
Application of Metal–Organic Frameworks-Based Functional
Materials for Gas Separation
Haoming Li
1,†
, Yuehan Lyu
2,†
, Yanqi Shao
3,†
and Tianye Sheng
4*,†
1
Changwai Bilingual School, Changzhou 213100, China
2
The Affiliated International School of Shenzhen University, Shenzhen 518066, China
3
Malvern College Qingdao, Qingdao 266109, China
4
WLSA Shanghai Academy, Shanghai 200433, China
Keywords: MOFs, Functional Materials, Gas Separation, Application.
Abstract: Energy scarcity is becoming the biggest problem of scientific research and industrial production in this
century. And gas separation, as an essential part of production activities, naturally needs to be considered a
more sustainable and environmentally friendly change. As an emerging porous material, metal–organic
frameworks (MOFs) are one of the candidates to replace the traditional distillation process due to their special
structural characteristics, such unique porous structure and adjustable surface properties. Therefore, MOFs-
based functional materials have been widely used for a diverse of various fields, such as fuel cell construction,
antibacterial agent development and gas separation. Due to each type of gas’s chemical/physical properties, a
variety of promising and practical separation functional materials based on MOFs are prepared by using the
advanced laboratory techniques, like UTSA-68 for C
2
H
2
/CO
2
separation, r-FUM 67-MES 33-FCu-MOF for
CH
4
/N
2
and IRMOF-1 for Xe and Ar separation. Herein, this research will not only summarize the current
importance and status of the application of functional materials in the field of gas separation, which include
CH
4
/N
2
, C
2
H
2
/CO
2
, Ar/Kr, Ar/Xe and Xe/Kr.
1 INTRODUCTION
A diverse of different functional materials have been
used for gas separation over the past century, such as
zeolite, which are now being utilized extensively in
industrial development. In any event, developing new
functional materials, such as metal-organic
frameworks (MOFs), is currently making significant
strides forward. Scientists began to realize the
commercial potential of MOFs around the year 1990,
which coincided with several significant
technological advances. For instance, the high
ductility, flexibility and efficient gas separation
offered by MOFs contribute to the material’s high
industrial value. The chemical properties of various
MOFs are different, and some changes typically
influence these differences in temperature and
pressure. As a result, this opens a wide variety of
opportunities for gas separation. Recently, MOFs-
based functional materials have been the subject of
extensive exploration and research (Wang, 2022), and
now their industrial potential is being realized. As
more time passes, MOFs will be used in more official
capacities in industrial applications.
Traditional thermal drive technology is based on
distillation which is relatively energy-intensive by a
continuous cycle of evaporation and condensation.
The separation of similar volatile gas mixtures (e.g.,
hydrocarbons) are processed industrially to obtain the
desired purity. To solve energy shortage and
environmental pollution, more and more attention is
paid to the energy consumption and sustainable
development of the process. The traditional
distillation separation process is bound to be replaced
by a more environmentally friendly and efficient non-
thermal process. Among these, porous materials are
one of the focuses of future development.
MOFs are one of typical porous materials that
involve single metal ions and coordination bonds
connected by organic ligands. In gas separation
applications, MOFs have plenty of benefits based on
their special and unique structures and properties
(Cui, 2021). The crucial characteristic of MOFs that
enables the absorption of several guest gas species is
high surface area, and an efficient procedure will be
viable since less stuff is required. Moreover, MOFs
can accept variable functional sites and highly adjust
the interaction between host and guest species. These
Li, H., Lyu, Y., Shao, Y. and Sheng, T.
Application of Metalâ
˘
A ¸SOrganic Frameworks-Based Functional Materials for Gas Separation.
DOI: 10.5220/0012003400003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 121-127
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
121
open metal sites can bind with guest species, forming
interactions with ligands. MOFs are controlled easily
due to their adjustable pore size and tunable porosity.
In other words, scientists can regulate the
functionalization of MOFs for specific applications
such as capturing particular species. The controllable
chemical properties of pores make various mixtures
of this material, which means lots of different and
advanced designs so that higher selectivity and
efficiency of gas species can be achieved. Overall,
MOFs will play a significant role in industrial
progress in reducing energy consumption and
improving industrial production efficiency.
