
Cancer Immunology: CAR T-cell Therapy on Renal Cell Carcinoma
Qingyang Huang
1,*
and Song Ying
2,*
1
School of Human Nutrition, McGill University, Montreal H9X 3V9, Canada
2
Department of Health, Kinesiology & Applied Physiology, Concordia University, Montreal H4B 1R6, Canada
Keywords:
CAR, Renal Cell Carcinoma, RCC.
Abstract: Chimeric antigen receptor T (CAR-T) cell therapy is a revolutionary tool for cancer treatment. Studies have
shown its potential in treating hematological malignancies. Renal cell carcinoma (RCC) as the most common
kidney cancer, would develop malignant tumor in the lining of kidney tubules. It has the highest mortality
rate among all genitourinary cancers. The main issue for RCC is its recurrence rate after surgery treatment.
As CAR T-cell therapy has shown its benefits in treating the leukemia and lymphomas, its use in RCC is
taking in consideration to reduce the chance of recurrence. Currently, there are some clinical trials are ongoing
to measure its feasibility. It is also important to pay attention to the CAR T-cell associated toxicities, on-target
off-tumor effect and tumor infiltration that would affect CAR T-cell therapy during the clinical trials. This
review focused on presenting ongoing clinical trials of CAR T cell on RCC and its limitations. Some future
perspectives are introduced to provide goals in later research.
1 INTRODUCTION
Renal cell carcinoma (RCC) is an aggressive kidney
disease originates in the lining of tubules. (Motzer,
1996) The ratio for both affected men and women is
about 2:1. Other than age and sex, cigarette smoking
and obesity are also risking factors. According to
studies, for cigarette smokers, the increase in risk for
RCC doubles. Obesity in both women and men
increases in mortality rate of RCC. (Yu, 1986)
Moreover, about 2% of the RCC has hereditary
syndrome. (Cohen, 2005) When a first-degree family
member has RCC, the risk for the second-degree
family member would be fourfold. (Motzer, 1996)
Generally, clear cell renal cell carcinoma (ccRCC) is
one of the typical RCC which represents around 3/4
of the cases. However, some patients would develop
the advanced RCC including locally invasive or
metastatic RCC (mRCC) as the cancer cells would
spread with a fast speed in the body. (Cohen, 2005)
Normally, surgery resection is the only effective
treatment for renal cell carcinoma because of its
chemoresistance. The patient would need to remove
the cancer cell affected kidney or having a kidney
transplant. Partial and radical nephrectomy are used
to treat localized RCC. For metastatic RCC, it
requires systemic therapies which are associated with
high mortality. (Hsieh, 2017) The approval of
cytokine-based immunotherapies (IFN-a and IL-2)
and tyrosine kinase inhibitors (TKIs) show effect on
reducing the mortality of metastatic RCC. (Schepisi,
2020) Other treatment including radiation therapy
and arterial embolization as the palliative therapy are
for patients who cannot have surgery. Patient’s health
condition and the stage of RCC are the general factors
that affect the chance of recovery and treatment
methods.
The recurrence rate of renal cell carcinoma
after nephrectomy is about 30%. (Chin, 2006)
In recent years, CAR T-cell therapy has become
one of the effective cancer treatments. It was
originally designed to treat hematologic neoplasms,
and have been used to treat solid tumors, including
RCC. (Schepisi, 2020) CAR-T cells are T cells that
have been genetically modified to create an artificial
T cell receptor for use in immunotherapy, which are
recombinant cell surface antigen receptors that have
changed the specificity and activity of T lymphocytes
and other immune cells in the blood. (Curran, 2012)
CARs have an extracellular antigen-identifying
domain made up of monoclonal antibody fragments
that recognize a particular protein on the cell
membrane of malignant cells and an intracellular
stimulating region that triggers CAR T-cell activation
and activity by signaling through the T-cell receptor
(TCR). (Minutolo, 2019) T cells must grow before
they can be used for CAR transduction and
Huang, Q. and Ying, S.
Cancer Immunology: CAR T-Cell Therapy on Renal Cell Carcinoma.
DOI: 10.5220/0012015600003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 171-177
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
171

amplification. In this process, transduction can take
many forms, but in clonal growing and durable T
cells. Based on this principle, CAR can target
antigens expressed on the cell surface, as well as
various T cell subpopulations, T cell progenitors, and
other immune cells.
