A Preliminary Introduction to the Nature of Phenylalanine and Some
Basic Reactions Related to It
Xiaoyue Shi
St John’s College Cardiff, Cardiff, CF3 5YX, U.K.
Keywords:
Phenylalanine, Biosynthesis, Metabolism Route, Phenylketonuria.
Abstract: The primary object of this study is to survey the nature of phenylalanine, and to study some basic reactions
or other information related to it. This is a working which is based on theoretical research. Detailed
information has been acquired about dehydration and condensation reaction, ionisation in water, important
metabolisms, or a disease called phenylketonuria and so on. The work has contributed to our present
understanding of this aromatic amino acid, and is a good reference for beginners in Chemistry who are
interested in this topic.
1 INTRODUCTION
In recent years, with the deepening of biochemical
research, more and more properties of amino acids
have been recognized by people.
Phenylalanine(C
9
H
11
NO
2
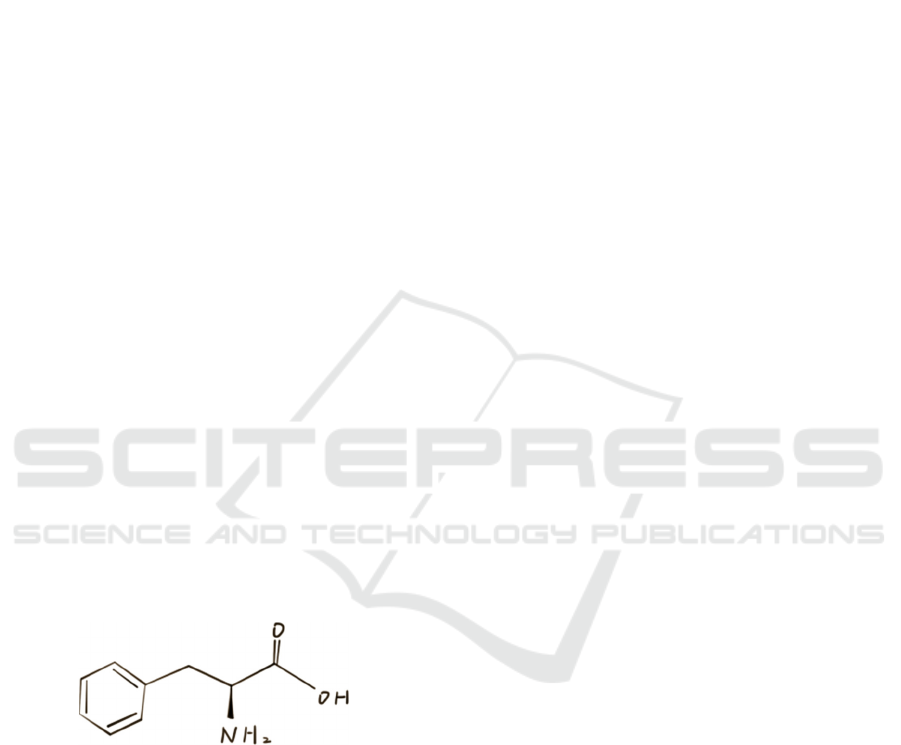
), with the bonding
structure shown in Figure 1, as an aromatic amino
acid and one of the essential amino acids that human
bodies need, is also being developed in more and
more fields, such as biosynthesis, microbio
fermentation, treatment of relevant diseases.
Figure 1: The Bonding Structure of Phenylalanine.
Unlike those professional researches, this article
will provide an introduction to phenylalanine by
giving a dossier of important information and facts.
In the work, we introduce phenylalanine from four
main aspects: the mode of obtaining, the contrast of
solubility, basic reactions related and the metabolism
in the human body. This work is a good source of
useful information for those who are just beginning
to do research in relavant field.
2 HOW IS PHENYLALANINE
OBTAINED?
2.1 In Nature
Figure 2 shows the biosynthesis of phenylalanine.
Aromatic amino acid, which includes phenylalanine,
tyrosine, and tryptophan, can only be produced by
plants and microbes. There is a common path in their
synthesis, and shikimic acid is a common precursor
to the synthesis of these aromatic acid. In this case we
can call the common pathway shikimic acid pathway,
that is, shikimic acid as the starting material until the
formation of chorismic acid. The aromatic amino acid
production pathway branches at chorismic acid.
