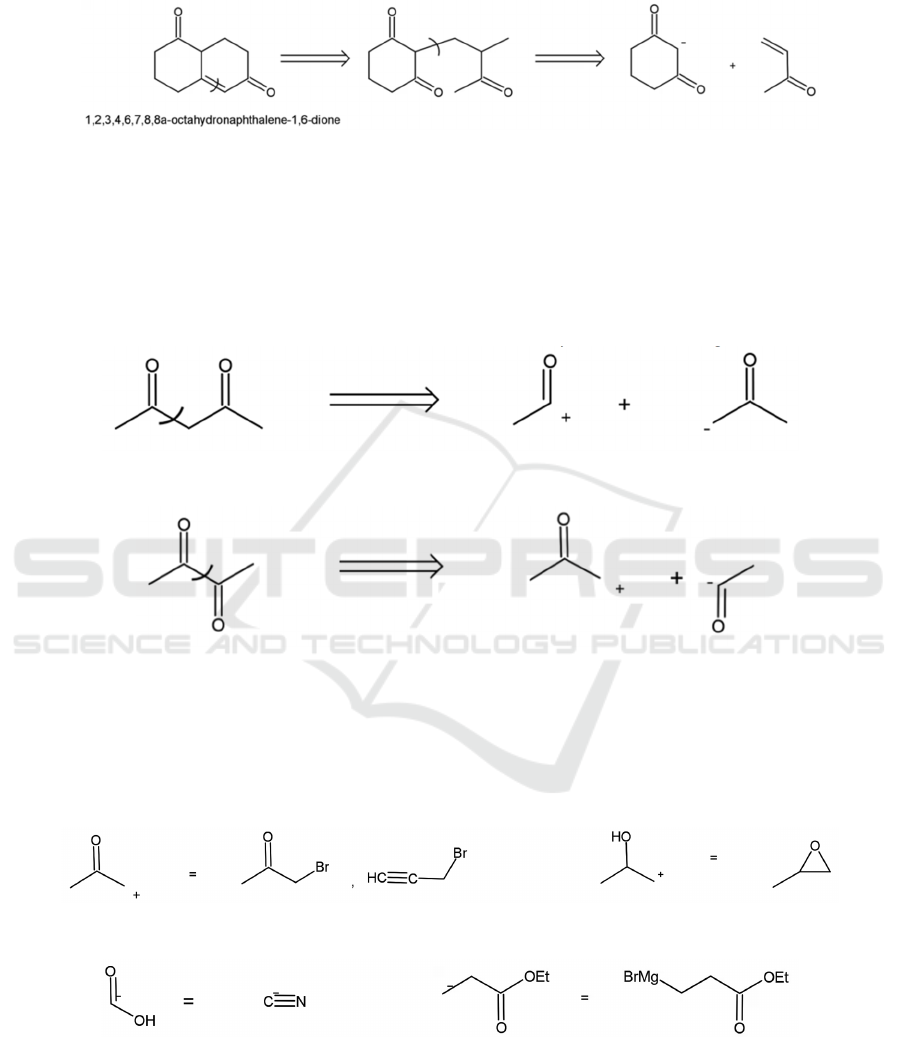
then the 𝛽, 𝛾 bond in the 1,5-dioxygenation pattern,
as shown in Figure 22.
Figure 22: Integrated example of disconnection for a compound with 1,3- and 1,5-dioxygenation patterns.
4.3 Illogical Disconnections
Disconnections resulting in synthons in which the
normal polarity is reversed are called illogical
disconnections (Ghosh, 2020). As a rule of thumb,
compounds with an even number of C atoms between
two functional groups produce illogical synthons
because of a dissonance of charges arising from their
oxidation patterns (Šunjić, 2016). For instance, while
disconnecting the middle bond of a 1,3-dicarbonyl
results in logical synthons (see Figure 23), a 1,2-
dicarbonyl produces two illogical synthons that react
unfavorably due to a like charges (see Figure 24).
Figure 23: Disconnection of a 1,3-dicarbonyl.
Figure 24: Disconnection of a 1,2-dicarbonyl.
