
Introduction to Retrosynthesis: Approach to Do Disconnection and
Practical Methods to Deal with Complex Molecules
Xinyu He
1,*
and Yuchen Chang
2
1
Sendelta International Academy, Shenzhen, 518108, China
2
The College of Arts and Sciences, University of Washington, Seattle, 98195-4550, U.S.A.
Keywords: Disconnection, Sythons, FGI, FGR, FGA, Dioxygentation Patterns, Synthetic Equivalent.
Abstract: Retrosynthesis analysis is one of the most important ways to do full synthetic routes designing. All of its
concept mainly include disconnection approach and sythons analysis respectively. In this passage, we want
to introduce some of the effective approaches to do disconnection and explain the concept to the people who
want to learn organic synthesis in the future or interested in this area. All about how to do disconnection,
how to find and use synthetic equivalent, how to deal with one and two groups, Electrocyclic, and Illogical
disconnection, how to use FGI, FGA, FGR to make those molecules which cannot be disconnected become
possible, and how to deal with Dioxygentation Patterns etc. Also, at the end, we will discuss some examples
that could enhance the memories.
1 INTRODUCTION
Imagine you're gluing bricks together when you see
a photo of the finished artwork, and that's the end
product. What is the first thing that springs to mind?
The answer is to locate the materials you require and
tie them according to the instruction book. Yes, this
is the conventional technique, as well as how we
normally think about synthesis. Retrosynthesis, on
the other hand, will take the end product and
imagine them into the fragment we already have,
and it is similar to the 'finding materials' step we
discussed before. Retrosynthesis may be thought of
in this way at its most fundamental level.
Retrosynthesis, often known as "the
disconnection approach," is an analytical process in
which a targeted organic molecule is deconstructed
or fragmented to obtain starting material, or
"Synthon". For a long term, many people have used
this train of thought to design their way to synthesis,
but there was not a clear definition. However, in
1964, Prof. Elias J. Corey, who was awarded Nobel
Prize in chemistry due to his great contribution to
synthetic organic chemistry. He was the first to
formalize 'Retrosynthesis' this concept in his book
‘The Logic of Chemical Synthesis’ (Tutor, 2020;
Corey, 1995). It provided different ideas to
synthesize single and complicated target molecules.
For some extremely complex molecules, the basic
goal is to generate precursors that correspond to
available starting materials. In other words,
retrosynthetic analysis is directed towards molecular
simplification. Often, a synthesis will have more
than one possible synthetic route. Retrosynthesis is
well suited for discovering different synthetic routes
and comparing them logically and straightforwardly.
Retrosynthetic analysis is a problem-solving
technique for transforming the structure of a
synthetic target molecule to a sequence of
progressively simpler structures along a pathway
which ultimately leads to a simple or commercially
available starting material for chemical synthesis
(Corey, 1995; Corey, 1988; Retrosynthetic analysis).
Take letters as examples:
Forward Synthesis Retrosynthesis
*A + B → AB *AB => A + B
In order to get AB, A and B should be found first.
Furthermore, retrosynthetic analysis is particularly
effective because there are so many different
intellectual paths to pursue (Wang, 2022). By
accessing its multiple possibilities of approaching
routes, the most cost-effective, environmentally
friendly, and concise path will be selected (Dmitrii A
He, X. and Chang, Y.
Introduction to Retrosynthesis: Approach to Do Disconnection and Practical Methods to Deal with Complex Molecules.