This research will describe the research and
development of MOFs-based functional materials for
gas separation in recent years. MOFs and their
composites have excellent application prospects in
gas separation. MOFs and membrane separation
technology are used in gas separation. These
applications are easy to operate and reduce energy
consumption by more than 30% compared with other
methods, which meets the requirements of energy-
saving society development. In the research process,
it is also found that methane and nitrogen showed
different pair interaction intensities in MOFs,
resulting in a more significant pair surface diffusion
rate. The combination of MOFs and membrane makes
membrane have excellent separation performance,
providing a new way for methane concentration and
purification in natural gas and coalbed methane. The
existing MOFs-based membrane materials are
studied, and the selection of MOFs separation
membranes and their synthesis process are discussed
from the perspective of the surface diffusion rate of
gas, as well as the application of MOFs-based
functional materials in gas separation.
2 APPLICATION OF
MOFs-BASED FUNCTIONAL
MATERIALS FOR GAS
SEPARATION
2.1 C
2
H
2
/CO
2
Separation
Acetylene (C
2
H
2
) and carbon dioxide (CO
2
)
separation is an essential process in the industry since
C
2
H
2
acts as a crucial raw material and has a broader
application. C
2
H
2
can be produced from the cracking
process of hydrocarbon under high temperatures or
the incomplete combustion of methane (Wang, 2022).
According to public statistics, the global market value
of acetylene is approximately 11.42 billion dollars,
and it is expected to increase by 6% each year which
displays the importance of this stock (Cui, 2021). As
for the application of C
2
H
2
, it can be widely used in
producing chemical substances such as acetic acid
and benzene. It is also used for welding and cutting
metals (Guo, 2017). These reactions need high purity
of acetylene to be achieved, instead, carbon dioxide
always exists as an impurity to affect
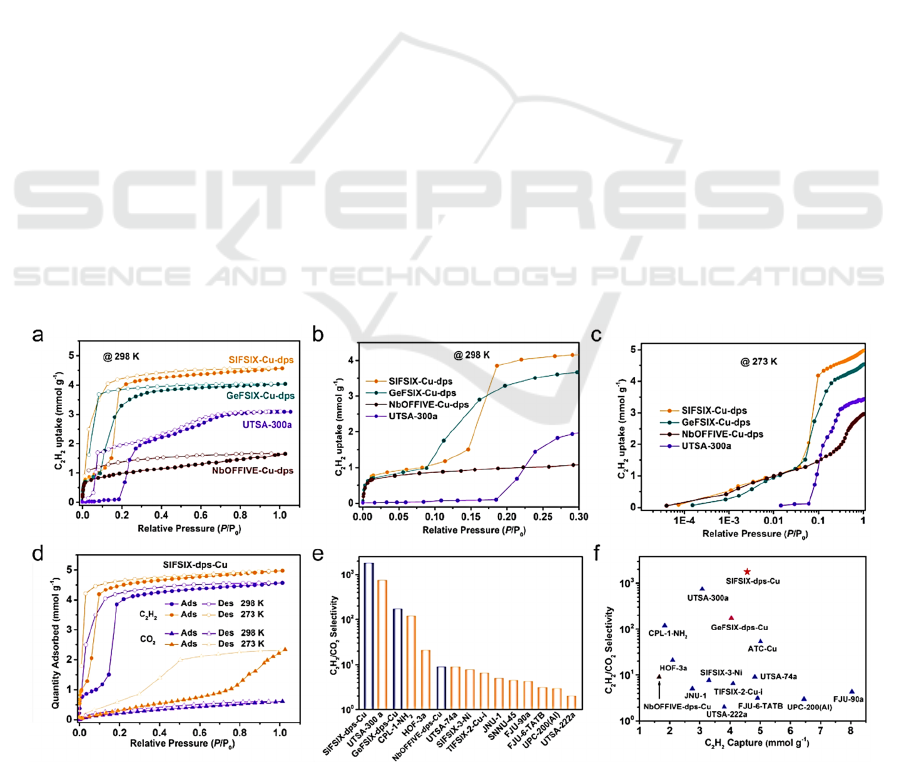
Figure 1: Application of MOFs for C
2
H
2
/CO
2
separation (Wang, 2022).