CAR T-cell therapy is also known as a "living
drug," since its effects might last for years. Because
the cells may live in the body for a long period, they
may be able to detect and target cancer cells if and
when they reoccur. (Sadelain, 2013) Importantly,
CARs with high potential and signaling quality can
control T cell proliferation and persistence, as well as
the degree of engineered T cell activation in the
cancer microenvironment, both of which are traits
that have a significant impact on cancer-targeted T
cell effectiveness and safety. (Watanabe, 2018)
Nowadays, techniques have been implemented to
improve cancer treatment for solid tumors, primarily
by overcoming challenges posed by T cell features
and the tumor environment. Companion diagnostics,
such as IHC and CTC detection tests, can be used to
improve CAR T-cell therapy of solid tumors. (Ma,
2019)
Therefore, to overcome this hurdle, CAR T-cell
treatment show its prospect in treating RCC. This
review summarizes the current CAR T-cell treatment
clinical trials on renal cell carcinoma and its
limitations. Moreover, the future perspectives for the
use of CAR T-cell therapy on RCC are explored.
2 CURRENT TRIALS OF CAR T-
CELL THERAPY ON RENAL
CELL CARCINOMA
For decades, surgery has been the only effective
treatment because of its chemoresistance. To
overcome this hurdle, CAR T-cell therapy shows its
advantages in treating tumors compared to the
previous modalities of adoptive cell therapy such as
TCR and TIL. Unlike TCR and TIL, CAR T-cell
therapy is a non-MHC-restricted approach, therefore
it can target the tumor cells without recognition of
MHC, as most of cancer cells would trigger the loss
of MHC expression to escape the T-cell immune
response. (Rohaan, 2018) Currently, FDA has
approved CD19 CAR T-cell therapy for Acute
Lymphoblastic Leukemia (ALL), Diffused large B-
cell lymphoma (DLBCL) and many other NHL.
Although there is no CAR-T cell therapy for RCC
approved by FDA right now, some clinic trials are
still ongoing.
2.1 CAR T-cells CCT 301-38 or CCT
301-59 in Relapsed /Refractory
Stage IV RCC
The recent clinical trial evaluates the safety and
efficacy of the two autologous CAR T-cell CCT301-
38 and CCT301-59 with escalation and expansion on
does. CCT301-59 targets the neurotrophic tyrosine
kinase, receptor-related 2 (ROR2), as the CCT301-38
directed against AXL gene expression. In patients
with kidney cancer, ROR2 expression is associated
with genes responsible for mitosis and metastasis.
AXL gene is involved in cell proliferation,
angiogenesis, immunity, stem cell maintenance and
other therapeutic processes. A total of 66 patients
participated in the clinical trial. Patients who tested
positive for ROR2 were treated with CCT301-59,
while patients who tested positive for AXL but
negative for ROR2 were treated with CCT301-38.
Peripheral blood mononuclear cells (PBMCs) are
needed to produce CCT301-38 and CCT301-59.
During the production of CCT201-38 and CCT301-
59, the trial requires patients to undergo a conditional
chemotherapy of regiment of cyclophosphamide and
fludarabine for lymphodepletion. After the injection
of CCT301-38 or CCT301-59 to After depleting
lymphocytes, patients would intravenously inject one
dose of CCT301-38 or CCT301-59. The estimate
primary completion date for these phases I and II
trials are in June 2022. (ClinicalTrials.gov, 2018)
2.2 CAR T-cell Therapy with PARPi
This research shows the application of CAR T-cell
therapy on targeting CD70 is through introducing a
single chain antibody against CD70 and generation of
CD70 CAR-T cells that have an effective anti-tumor
function both in vitro and vivo. (Ji, 2021) Moreover,
the PARP inhibitor (PARPis) is used to enhance the
effectiveness of this CAR T-cell therapy by
increasing the recruitment of CD8+ T cells to the
TME in this trial. The second-generation humanized
CAR was first used to augment its anti-tumor
efficacy. (Pantelidou, 2019) The introduction of
PARPi olaparib (OLA) to CD70 CAR T-cell therapy
in RCC show its effects on promoting the apoptosis
of RCC cells but protecting CAR-T cells from
apoptosis. It also shows a more effective repression
of RCC cells with a better survival rate. The OLA-
mediated CAR T-cell therapy starts with the
cGAS/STING signaling pathway, which is a key
activator for PARPi treatment to promote infiltration
of CD8+ CAR T-cell. It then up-regulates the IFN-B
expression and the expression of both CCL5 and
ICBB 2022 - International Conference on Biotechnology and Biomedicine
172

CXCL10. Both would improve the amount of CD8+
CAR T-cells and enhance the tumor lysis due to the
secretion of granzyme B. These demonstrated that
cGAS-STING pathway determines the Penetration
and permanence of OLA-mediated CAR T-cell
therapy in TME. This approach shows its potential in
treating the solid tumors. (Ji, 2021)
2.3 Metastatic Renal Cell Carcinoma
with CAIX CAR-engineered T cells
Metastatic renal cell carcinoma (mRRC) is well
known for its treatment-resistance. The patients with
mRRC often with poor survival of less than one year
after diagnosis. (Gupta, 2008) Therefore, the CAR T-
cell therapy is applied to treat mRRC. However, CAR
T-cell therapy may develop a toxicity in the body due
to its “on-target” effect. Carbonic anhydrase-IX
(CAIX) is a transmembrane protein that is over-
pressed in RCC. It is generally used as a diagnosis
marker or drug target. In the trial, 12 mRCC patients
with CAIX-pressing were injected by CAR-T cell that
is engineered against CAIX. The patients would pre-
treat with a CAIX monoclonal antibody (mAb) G250.