DOI: 10.5220/0012004000003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 153-159
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
153
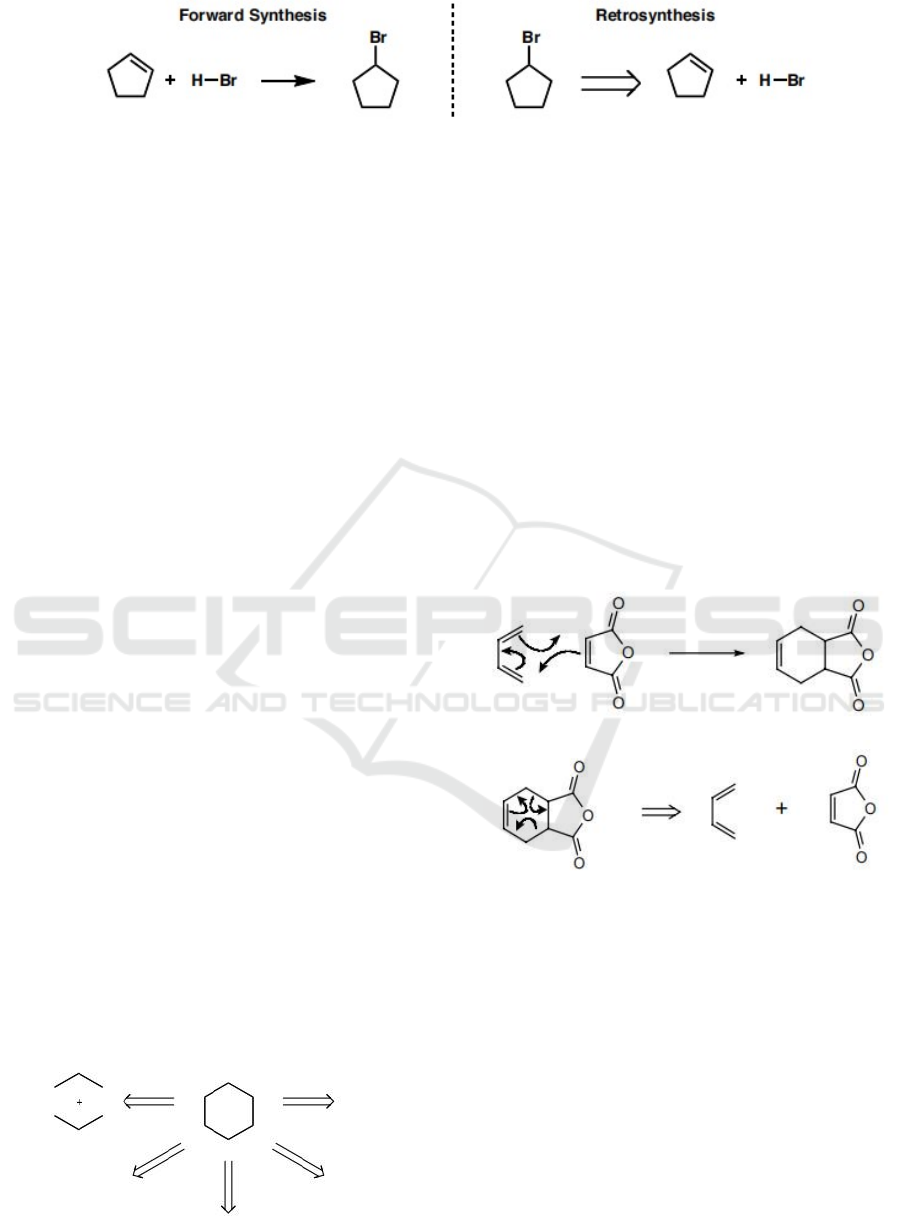
(A) (B)
Figure 1: The example of the forward (A) and reverse (B) process of the hydrobromination of olefins.
, 2020). Take letters as examples again. In order to
gain molecule ABCD, several ways to synthesis can
be taken:
Ⅰ. A + B → AB => AB + C → ABC =>
ABC + D → ABCD
Ⅱ. A + B →AB => C + D → CD => AB +
CD → ABCD
...
As can be seen, there are many ways to arrange
and design paths to synthesis. Only thing need to be
considered is comparing the advantages and
disadvantages of each way, and select the best way
to be taken.
2 STARTING TO
DISCONNECTION
In the beginning, we mentioned that we may
consider binding bricks as the way to synthesis.
Now, we begin to disconnect some real molecules.
Disconnection means 'breaking' the bond of a
molecule to generate simpler fragments. Actually,
when disconnection occurs, instead of real bond
breaking, it is more like brainstorm and a process in
your head.
More simply, considering the analogy of a
simple game, cutting things into pieces. The
hexagon below shows that disconnection occurs, this
shape is cut into some fragments as the right
example denoted by the arrow. In figure 2, the two
sticks are broken in the middle, and two lines are
made up and down. There are several ways to cut
this shape into pieces. But we need to obey the rules
that keep the number of points at 6.