There are two pathways after this branch point: one
to generate phenylalanine and tyrosine, and the other
to form tryptophan. Chorismic acid is transformed to
prephenic acid by chorismate mutase, which is then
dehydrated and decarboxylated to form
phenylpyruvic acid for phenylalanine production
(Xiao, 2014).
From phenylpyruvic acid to L-phenylalanine, the
molecules react under transamination. The
mechanisms are shown in Figure 3.
138
Shi, X.
A Preliminary Introduction to the Nature of Phenylalanine and Some Basic Reactions Related to It.
DOI: 10.5220/0012003700003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 138-142
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
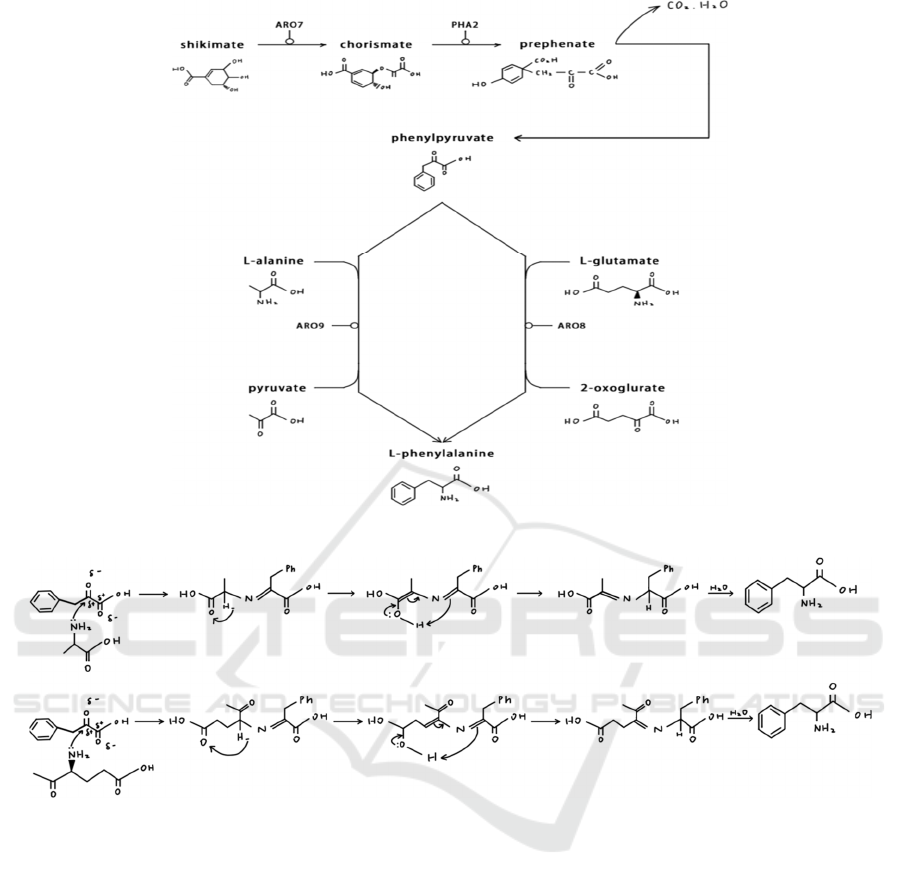
Figure 2: The Biosynthesis of Phenylalanine
Figure 3: The Mechanisms for Synthesis of Phenylalanine (Hu, 1985).
2.2 None-Nature
Chemical synthesis, enzymatic technique, microbial
fermentation, and asymmetric hydrogenation are the
four primary approaches of non-natural
phenylalanine synthesis.
As for chemical synthesis, somebody has already
done it successfully as early as 1882. However, the
route was too long and expensive.
For enzymatic method, there are two ways:
Transaminase is used to make phenylalanine from
phenylpyruvic acid, or phenylalanine ammonia-lyase
is used to make phenylalanine from cinnamic acid.
There are several advantages such as higher product
concentration, fewer steps, and stronger production
capacity. However, the cost of raw materials is
excessive.
For microbial fermentation, there are two types:
Precursor fermentation and direct fermentation. The
former uses some intermediates in the pathway of
amino acid biosynthesis as raw materials for
fermentation to produce amino acids. Unfortunately,
the cost is too high. In the latter, amino acids are
produced directly from inorganic carbon and nitrogen
sources, which is cheap and easy to obtain.
Furthermore, because the reaction may be performed
at normal temperature and pressure, this is one of the
most common methods for producing amino acids
(Lu, 2011; Pan, 1996).