The results indicate that there are no toxicities in the
liver. Moreover, this trial surprisingly enhances the T-
cell persistence in the therapy. Therefore, by pre-
treating patients with CAIX mAb, it helps to prevent
on-target toxicity from CAR T-cell therapy on RCC
and improve the peripheral persistence. This strategy
can be applied on the use of CAR T-cell therapy in
other cancer. More research is needed. (Lamers,
2013)
3 LIMITATION
CAR-T cells binding to target antigens expressed on
the cell surface is independent of MHC receptors. It
caused T cell activation and a powerful antitumor
response, which is revolutionary for leading a
remarkably effective and long-lasting clinical
response. When targeting solid tumors, CAR-T cells
have shown mild clinical efficacy in malignancies.
(Neelapu, 2017) The development of T cell in vitro
growth technology and genetic engineering enables
the rapid generation of tumor antigen effector cells,
which promotes the applicability of cancer
immunotherapy. However, the serious toxicity of
CAR T-cell therapy, targeting of non-tumor cells, cell
metastasis and tumor invasion remain unresolved.
3.1 CAR-T cell-associated Toxicities
CAR-T cells recognize hLA-independent and reduce
the risk of cross-reaction by binding to larger
epitopes. (Casucci, 2014) Genetic engineering may
improve the toxicity characteristics of traditional
chemotherapeutic drugs by improving the accuracy of
T cell recognition of targets. Nonetheless, cell
therapies are unique in that up to 10 years of
extraordinary long-term persistence can be achieved
using adoptive cellular therapy in human trials. The
timeline for this persistence will potential toxicity to
extend far beyond traditional small-molecule drugs
timeline. (Scholler, 2021)
Side effects after CAR T-
cell therapy may be rapid, slow, moderate, severe,
and even persist throughout the T cell cycle, thus
preventing the development of CAR T-cell therapy.
Data showed that nearly all patients with acute
lymphoblastic leukemia and lymphoma treated with
CAR-T cells had at least some mild toxicity, while
23-46% showed production of parapsychological
cytokines and severe in vivo T cell proliferation.
(Frey, 2016)
Cytokine release syndrome (CRS) is a systemic
inflammatory response that leads to immune
activation caused by exogenous cytokines produced
by CAR-T cells, leading to dysfunction in most
organs. (Lee, 2014) Specifically, patients with
leukemia (77-93%) and with lymphoma (67-91%)
treated with CAR-T cells experienced more CRS of
any grade, compared with patients treated with
axicabtagene ciloleucel and tisagenlecleucel for
relapsed or refractory B-All. (Halford, 2020)
CRS-
associated cytokines may be produced directly by
injected CAR-T cells or macrophages, which may
produce cytokines in response to injected CAR-T
cells. (Brudno, 2016)[24] And elevated serum levels
of several cytokines, including interleukin-2 (IL-2),
IL-2, IL-2-receptor -α, IL-8, IL-10, tumor necrosis
factor and so on, are associated with fever,
tachycardia, hypotension, and other toxicity after
CAR-T cell infusion. The severity of CRS and the
increase of serum cytokines are associated with the
disease burden, and the higher the disease burden, the
greater the toxicity. Clinically, the characteristics of
CRS ranging from mild to severe include fever,
creatine, aversion to eating, rapid heartbeat,
decreased blood pressure, increased capillary osmotic
pressure, loss of cardiac function, abnormal renal
function, weakened liver function, and disseminated
coagulation in the blood vessels. (Bonifant, 2016)
Patients in clinical trials of CAR-T cells often
develop neutropenia and lymphocytopenia after
chemotherapy and CAR-T cells. After CAR-T cell
Cancer Immunology: CAR T-Cell Therapy on Renal Cell Carcinoma
173
infusion, bacteremia, salmonella, urinary tract
infections, and viral infections such as influenza,
respiratory syncytial virus, and shingles virus have
been observed. (Brentjens, 2010) Besides, elevated
cerebrospinal fluid cytokine levels can induce ICANS
presenting with delirium, encephalopathy, aphasia,
drowsiness, inattention, agitation, tremors, seizures,
and rare cerebral edema.