Figure 2: Showcase of a simple game.
In a similar way. Consider that this hexagon is a
genuine Cyclohexane. Now you must recognize that
more rules or restrictions have appeared. Following
the same procedure to break the C-C bond.
However, if you want to weaken the C-H bond, you
must keep the skeleton formula's amount of invisible
hydrogen constant. It's similar to breaking bonds,
yet they're fundamentally different. As Figure 3
shows, now consider a relatively complex molecule.
In (1,1), we know a reaction to produce the
molecule, then we can also do disconnection on it.
But this is not the only way to produce the target
molecule or the only way to disconnect. Thus, this
lead us to find or try more reaction mechanisms in a
long term. The more we know, the more simply the
synthesis processes.
FXP 1:
(1,1)
(1,2)
Figure 3: Reaction procedure.
3 APPROACH TO
DISCONNECTION
3.1 One Group Disconnection
By now, we know how to do disconnection. Thus,
we now need to consider how to allocate the
electrons when bonds are broken. This is what we
called bond cleavage. We have 3 situations to
consider---Heterolytic cleavage (Left-side),
Heterolytic cleavage(right-side), and homolytic
FSB 2022 - The International Conference on Food Science and Biotechnology
154
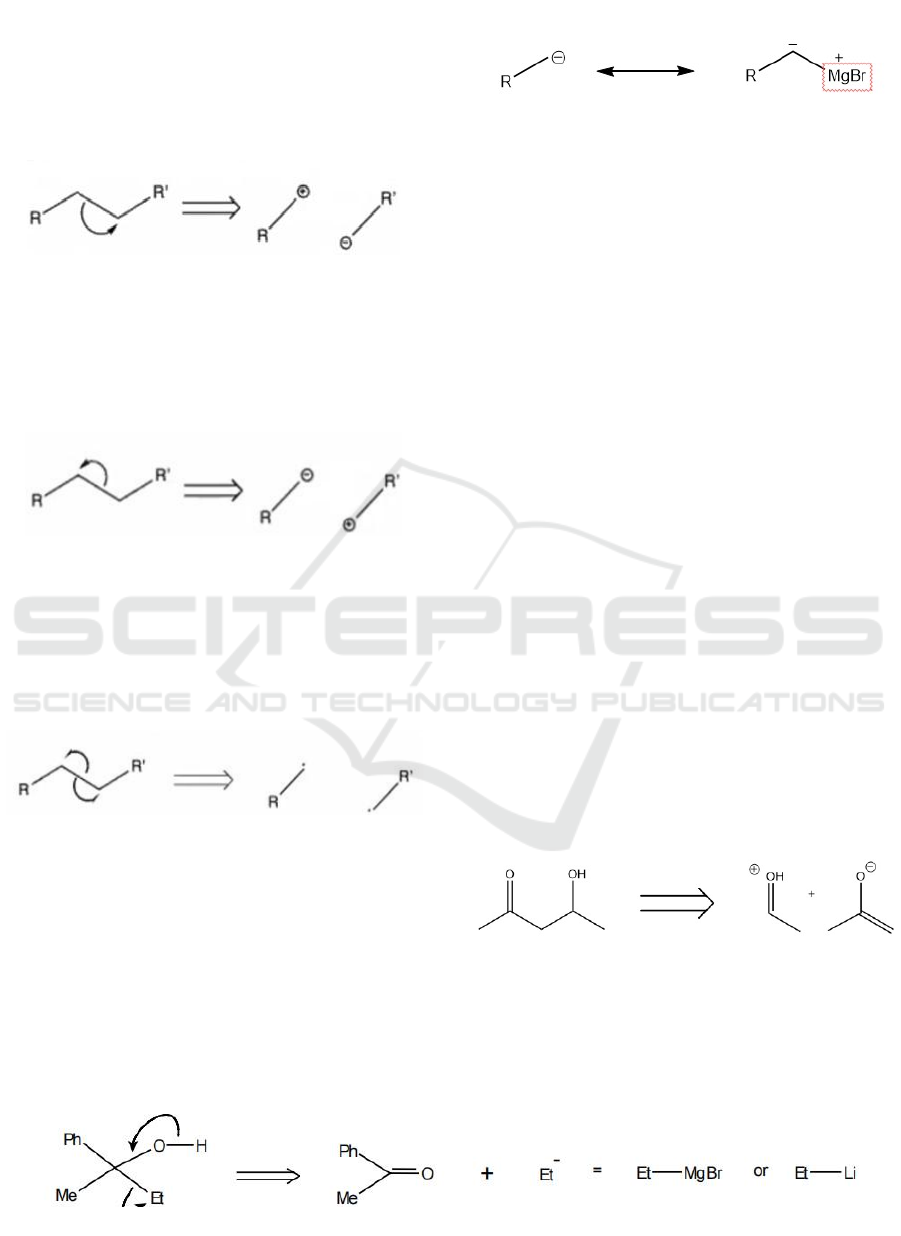
cleavage. In Figure 4, Heterolytic cleavage
(right-side), the arrows show the direction of the
electron's movement. When we break bonds, the two
electrons are removed from the left-side molecule
and pulled to the right-side molecule, making the
left-side molecule cationic and the right-side
molecule anionic.