For asymmetric hydrogenation, it adds a molecule
of hydrogen across the double bond between α and β
carbon of the amino acid. This is a method that people
use extensively. This work was also recognised with
the Nobel Prize in Chemistry (Li, 2002).
A Preliminary Introduction to the Nature of Phenylalanine and Some Basic Reactions Related to It
139
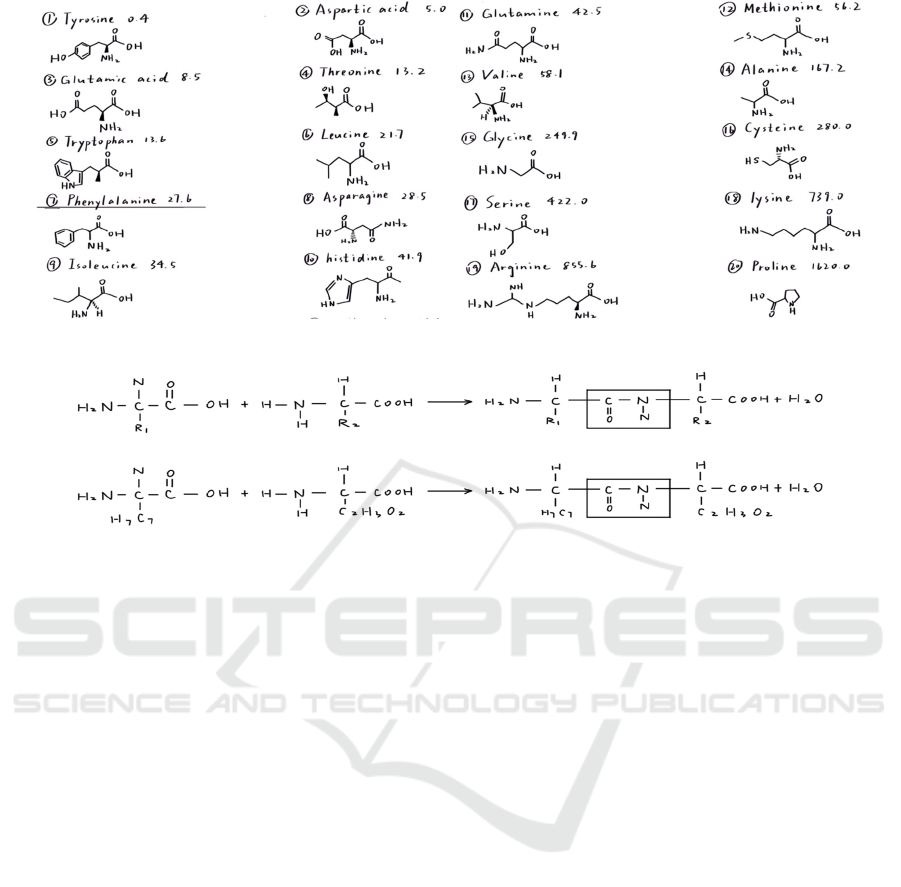
Figure 4: Solubility of 20 Amino Acids.
Figure 5: The Dehydration and Condensation Reaction of Two Amino Acid Molecules.
3 COMPARISON OF
SOLUBILITY OF 20 AMINO
ACIDS
Figure 4 shows the solubility of these amino acids,
which is one measure of their characteristics. Even
though there are only 20 of them, because they have
such a wide range of properties, when starting to
combine them, an infinite number of possibilities can
be made which leads to quite a considerable number
of differences in properties. Nature is guiding
evolution by property from an evolutionary
standpoint. Whatever the thing is, it needs to do
something more efficiently and more effectively so
that it can survive. And the fact that it has so many
options feeds into this evolutionary development,
which is beneficial to nature. For instance, nature
finds a way to resist to medicine over time. The
organism is trying to overcome whatever fighting.
Often, it is just a single amino acid reaction.
4 SOME BASIC REACTIONS
4.1
The Dehydration and Condensation
Reaction of Two Amino Acid
Molecules
This is a reaction that join two amino acid molecules
together. The general formula of reaction and the
reaction of phenylalanine are shown in Figure 5.
The condensation reaction between two amino
acid molecules is known as amino acid dehydration
and condensation. Amino and carboxyl groups also
generate a water molecule together.