3.2 On-Target off-Tumor Effects
Solid tumor antigens are usually expressed at
different levels in normal tissues, so antigen selection
is critical for CART engineering, not only to protect
the therapeutic effect, but also to inhibit the
extratomatous cross-reaction of overactive toxic
engineered T cells. Exposure of high doses of CAR-
T cells to heart, lung, or liver tissue at the time of
initial cell injection leads to rapid death. Even if the
tumor is successfully targeted, a rapid increase in
overall T-cell activity driven by CAR signals during
treatment may result in tumor-lysis syndrome, which
rapidly removes large numbers of tumor cells in a
short period of time, endangering cell life. The
differences between patients in T cell response and
risk of toxicity make it challenging to predict the
optimal number of T cells to be transfused. (Morgan,
2010) Therefore, allowed to control the regulation of
the dose and time T cell function of system
engineering is an important priority. (Sadelain, 2013)
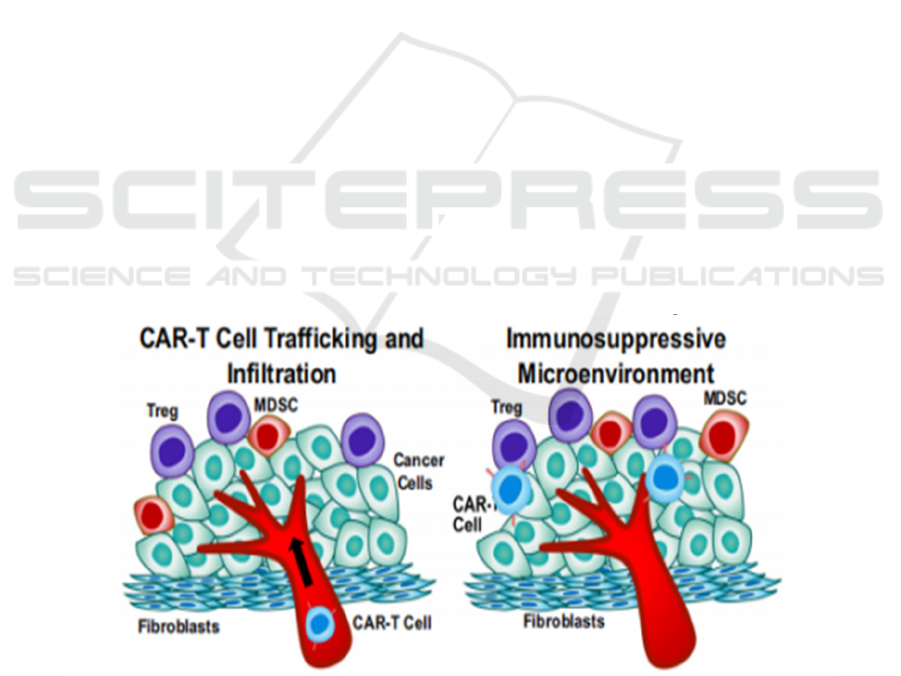
3.3 CAR-T cell Trafficking and Tumor
Infiltration
Compared with hematological malignancies, CAR T-
cell therapy in solid tumors is limited by the ability of
CAR T cells to transport and infiltrate solid tumors
because of physical tumor barriers such as
immunosuppressive tumor microenvironment and
tumor stroma that limit CAR T-cell penetration and
mobility in Figure 1. (Murad, 2018) After infusion,
CAR-T cells need to enter the malignant site, navigate
the complex tumor environment, form effective
interactions with cancer cells, exert their cytotoxic
activity, and ultimately persist. Many
immunosuppressive cells can infiltrate solid tumors
in the tumor microenvironment, including medullary
suppressor cells (MDSCs), tumor-associated
macrophages (TAMs), and regulatory T cells (Tregs),
which promote the production of cytokines,
chemokines, and growth factors. In addition, immune
checkpoint pathways such as PD-1 or CTLA-4 can be
used to modulate antitumor immunity, causing T cell
dilation and short-term T cell persistence, resulting in
less effective CAR T-cell therapy. (Quail, 2013)
Furthermore, increased tumor hardness, an increased
deposition of tumor ECM proteins and the significant
presence of cancer-associated fibroblasts have been
shown to promote immunosuppression through
various mechanisms. More simply, the thick network
of collagenous fibers surrounding some tumor islets
may constitute a physical barrier to t-cell lymphocyte
invasion of tumor cell regions. (Yamauchi, 2018)
Figure 1: Tumor metastasis and invasion and immunosuppressive tumor microenvironment. (Sterner, 2021) CAR-T cell
trafficking to tumors may be hampered by aberrant tumor blood vasculature with pericyte detachment, deregulation of
chemokine-chemokine receptor interaction, extracellular matrix (ECM) protein deposition by cancer-associated fibroblasts