Figure 4: Heterolytic cleavage (right-side).
In Figure 5, there's another way to assign
electrons. And it is similar to Figure 4. Two
electrons are taken and pulled into the left-side
molecule and make it anionic, and the other side
becomes cationic.
Figure 5: Heterolytic cleavage (left-side).
Otherwise, all of the above are synth ions, except
for the final one. It is homolytic cleavage. When we
break a bond, the electrons split and become free
radicals on both sides. However, it is less common
to make free radical (Figure 5) when bond
disconnecting happens.
Figure 6: Homolytic cleavage.
When we finish our retrosynthesis analysis, it's
not easy to find all of the materials we need,
especially for some very complex molecules. So,
how do we go on with our work? Normally, we will
substitute other groups for the synthons we desire,
and these groups are referred to as synthetic
equivalents. A reagent that performs the function of
a synthon but cannot be utilized itself, usually due to
its instability.
For example:
Figure 7: Equivalent function using Grignard reagent.
In Figure 7, it is obvious to see the carbon on the
left is negative. We always use Grignard reagent to
show an equivalent function when we need to do
synthesis of some complex molecules. Since it
shows a negative property in the middle.
In this example, we use the synthetic equivalent
of the anion, the Grignard reagent or alkyl lithium,
in this case because none of the stable anions are
available. And in real retrosynthesis, when we talk
about Et as a synthon, we're talking about EtMgBr
or EtLi. Cause it will lead us to do disconnection
and the whole synthesis process.
3.2 Two Group Disconnection
So far, we finished the one group disconnection,
then how can we deal with the molecules with more
functional groups? We must now consider the
disconnection of the two groups, and I will
demonstrate how to do so using the examples below.
For example, in Figure 9, we have ketone and
alcohol groups, and there are a lot of ways to do
disconnection. The way we are easy to find is
breaking the carbon in the middle and separating it
into two parts. One is ketone and another is alcohol.
Nevertheless, we have a better way to approach
it. The oxygen's delocalized electrons will offer us a
more appropriate approach to detach. Then we
obtained a positive and a negative synthon which is
more common to find.
Figure 9: Two group disconnection.
Furthermore, we need to know what is FGI,
FGA and FGR. And all of these are significant
methods to do disconnection.
Figure 8: Example of retrosynthesis.