The bonds that bind two amino acid molecules are
called peptide bonds. A compound which is formed
by the dehydration and condensation of two amino
acid molecules is called a dipeptide, which contains a
peptide bond. This process can be performed
hundreds of thousands of times to produce a molecule
that is extremely large. A polypeptide is a substance
that is generated by the dehydration and condensation
of several amino acid molecules and contains
multiple peptide linkages. A peptide chain is a type of
polypeptide that has a chain structure. A protein
molecule with a specific spatial structure can be
formed by twisting and folding peptide chains. A cell
contains hundreds of thousands of amino acids of
various kinds. The order of amino acids varies greatly
FSB 2022 - The International Conference on Food Science and Biotechnology
140

Figure 6: Reactions for Ionization of Phenylalanine in Water.
Figure 7: The Concentration of Different Ions in the Process of Phenylalanine Ionization (Fu, 2010).
when forming a peptide chain, and the way of
twisting, folding, and forming the spatial structure
also differs in thousands of ways. As a result, protein
molecules have a wide range of structures (Chen,
2015).
4.2
Ionization of Phenylalanine in
Water
The presence of R
-
NH
2
and R
-
COOH in amino acids
makes them amphoteric. According to the quantity of
amino and carboxyl groups linked, all amino acids
can be categorised into three types: neutral amino
acid, acidic amino acid, and basic amino acid.
For phenylalanine, it is a neutral amino acid.
Figure 6 shows the two steps of ionization.
When pH < pKa
1
, the main form in solution is
H
3
N
+
C
8
H
8
COOH.
When pH = pKa
1
, there is about 50% of
H
3
N
+
C
8
H
8
COOH and 50% of H
3
N
+
C
8
H
8
COO
-
.
When pKa
1
< pH < pKa
2
, the main form in
solution is H
3
N+C
8
H
8
COO
-
.
When pH = pKa
2
, there is about 50% of
H3N
+
COO
-
and 50% of H
2
NC
8
H
8
COO
-
.
When pH > pKa
2
, the main form in solution is
H
2
NC
8
H
8
COO
-
(Qie, 2000).
This can be converted into a graph, as shown in
Figure 7.
5 METABOLISM IN THE HUMAN
BODY AND A RELATED
DISEASE
5.1
Metabolism Route
Figure 8 shows the metabolism route of
phenylalanine. Inside the human body, most of the
phenylalanine are oxidized to tyrosine by the
catalysis of phenylalanine hydroxylase, and together
with tyrosine, they synthesize important
neurotransmitters and hormones, and participate in
the body’s glucose and fat metabolism. The
remaining phenylalanine is converted into
phenylpyruvic acid.
1
A Preliminary Introduction to the Nature of Phenylalanine and Some Basic Reactions Related to It
141
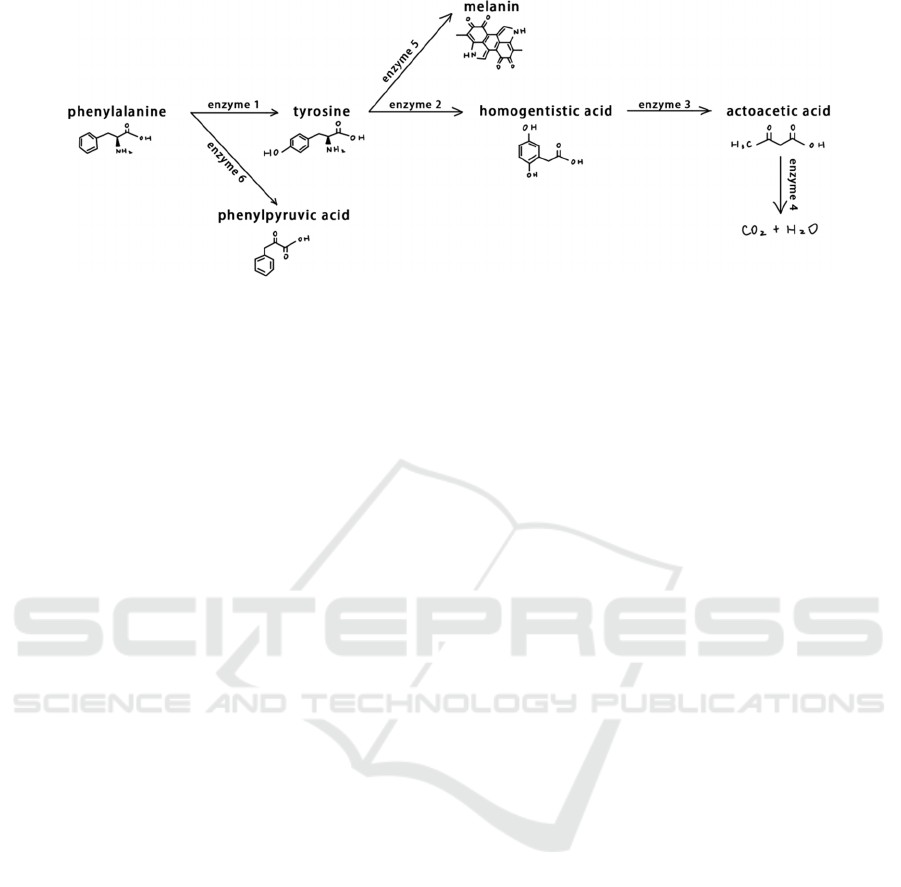
Figure 8: The Metabolism Route of Phenylalanine.