(CAF), MDSCs, Tregs, and encounters with TAMs.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
174
4 FUTURE DEVELOPMENT
4.1 Reduction of CAR-T cell-associated
Toxicities
Cytotoxicity can be reduced by altering the affinity of
CAR-T cell antigen binding domains. In this way,
tumor cells can increase their response to higher
concentrations of antigen and achieve higher levels of
activation. (Sterner, 2021) As a result, decreased
antigen affinity is predicted to avoid targeting healthy
tissues with low levels of antigen. Compared with
antigen binding regions with low affinity, antigen
binding regions with micromolar affinity were more
selective for tumors with higher antigen expression
levels. (Liu, 2015) The 4-1BB domain receptor was
associated with reduced toxicity and increased T cell
tolerance, while the CD28 costimulatory domain was
associated with CAR-T cell activity. The 4-1BB
costimulation domain can be specifically targeted at
tumors with high burden and antigen density. In
contrast, in the low affinity antigen binding domain
CAR, the CD28 costimulatory domain may be
required to reach the desired T cell activation
threshold. (Salter, 2018)
Changes in hinge and transmembrane regions that
activate CAR-T cells can also affect cytokine
production. For example, CD8-derived hinge and
transmembrane amino acid sequence changes can
reduce cytokine levels and reduce THE proliferation
of CAR-T cells targeting CD19 CAR93.These
improved hinged and transmembrane region CARs
resulted in complete remission in 50% of B-cell
lymphoma patients in a Phase 1 clinical study, so
optimizing these domains may be an effective way to
reduce toxicity. (Ying, 2019)
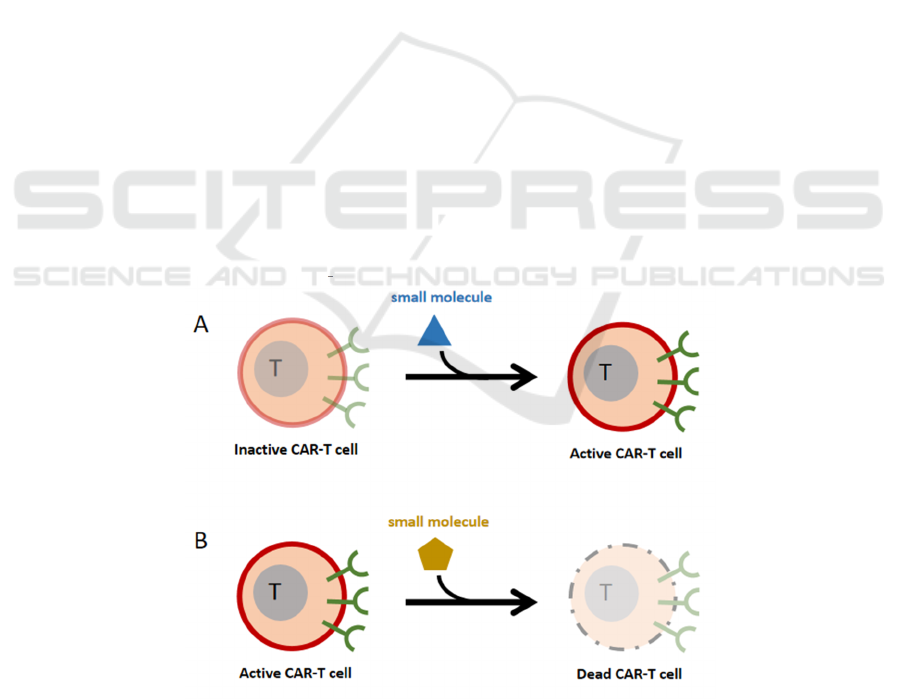
4.2 ON-Switch Control Mechanisms
Adoptive transfer of T-cells expressing the CAR has
yielded extraordinary in the treatment of B-cell
malignancies. Other cancers are less responsive to
this technique. In the case of solid tumors, CAR T-
cell metabolic fitness must be ideal in order for them
to reach the tumor and carry out their cytolytic
activity in an often-hostile environment. (Pellegrino,
2020)
However, there is a risk that shutting off all CAR-
T cells in a patient may allow residual tumor cells to
proliferate unregulated and fast. A mechanism was
discovered to switch off CAR-T cells selectively,
using a small molecule-controlled caspase that
promotes T cell death. (Ciceri, 2009) Another way to
supplement is to keep the cells dormant until the small
molecule drug signalling is introduced (Fig. 2). This
ON switch provides for titratable T cell activity
regulation (dial-up or down). When certain "no-kill"
ligands are found, another strategy is to develop
negatively regulated co-receptors that can overcome
the death response. (Fedorov, 2013)
Figure 2: ON-switch control mechanisms. (Wu, 2015) (A). The small molecule medication signal is used to trigger CAR-T
cell active. (B). CAR-T cells are switched off selectively using a tiny chemical that causes T cell death.