Introduction to Retrosynthesis: Approach to Do Disconnection and Practical Methods to Deal with Complex Molecules
155
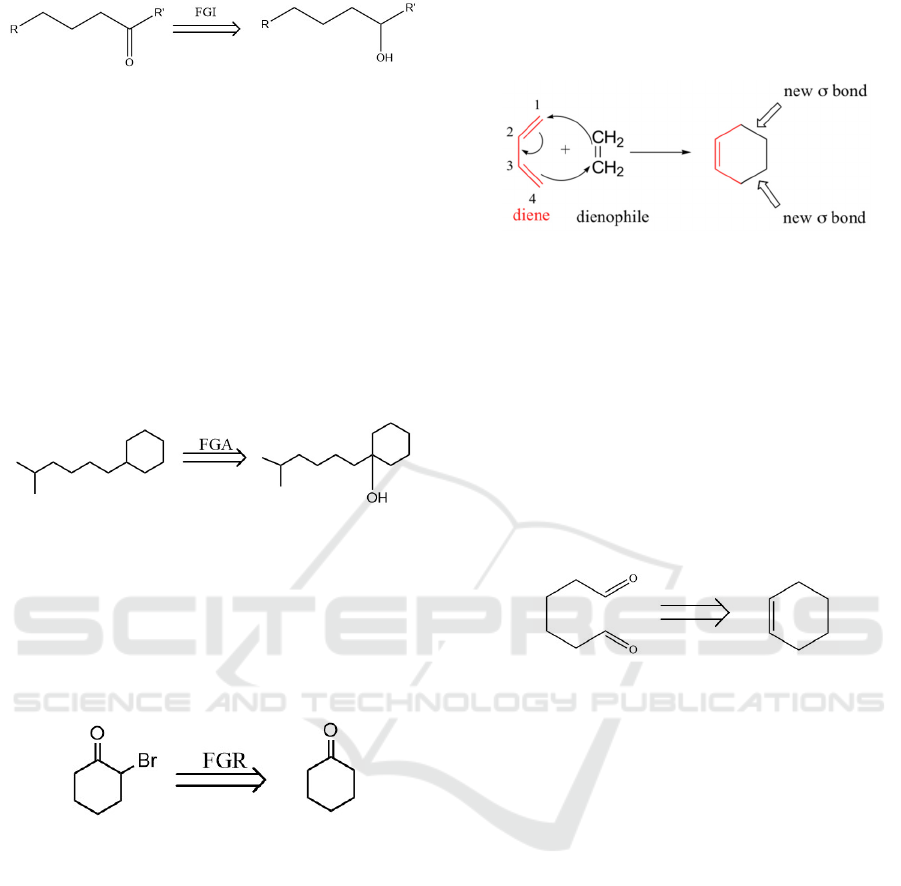
Figure 10: Example of FGI.
FGI fully spelled Functional Group
interconversion. For this step, we always change
our functional group to others in order to make sure
we can disconnect the bond successfully. In Figure
10, the Carbonyl group was converted to an
Oxhydryl group. And allowing the disconnection to
occur.
FGA is the shorthand of Functional Group
Addition. In real synthesis designing, it is
sometimes necessary to add a functional group in
order to enable the interchange of functional groups
or later cut-off.
Figure 11: Example of FGA.
FGR is an abbreviation for Functional Group
Removal. It means remove a functional group from
a molecule. No like FGI, we need to remove some
certain functional groups and make sure we can do
disconnection. For instance, in Figure 12, we cut off
Br group by doing FGR.
Figure 12: Example of FGR.
3.3 Electrocyclic Disconnection
In this part, I will explain to you a very important
reaction mechanism that can dispose the
complicated molecules effectively. It is Diels -
Alder Reaction. And when we do retrosynthesis, it
is the typical 4+2 disconnection.
Figure 13 below, it is the basic way to display
how the process was. The left diene gets 2 double
bonds, and the dienophile only has one double bond.
As the arrow denotes, electrons from the dienophile
attack the carbon (1) and make a bond between the
diene and dienophile over there.
The double bond on the top of the diene
(between C (1) and C (2)) will further relocate to
carbon (2) and (3), then the double bond below will
connect with dienophile. All the six π bonds will
shift at the same time, and eventually formed
cyclohexene.
Figure 13: Basic process of electrocyclic disconnerction.
3.4 Illogical Disconnection (Connection)
Illogical disconnection is not a common way to do
disconnection, since it is making a bond rather than
breaking a bond. The reaction below is a
representative example to introduce this method.
Using the double bonds on the aldehydes and
combining them to form a single double bond and
abandon the oxygen. Finally, we'll get cyclohexene.
We may achieve this by using ozone, and the
reaction is known as Ozonolysis.
Figure 14: Example of illogical disconnection.