4.3.1 Illogical Synthons
The key to illogical disconnections is recognizing
illogical synthons and replacing them with their
corresponding synthetic equivalents. The synthetic
equivalent of a positively charged illogical synthon is
called an illogical electrophile (see Figure 25), while
that of a negatively charged illogical synthon is an
illogical nucleophile (see Figure 26).
Figure 25: Illogical electrophiles and their synthetic equivalents.
Figure 26: Illogical nucleophiles and their synthetic equivalents.
4.3.2 1,2-dioxygenation
An 𝛼-hydroxy-carbonyl can be disconnected in the
middle, resulting in a benzyl alcohol electrophile and
an illogical nucleophile equivalent to a benzaldehyde,
as shown in Figure 27.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
306
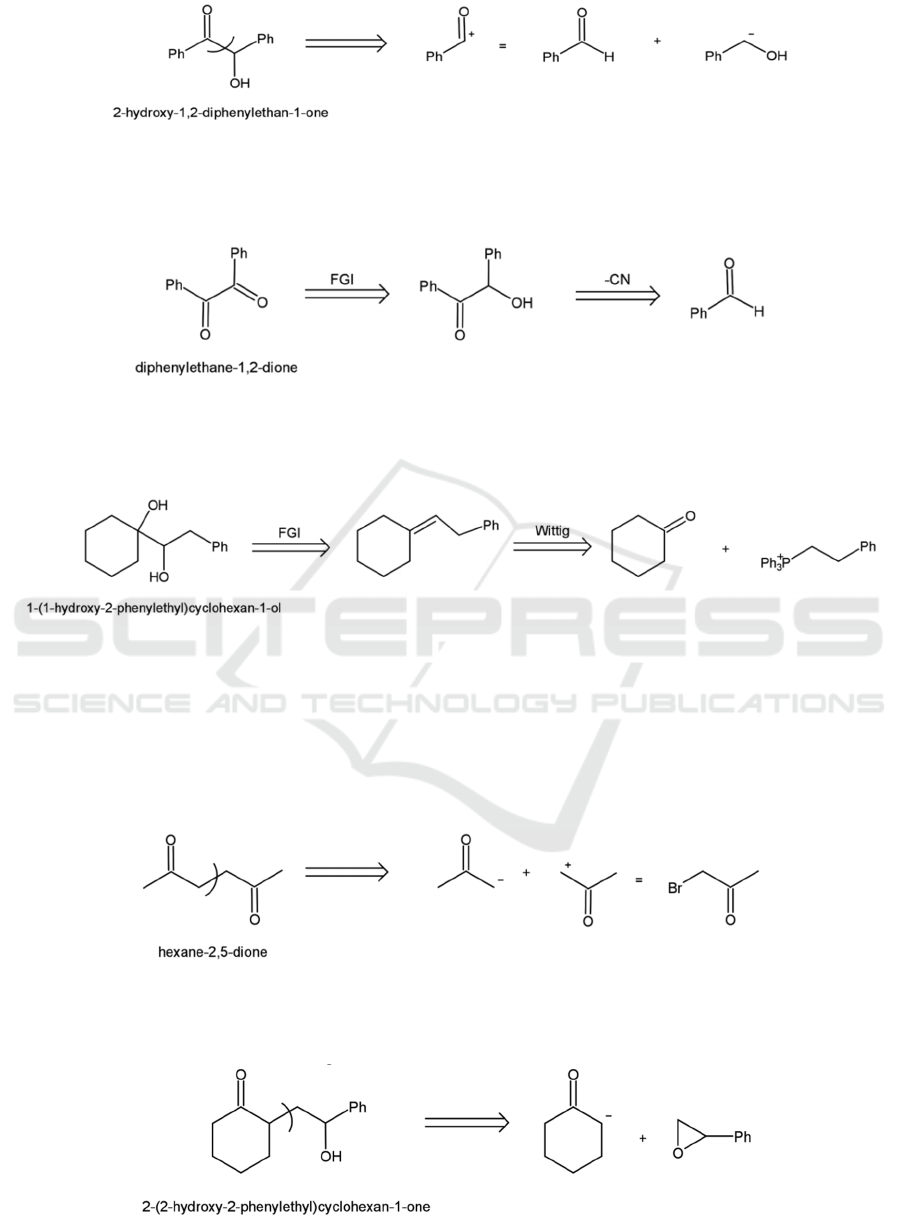
Figure 27: Disconnection of an α-hydroxy-carbonyl.