Cancer Immunology: CAR T-Cell Therapy on Renal Cell Carcinoma
175

4.3 Improvement of CAR-T cells
Trafficking
Tumor and T cell metabolism, via modulating tumor
microenvironment and T cell destiny and activity,
have now been discovered to function in determining
immune response. CAR-T cell treatment can be
enhanced by focusing on this element. The expression
of chemokine receptors on CAR-T cells is a recently
established technique that looks to dramatically
increase CAR-T cell trafficking. (Whilding, 2019) In
animal models, CAR-T cells targeting the fibroblast
activating protein (FAP) exhibited improved
cytotoxic efficacy by decreasing tumor fibroblasts.
(Wang, 2013)
5 CONCLUSION
CAR T-cell therapy on RCC has showed its effect in
treating the solid tumor or reducing the “on-target”
effect. However, its limitation still needs sustainable
investigation to improve the efficacy. Currently,
some studies have given ideas to reduce its toxicity
and improvement of trafficking. Therefore, these
methods may take in consideration for their
application on CAR T-cell therapy on RCC. The
approval of designated CAR T-cell therapy for
specific cancer has confirmed its importance in
certain tumors as the first-line treatment. It is
encouraging to have more CAR T-cell therapy related
to cancer treatment to broaden the horizons.
REFERENCES
Brudno, J. N., & Kochenderfer, J. N. (2016). Toxicities of
chimeric antigen receptor T cells: Recognition and
management. Blood, 127(26), 3321–3330.
Bonifant, C. L., Jackson, H. J., Brentjens, R. J., & Curran,
K. J. (2016). Toxicity and management in car T-cell
therapy. Molecular Therapy - Oncolytics, 3, 16011.
Brentjens, R., Yeh, R., Bernal, Y., Riviere, I., & Sadelain,
M. (2010). Treatment of chronic lymphocytic leukemia
with genetically targeted autologous T cells: Case report
of an unforeseen adverse event in a phase I clinical trial.
Molecular Therapy, 18(4), 666–668.
Casucci, M., Hawkins, R. E., Dotti, G., & Bondanza, A.
(2014). Overcoming the toxicity hurdles of genetically
targeted T cells. Cancer Immunology, Immunotherapy,
64(1), 123–130.
Chin, A. I., Lam, J. S., Figlin, R. A., & Belldegrun, A. S.
(2006). Surveillance strategies for renal cell carcinoma
patients following nephrectomy. Reviews in urology,
8(1), 1–7.
Ciceri, F., Bonini, C., Stanghellini, M. T. L., Bondanza, A.,
Traversari, C., Salomoni, M., Turchetto, L., Colombi,
S., Bernardi, M., Peccatori, J., Pescarollo, A., Servida,
P., Magnani, Z., Perna, S. K., Valtolina, V., Crippa, F.,
Callegaro, L., Spoldi, E., Crocchiolo, R., . . . Bordignon,
C. (2009). Infusion of suicide-gene-engineered donor
lymphocytes after family haploidentical haemopoietic
stem-cell transplantation for leukaemia (the TK007
trial): a non-randomised phase I–II study. The Lancet
Oncology, 10(5), 489–500.
Cohen, H. T., & McGovern, F. J. (2005). Renal-Cell
Carcinoma. New England Journal of Medicine, 353(23),
2477–2490.
Curran, K. J., Pegram, H. J., & Brentjens, R. J. (2012).
Chimeric antigen receptors for T cell immunotherapy:
Current understanding and future directions. The
Journal of Gene Medicine, 14(6), 405–415.
Fedorov, V. D., Themeli, M., & Sadelain, M. (2013). PD-1–
and CTLA-4–Based Inhibitory Chimeric Antigen
Receptors (iCARs) Divert Off-Target Immunotherapy
Responses. Science Translational Medicine, 5(215).
Frey, N. V., & Porter, D. L. (2016). Cytokine release
syndrome with Novel Therapeutics for Acute
Lymphoblastic Leukemia. Hematology, 2016(1), 567–
572.
Gupta, K., Miller, J. D., Li, J. Z., Russell, M. W., &
Charbonneau, C. (2008). Epidemiologic and
socioeconomic burden of metastatic renal cell
carcinoma (mRCC): A literature review. Cancer
Treatment Reviews, 34(3), 193–205.
Halford, Z., Anderson, M. K., & Bennett, L. L. (2020).
Axicabtagene CILOLEUCEL: Clinical data for the use
of car T-cell therapy in relapsed and refractory large B-
cell lymphoma. Annals of Pharmacotherapy, 55(3),
390–405.
Hsieh, J. J., Purdue, M. P., Signoretti, S., Swanton, C.,
Albiges, L., Schmidinger, M., Heng, D. Y., Larkin, J., &
Ficarra, V. (2017). Renal cell carcinoma. Nature
Reviews Disease Primers, 3(1).