3.5 Dioxygentation Patterns
In this part, it is clear to see that the main purpose of
this process is dealing with ‘oxygen’. We will face
to a following of reactions, and I think the basic
synthetic way is to simplify problems into the model
we have worked out in the past.
3.6 1dioxygentation Patterns
As shown, once we see these patterns, we should
convert them into OH-groups by using FGI, then
OH-groups could disconnect to olefin.
3.7 2dioxygentation Patterns
For these molecules, FGI is used and we will have a
carbonyl and a hydroxyl group. Then we can repeat
the steps (Two groups disconnection) we mentioned
before.
FSB 2022 - The International Conference on Food Science and Biotechnology
156
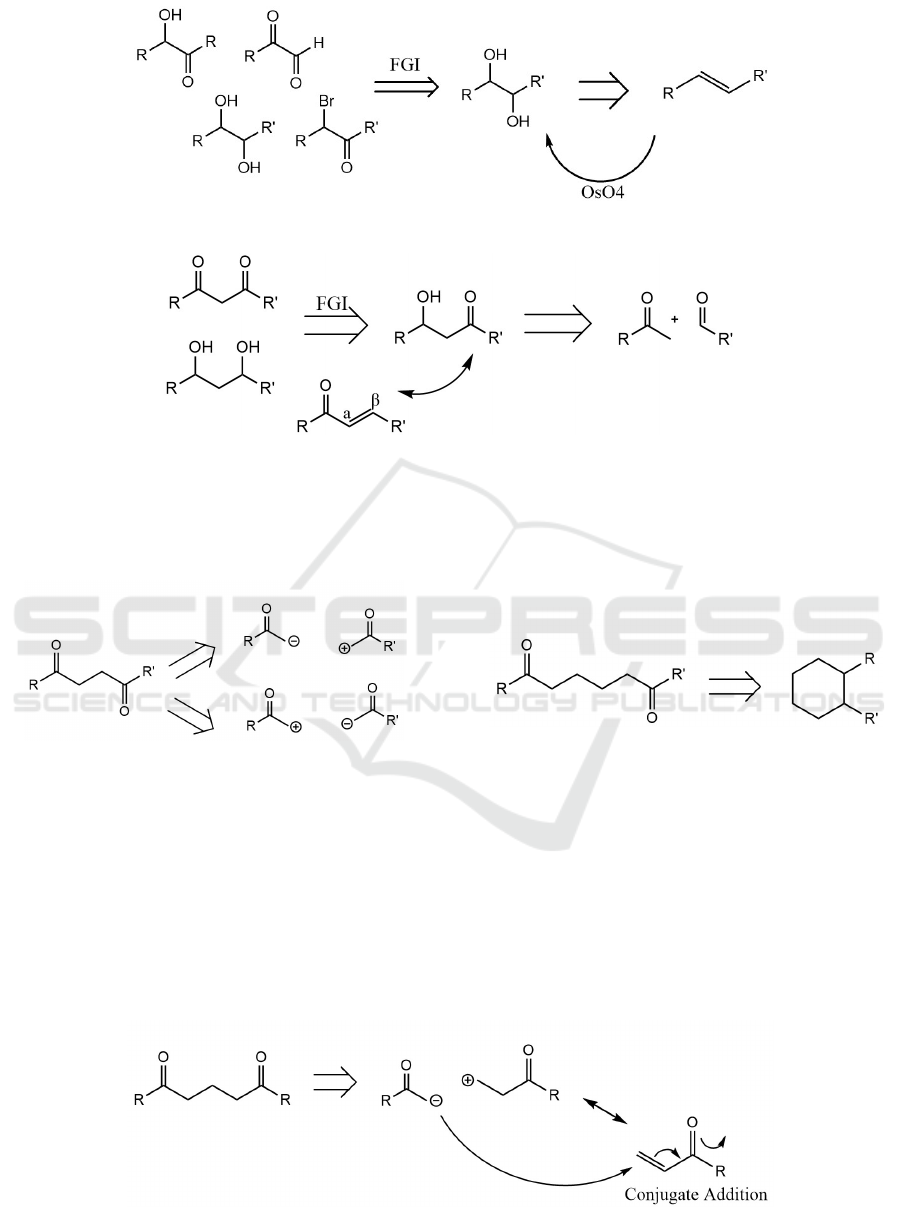
Figure 15: 1dioxygentation patterns.