The strategy for 1,2-dicarbonyl compounds is
similar. The most convenient way to make a 1,2-
dicarbonyl is to convert it to an 𝛼-hydroxy-ketone
first, and then synthesize it from there (see Figure 28).
Figure 28: Disconnection of a 1,2-dicarbonyl.
The disconnection of 1,2-diols is slightly different.
Figure 29 shows a good approach, which is to use an
olefin as the intermediate and disconnect the double
bond by the Wittig reaction.
Figure 29: Disconnection of a 1,2-diols.
4.3.3 1,4-dixoygenation
The disconnecting strategy for the 1,4-dioxygenation
pattern is similar to that of 1,2-dioxygenated
compounds in essence: disconnection of the 𝛽, 𝛾
bond results in two synthons, one of them illogical.
The disconnection of the 1,4-dicarbonyl shown in
Figure 30 results in a stable acetone nucleophile and
an illogical electrophile, which can be substituted by
its corresponding synthetic equivalent.
Figure 30: Disconnection of a 1,4-carbonyl.
𝛾 -hydroxy-carbonyl compounds can be
disconnected in a similar fashion (see Figure 31).
Figure 31: Disconnection of an α-hydroxy-carbonyl.
A Categorical Guide to Basic Terminologies, Principles, and Disconnections in Retrosynthesis
307
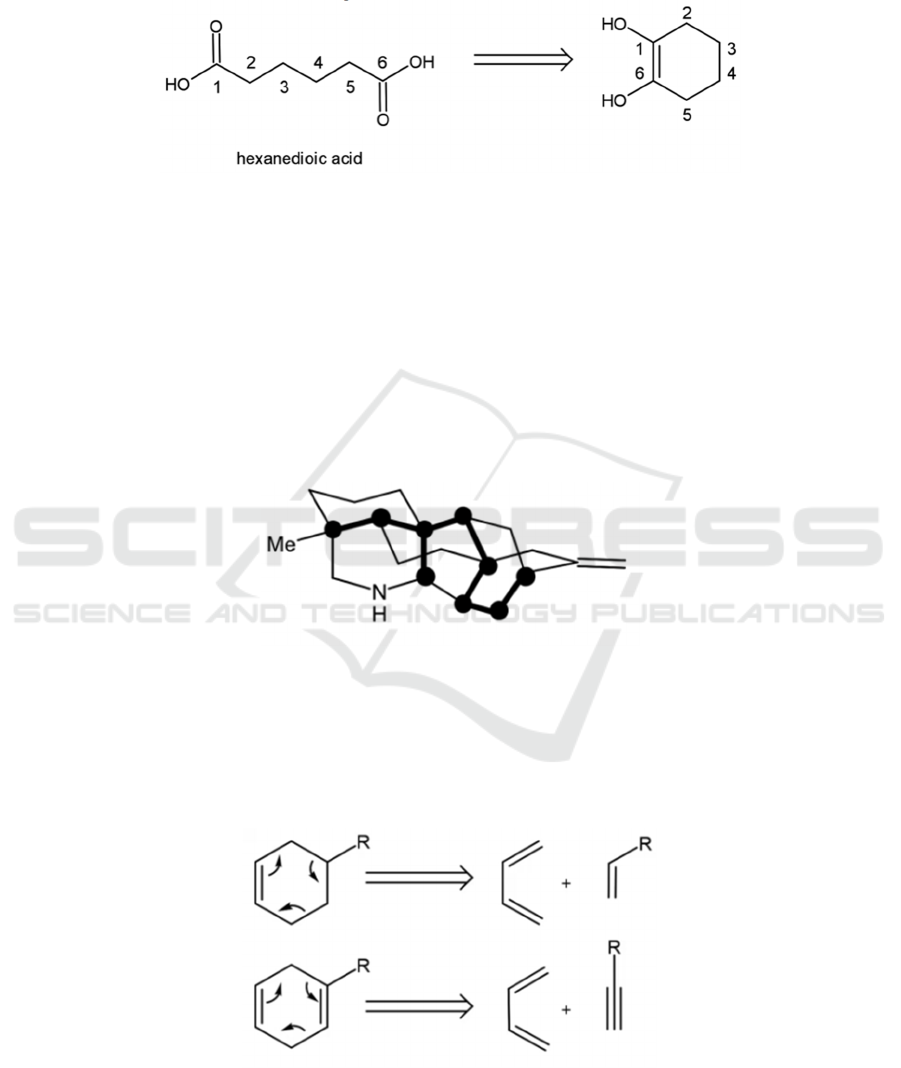
4.3.4 1,6-Dioxygenation
1,6-dioxygenated compounds have a different
synthetic strategy. Instead of disconnecting bonds in
the usual sense, a general strategy is to disconnect the
two oxygenation groups, as shown in Figure 32.