Ji, F., Zhang, F., Zhang, M., Long, K., Xia, M., Lu, F., Li,
E., Chen, J., Li, J., Chen, Z., Jing, L., Jia, S., Yang, R.,
Hu, Z., & Guo, Z. (2021). Targeting the DNA damage
response enhances CD70 CAR-T cell therapy for renal
carcinoma by activating the cGAS-STING pathway.
Journal of Hematology & Oncology, 14(1).
Lamers, C. H., Sleijfer, S., van Steenbergen, S., van
Elzakker, P., van Krimpen, B., Groot, C., Vulto, A., den
Bakker, M., Oosterwijk, E., Debets, R., & Gratama, J.
W. (2013). Treatment of Metastatic Renal Cell
Carcinoma with CAIX CAR-engineered T cells:
Clinical Evaluation and Management of On-target
Toxicity. Molecular Therapy, 21(4), 904–912.
Lee, D. W., Gardner, R., Porter, D. L., Louis, C. U., Ahmed,
N., Jensen, M., Grupp, S. A., & Mackall, C. L. (2014).
Current concepts in the diagnosis and management of
cytokine release syndrome. Blood, 124(2), 188–195.
Liu, X., Jiang, S., Fang, C., Yang, S., Olalere, D., Pequignot,
E. C., Cogdill, A. P., Li, N., Ramones, M., Granda, B.,
Zhou, L., Loew, A., Young, R. M., June, C. H., & Zhao,
Y. (2015). Affinity-tuned ErbB2 or EGFR chimeric
ICBB 2022 - International Conference on Biotechnology and Biomedicine
176

ntigen receptor T cells exhibit an increased therapeutic
index against tumors in mice. Cancer Research, 75(17),
3596–3607.
Ma, S., Li, X., Wang, X., Cheng, L., Li, Z., Zhang, C., Ye,
Z., & Qian, Q. (2019). Current progress in car-T cell
therapy for solid tumors. International Journal of
Biological Sciences, 15(12), 2548–2560.
Minutolo, N. G., Hollander, E. E., & Powell, D. J.
(2019). The emergence of universal immune receptor T
cell therapy for cancer. Frontiers in Oncology, 9.
Motzer, R. J., Bander, N. H., & Nanus, D. M. (1996). Renal-
cell carcinoma. New England Journal of Medicine,
335(12), 865–875.
Morgan, R. A., Yang, J. C., Kitano, M., Dudley, M. E.,
Laurencot, C. M., & Rosenberg, S. A. (2010). Case
report of a serious adverse event following the
administration of T cells transduced with a chimeric
antigen receptor recognizing ERBB2. Molecular
Therapy, 18(4), 843–851.
Murad, J. P., Kozlowska, A. K., Lee, H. J., Ramamurthy, M.,
Chang, W.-C., Yazaki, P., Colcher, D., Shively, J.,
Cristea, M., Forman, S. J., & Priceman, S. J. (2018).
Effective targeting of TAG72+ peritoneal ovarian
tumors via regional delivery of car-engineered T cells.
Frontiers in Immunology, 9.
Neelapu, S. S., Locke, F. L., Bartlett, N. L., Lekakis, L. J.,
Miklos, D. B., Jacobson, C. A., Braunschweig, I.,
Oluwole, O. O., Siddiqi, T., Lin, Y., Timmerman, J. M.,
Stiff, P. J., Friedberg, J. W., Flinn, I. W., Goy, A., Hill,
B. T., Smith, M. R., Deol, A., Farooq, U., … Go, W. Y.
(2017). Axicabtagene CILOLEUCEL car T-cell therapy
in refractory large B-cell lymphoma. New England
Journal of Medicine, 377(26), 2531–2544.
Pantelidou, C., Sonzogni, O., de Oliveria Taveira, M.,
Mehta, A. K., Kothari, A., Wang, D., Visal, T., Li, M.
K., Pinto, J., Castrillon, J. A., Cheney, E. M., Bouwman,
P., Jonkers, J., Rottenberg, S., Guerriero, J. L., Wulf, G.
M., & Shapiro, G. I. (2019). PARP Inhibitor Efficacy
Depends on CD8+ T-cell Recruitment via Intratumoral
STING Pathway Activation in BRCA-Deficient Models
of Triple-Negative Breast Cancer. Cancer Discovery,
9(6), 722–737.
Pellegrino, M., del Bufalo, F., de Angelis, B., Quintarelli, C.,
Caruana, I., & de Billy, E. (2020). Manipulating the
Metabolism to Improve the Efficacy of CAR T-Cell
Immunotherapy. Cells, 10(1), 14.
Quail, D. F., & Joyce, J. A. (2013). Microenvironmental
regulation of tumor progression and metastasis. Nature
Medicine, 19(11), 1423–1437.