5.2 The Disease: Phenylketonuria
Mutations in any of the genes that encode the
enzymes needed can lead to defects in the activity of
those enzymes, which may lead to metabolic
disorders in phenylalanine.
There are two types of phenylketonuria:
deficiency of PKU or deficiency of BH
4
.
Phenylalanine cannot be turned into tyrosine in the
former, resulting in brain cell destruction. However,
the latter not only makes phenylalanine unable to be
converted into tyrosine, it also blocks important
neurotransmitters and therefore exacerbates the
damage to the nervous system. When
phenylketonuria patients are born, they normally
have no abnormalities and are easy to be
misdiagnosed (Zhang, 2022). Symptoms normally
appear between the ages of 3 and 6 months, and they
are most noticeable at the age of one year.
Phenylketonuria patients should avoid
phenylalanine-containing foods.
Phenylalanine (C
9
H
11
NO
2
) can form
phenylpyruvic acid (C
9
H
8
O
3
) under transamination,
which can be further converted into derivatives such
as phenylacetic acid (C
8
H
8
O
2
). At this point, the urine
is full of various metabolites including big amount of
phenylpyruvic acid. That is why the disease is called
phenylketonuria (Xie, 2011).
6 CONCLUSION
In this investigation, the aim is to provide basic
information and important facts of phenylalanine for
beginners on this topic, which includes the way of
obtaining in both nature and non-nature, the
comparison of solubility of 20 amino acids, two
typical reactions and its activity in the human body. It
is unfortunate that the study did not include any in-
depth knowledge, as they are too advanced for
beginners. The insights gained from this study may
be of assistance to future research into certain aspect
of phenylalanine. This would be a fruitful area for
further work.
REFERENCES
Chen YG. (2015). Dehydration Condensation Reaction in
Inorganic Chemistry. University Chemistry (02), 39-
43.
Fu CX. (2010). Relationship between the Existences of
Amino Acids in Solution and PH Values. Journal of
Science of Teachers’ College and University (04),73-
76.
Hu Q. (1985). How to Understand Deamination and
Transamination in Protein Metabolism?. Biology
Teaching (01),26.
Lu J. (2011). Studies on Coordinated Expression of
Multienzyme-genes in Phenylalanine Biosynthesis
Pathway, Inner Mongolia Agricultural University.
https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=
CMFD2011&filename=1011178388.nh
Li Y H. (2002). Advances in Biosynthesis of Phenylalanine.
Letters in Biotechnology (04),296-300.
Pan GL, He XJ, Zheng J. (1996). Biosynthesis of L-
Phenylalanine. Journal of Nanjing University of
Chemical Technology (Natural Science Edition) (01),
89-94.
Qie WJ, Wu YM, Huang HY.(2000).A Discussion on the
Form and Solubility of Amino Acid at Isoelectric Point.
Journal of Shijiazhuang University of Applied
Technology (04),35-39.
Xiao MR, Zhang L, Shi GY.(2014).Improvements of
Shikimic Acid Production in Escherichia Coli with
Ideal Metabolic Modification in Biosynthetic Pathway-
-----A Review. Acta Microbiologica Sinica (01),5-13.
doi:10.13343/j.cnki.wsxb.2014.01.002.
Xie W. (2011). About Phenylketonuria. Science &
Technology Information (13),814-815.
Zhang CF. (2022). Early Screening and Controlling
Phenylketonuria. Health Guide (01),28-29.
FSB 2022 - The International Conference on Food Science and Biotechnology
142