Figure 32: Disconnection of a 1,6-dioxygenated compound.
Subsequent disconnections of the ring structure
will be introduced in a later section.
All compounds with a 1,6-oxygenation pattern
can be made by first converting the corresponding
functional groups to carbonyl groups, and then
reconnecting the bonds in a similar fashion.
4.4 Pericyclic Disconnections
Pericyclic reactions are concerted reactions with
cyclic transition states.
4.4.1 The Common Atom Approach
The common atom approach is a guiding principle for
the retrosynthesis of polycyclic compounds. The
most strategic disconnections are made by breaking
bonds connecting atoms that are common to more
than one ring, for they lead to maximum
simplification. For instance, in the compound shown
in Figure 33, the common atoms are marked in bold
along with the most strategic bonds.
Figure 33: A hetidine analyzed with the common atom approach.
4.4.2 Diels-Alder Reaction
The Diels-Alder reaction is one of the most important
reactions in organic synthesis. It occurs between a
conjugated diene and a dienophile (an alkene or
alkyne), and is sometimes referred to as a [2+4]-
cycloaddition (Gunawardena, 2020). Its
disconnection is easily recognizable as the reverse of
the reaction. Below are the mechanisms of the
disconnection (Byrne, 2013):
Figure 34: Mechanisms of the Diels-Alder reaction.
Below are some examples (Figure 35):
ICBB 2022 - International Conference on Biotechnology and Biomedicine
308
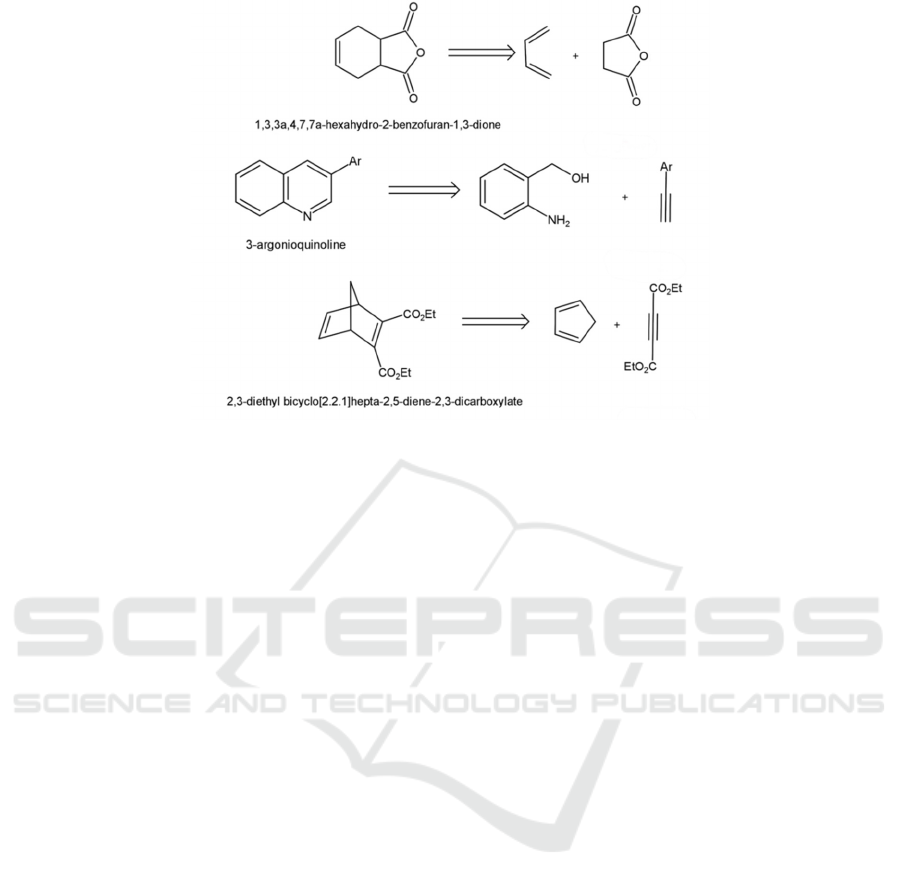
Figure 35: Examples of the Diels-Alder reaction.