Rohaan, M. W., Wilgenhof, S., & Haanen, J. B. A. G.
(2018). Adoptive cellular therapies: the current
landscape. Virchows Archiv, 474(4), 449–461.
Sadelain, M., Brentjens, R., & Rivière, I. (2013). The basic
principles of chimeric antigen receptor design. Cancer
Discovery, 3(4), 388–398.
Salter, A. I., Ivey, R. G., Kennedy, J. J., Voillet, V., Rajan,
A., Alderman, E. J., Voytovich, U. J., Lin, C.,
Sommermeyer, D., Liu, L., Whiteaker, J. R., Gottardo,
R., Paulovich, A. G., & Riddell, S. R. (2018).
Phosphoproteomic analysis of chimeric antigen receptor
signaling reveals kinetic and quantitative differences
that affect cell function. Science Signaling, 11(544).
Safety and Efficacy of CCT301 CAR-T in Adult Subjects
With Recurrent or Refractory Stage IV Renal Cell
Carcinoma - Full Text View - ClinicalTrials.gov.
(2018). ClinicalTrials.Gov.
https://clinicaltrials.gov/ct2/show/NCT03393936
Schepisi, G., Conteduca, V., Casadei, C., Gurioli, G., Rossi,
L., Gallà, V., Cursano, M. C., Brighi, N., Lolli, C.,
Menna, C., Farolfi, A., Burgio, S. L., Altavilla, A.,
Martinelli, G., & de Giorgi, U. (2020). Potential
Application of Chimeric Antigen Receptor (CAR)-T
Cell Therapy in Renal Cell Tumors. Frontiers in
Oncology, 10, 1909.
Scholler, J., Brady, T. L., Binder-Scholl, G., Hwang, W.-T.,
Plesa, G., Hege, K. M., Vogel, A. N., Kalos, M., Riley,
J. L., Deeks, S. G., Mitsuyasu, R. T., Bernstein, W. B.,
Aronson, N. E., Levine, B. L., Bushman, F. D., & June,
C. H. (2012). Decade-long safety and function of
retroviral-modified chimeric antigen receptor T cells.
Science Translational Medicine, 4(132).
Sterner, R. C., & Sterner, R. M. (2021). Car-T cell therapy:
Current limitations and potential strategies. Blood
Cancer Journal, 11(4).
Watanabe, K., Kuramitsu, S., Posey, A. D., & June, C. H.
(2018). Expanding the therapeutic window for car T cell
therapy in solid tumors: The knowns and unknowns of
Car T cell biology. Frontiers in Immunology, 9.
Wang, L. C. S., Lo, A., Scholler, J., Sun, J., Majumdar, R.
S., Kapoor, V., Antzis, M., Cotner, C. E., Johnson, L. A.,
Durham, A. C., Solomides, C. C., June, C. H., Puré, E.,
& Albelda, S. M. (2013). Targeting Fibroblast
Activation Protein in Tumor Stroma with Chimeric
Antigen Receptor T Cells Can Inhibit Tumor Growth
and Augment Host Immunity without Severe Toxicity.
Cancer Immunology Research, 2(2), 154–166.
Whilding, L., Halim, L., Draper, B., Parente-Pereira, A.,
Zabinski, T., Davies, D., & Maher, J. (2019). CAR T-
Cells Targeting the Integrin αvβ6 and Co-Expressing
the Chemokine Receptor CXCR2 Demonstrate
Enhanced Homing and Efficacy against Several Solid
Malignancies. Cancers, 11(5), 674.
Wu, C.-Y., Roybal, K. T., Puchner, E. M., Onuffer, J., &
Lim, W. A. (2015). Remote control of therapeutic T cells
through a small molecule–gated chimeric receptor.
Science, 350(6258).
Yamauchi, M., Barker, T. H., Gibbons, D. L., & Kurie, J. M.
(2018). The fibrotic tumor stroma. Journal of Clinical
Investigation, 128(1), 16–25.
Ying, Z., Huang, X. F., Xiang, X., Liu, Y., Kang, X., Song,
Y., Guo, X., Liu, H., Ding, N., Zhang, T., Duan, P., Lin,
Y., Zheng, W., Wang, X., Lin, N., Tu, M., Xie, Y.,
Zhang, C., Liu, W., … Chen, S.-Y. (2019). A safe and
potent anti-cd19 car T cell therapy. Nature Medicine,
25(6), 947–953.
Yu, M. C., Mack, T. M., Hanisch, R., Cicioni, C., &
Henderson, B. E. (1986). Cigarette Smoking, Obesity,
Diuretic Use, and Coffee Consumption as Risk Factors
for Renal Cell Carcinoma. JNCI: Journal of the National
Cancer Institute, 77(2), 351–356.
Cancer Immunology: CAR T-Cell Therapy on Renal Cell Carcinoma
177