Figure 16: 2dioxygentation patterns.
3.8 3dioxygentation Patterns
In this part, we need to convert the groups into two
carbonyl groups, and disconnect them into two parts.
Figure 17: 3dioxygentation patterns.
3.9 4dioxygentation Patterns
For these molecules, we want to make di-carbonyl
groups, thus we need to use two carbonyl groups.
And we have equivalent molecules. Using the
conjugate addition mechanism, when these two
molecules come into contact, the anion will attack
the double bond and form the di-carbonyl
compound.
3.10 5dioxygentation Patterns
For these molecules, we need to convert into alkene
with two carbonyl groups and repeat the step
(Illogical Disconnection) again.
Figure 19: 5dioxygentation patterns.
4 FOCUS ON MAXIMIZING
SIMPLIFICATION (FOCUS ON
SYMMETRY)
Efficient and concise synthetic routes based on the
symmetry of the molecule are gaining widespread
attention, and such synthetic routes can be
two-directional synthesis.
Figure 18: 4dioxygentation patterns.
Introduction to Retrosynthesis: Approach to Do Disconnection and Practical Methods to Deal with Complex Molecules
157
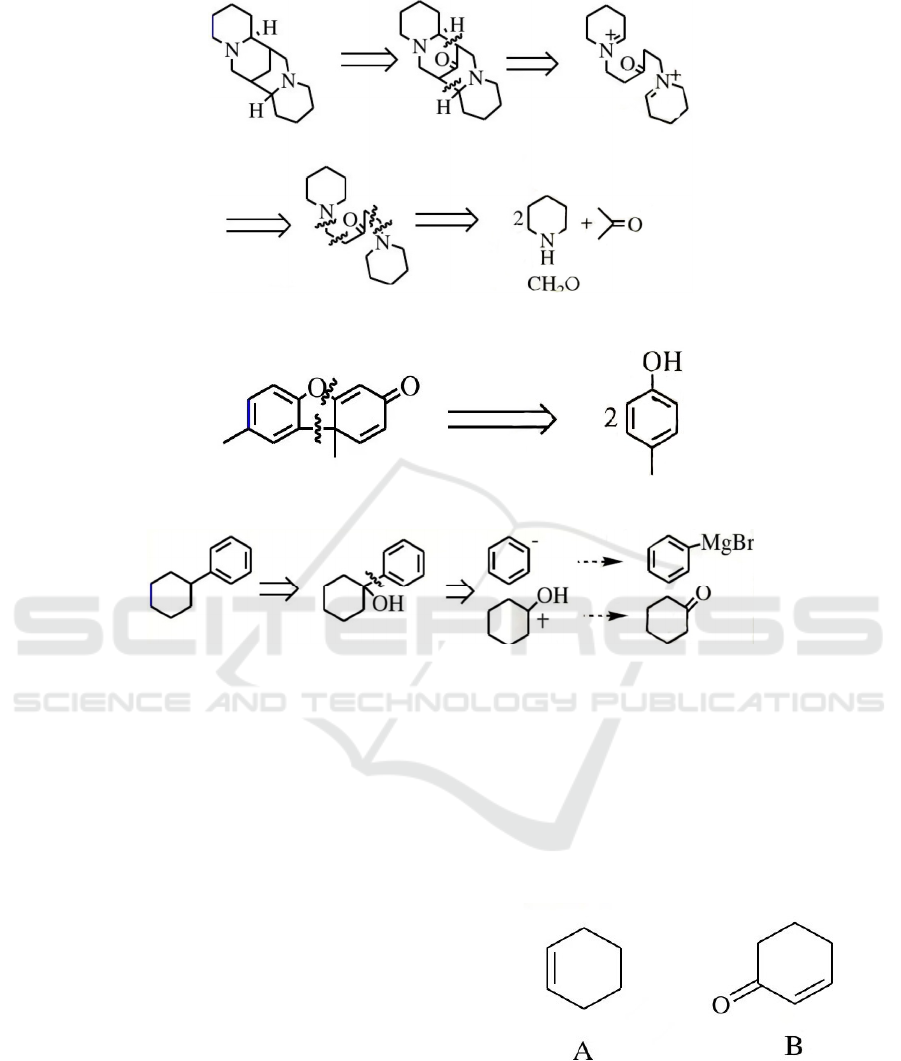
Figure 20: Synthetic routes of two-directional synthesis.