However, it is important to note that although
different types of disconnections have different
mechanisms, they are under the same theoretical
backdrop and are guided by the same principles, and
it is important, therefore, to look at them as a whole
instead of simply treating them as separate operations.
5 CONCLUSION
This work introduces basic retrosynthetic concepts
including terminologies and guiding principles, and
gives a categorical and detailed overview of
disconnections of molecules with different
functionalities. This work summarizes and builds
upon previous introductory works, and can serve as a
guide to retrosynthesis for beginning students of
organic chemistry, providing them with the
knowledge base which will help them in further
studies in the field. However, this paper only covers
representative concepts, disconnections, and
strategies currently, and there will be continued
efforts to make the work more comprehensive and
readable.
REFERENCES
Byrne, P. A., & Gilheany, D. G. (2013, May 14). The
modern interpretation of the Wittig reaction mechanism.
Chemical Society Reviews.
https://pubs.rsc.org/en/content/articlelanding/2013/cs/c
3cs60105f.
Divakaran, G. (2008). Retrosynthetic Analysis. science
blogs. https://science-blogs.ucoz.com/resources/notes/
msc/theory/Retrosynth.pdf.
Ghosh, D. C. (2020). The Logic of Organic Synthesis.
Sajaipuria College. http://www.sajaipuriacollege.in/
wp-content/uploads/2020/04/SEM-4_Chemistry-
Hons_Organic-Synthesis_Module-1_Dinesh-Ch.-
Ghosh-converted.pdf.
Gunawardena, G. (2020, August 24). Diels-Alder reaction.
Chemistry LibreTexts. https://chem.libretexts.org/
Ancillary_Materials/Reference/Organic_Chemistry_Gl
ossary/Diels-Alder_Reaction.
Rao, R. B. (2020, August 11). Chapter 18: Organic
synthesis. Chemistry LibreTexts. https://chem.
libretexts.org/Courses/Purdue/Purdue%3A_Chem_266
05%3A_Organic_Chemistry_II_(Lipton)/Chapter_18
%3A_Organic_Synthesis.
Shampo, M. A., Kyle, R. A., & Steensma, D. P. (2012).
Elias James Corey—Nobel Prize for Retrosynthetic
Analysis. Mayo Clinic Proceedings.
https://www.mayoclinicproceedings.org/article/S0025-
6196(12)01042-7/fulltext.
Singh, R., & Rarh, V. (2013.). 14: Organic Chemistry –IV
(Advance Organic Synthesis and Supramolecular
Chemistry and carbocyclic rings). BSc Chemistry.
http://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_cont
ent/S000005CH/P000669/M019143/ET/1515665975C
HE_P14_M1_etext.pdf.
Šunjić, V., & Petrović Peroković, V. (2016). Illogical
disconnections with participation of two groups.
Organic Chemistry from Retrosynthesis to Asymmetric
Synthesis, 103–141. https://doi.org/10.1007/978-3-319-
29926-6_5
Warren, S. G. (2002). Designing organic syntheses: A
programmed introduction to the synthon approach.
Wiley & Sons.
A Categorical Guide to Basic Terminologies, Principles, and Disconnections in Retrosynthesis
309