Figure 21: Process of reaction.
Figure 22: Process of reaction.
eg: sparteine has symmetry by introducing the
carbonyl group on the central methylene and then
symmetrically using the inverse Mannich cut on
both sides to highly simplify the molecule. This
yields three basic raw materials: piperidine,
formaldehyde, and acetone, all of which are
synthesized by the classical standard reaction.
Some target molecules do not have symmetry per
se, but have potential symmetry, and after certain
inverse synthetic transformations, a symmetric
molecule or a symmetric synthetic route can be
obtained, thus simplifying the synthetic design. For
example, there is no symmetry factor in Pummerer's
ketone molecule, but the two radicals obtained after
the cut is from the same precursor.
Addition of auxiliary functional groups followed
by cleavage (FGR).
Some target molecules can be cut off only after
the addition of appropriate functional groups to find
the correct route of synthesis. In the example figure
22, there is no functional group, and when a
hydroxyl group is introduced into the cyclohexyl
group, it can be cut further.
In the retrosynthetic transformation of the target
molecule, it is required that some necessary
structural unit exists in the target molecule, and only
when such a structural unit exists or such a
substructure can be generated, can the target
molecule be effectively simplified and
easy-to-access starting materials be deduced, such as
the following structural units of A, B is the basic
retron of Diels-Alder reaction, Robinson formation,
respectively.
Figure 23: Structural units.
The core problem of retrosynthesis analysis is
transformation, and inverse retron and synthon are
two aspects of this core problem, the former is the
necessary structural unit for transformation, and the
latter is the structural unit to be obtained by
transformation.
FSB 2022 - The International Conference on Food Science and Biotechnology
158

5 CONCLUSION
The trend of organic synthesis is not to blindly
pursue new compounds, but to design and synthesize
compounds that are expected to have excellent
properties or have great significance. The
retrosynthetic analysis method takes a complex
synthetic problem and decomposes it into several
simple synthetic problems by dissecting it step by
step from tedious to simple through the inverse
method, and then forms a synthetic route from
simple to complex molecules. It makes many
difficult applications possible and saves research
costs, such as the application of drug synthesis. Such
a mode of thinking can also be applied to different
disciplines, such as synthetic aperture radar for
meteorological and atmospheric motion studies,
mathematical studies of inverse matrices, economic
reverse logistics.
REFERENCES
Dmitrii A. Shabalin, Elena V. Ivanova, Igor’ A. Ushakov,
Elena Yu. Schmidt, Boris A. Trofimov, Retrosynthetic
Analysis of α-Alkenyl-β-Diketones: Regio- and
Stereoselective Two-Step Synthesis of Highly Arylated
Representatives from Acetylenes, Ketones, and Acyl
Chlorides, The Journal of Organic Chemistry, Volume
85, Issue 13, 2020, Pages 8429-8436.
E. J. Corey, X-M. Cheng (1995). The Logic of Chemical
Synthesis. New York: Wiley. ISBN 0-471-11594-0.
E. J. Corey (1988). "Retrosynthetic Thinking - Essentials
and Examples". Chem. Soc. Rev. 17: 111-133.
doi:10.1039/CS9881700111.
Retrosynthetic analysis https://www.chemeurope.com/en
/encyclopedia/Retrosynthetic_analysis.html#_note-0/
Tutor,13 Jul, 2020, Retrosynthesis, https://onlineorganic
chemistrytutor.com/retrosynthesis/
Wenlong Wang, Qilei Liu, Lei Zhang, Yachao Dong, Jian
Du, RetroSynX: A retrosynthetic analysis framework
using hybrid reaction templates and group
contribution-based thermodynamic models, Chemical
Engineering Science, Volume 248, Part B, 2022,
117208
Introduction to Retrosynthesis: Approach to Do Disconnection and Practical Methods to Deal with Complex Molecules
159