FSB 2022 - The International Conference on Food Science and Biotechnology
122

industrial manufacture, so it is necessary to separate
CO
2
from C
2
H
2
. However, C
2
H
2
/CO
2
separation is
difficult as they have similar molecular sizes and
physical properties like the critical temperature.
Overall, the appearance of MOFs-based functional
materials will play a significant role on C
2
H
2
/CO
2
separation, which will benefit the industries, as shown
in Fig. 1.
A previous study used molecular simulations to
identify the adsorption power of magnesium format
(Fischer, 2010). This light metal-organic framework
is called magnesium formate, which combines with
the most uncomplicated carboxylate formate. In
comparing the single-component isotherms in the
experiment, high absorption of 65.7 cm
3
/g at 298 K
and 1 bar of the pressure for acetylene is measured so
that high selectivity of acetylene over carbon dioxide
could be observed. Even though the theoretical
acetylene absorption is smaller than the single-
component isotherms, almost no carbon dioxide can
be adsorbed. This result shows that magnesium
formate’s capacity to separate C
2
H
2
and CO
2
molecules should be nearly constant at a suitable
pressure range. To explore the ability of adsorption
for magnesium formate, the calculated Henry
constants ratio was used, which is shown in a line
graph, and acetylene is highly adsorptive from carbon
dioxide at room or even lower temperatures.
Another method is to discuss the potential field of
these two species. As for the C
2
H
2
, the interaction
power ranges from -25 to over -40 KJ/Mol, whereas
less than -30 KJ/Mol interaction is measured for CO
2
.
Due to the greater power of interacting with the
framework for C
2
H
2
, the energetic areas will be taken
up absolutely by acetylene molecules rather than
carbon dioxide. These avoid the adsorption of carbon
dioxide molecules effectively. In summary,
magnesium formate should be efficient in C
2
H
2
/CO
2
separation. The UTSA-68 is an instance of an
unpenetrated framework and can adsorb 70.1 cm
3
/g
amount of C
2
H
2
due to the porosity at 296 K and 1
atm pressure (Chang, 2016). The C
2
H
2
molecules can
occupy inside the framework more frequently as the
increasing porosity, and high selectivity is also
possible for UTSA-68. The calculated range of
selectivity, which is 5-3.4 based on the Ideal
Adsorbed Solution Theory (IAST), is a comparatively
high result. Above all this evidence, it is believed that
UTSA-68 has beneficial properties such as a high
ability of adsorption to be applied for C
2
H
2
/CO
2
separation.
In terms of MOFs with ultra-micropores,
[Cu(hfipbb)(Hhfipbb0.5)] was studied by scientists
(Cui, 2021). The pore size of this framework is
suitable for acetylene and carbon dioxide molecules
contributing to the sieving effect. First, the
thermogravimetry analysis demonstrates that this
MOFs can be maintained at about 350°. Compare the
adsorption of guest molecules at 273 K and 298 K.
Their powers are similar, while there is a significant
difference (sevenfold) in the amount of acetylene and
carbon dioxide adsorbed by MOFs. In other words,
this microporous MOFs does have the potential to be
applied. In addition, the IAST calculation investigates
the adsorption capacity when the mixture contains the
same mole of two gas species. Moreover, 696
selectivity is concluded, which is higher than most of
the MOFs. The simulations suggest that CO
2
molecules can interact with the framework while the
hydrogen repulsion prevents the interaction of C
2
H
2
.
That is why the reverse separation of C
2
H
2
and CO
2
is observed. After that, column breakthrough
experiments are utilized to confirm the adsorption
power. Since no CO
2
is indicated at the exit, it is
possible to prove that a great ability of separation can
be achieved to obtain pure C
2
H
2
when using this type
of MOFs under the kinetic circumstance.
2.2 CH4/N2 Separation
Methane (CH
4
) is necessary for the world to reach
zero emissions, yet it cannot separate from nitrogen
(N
2
). Due to the identical polarizability and kinetic
dimensions of CH
4
and N
2
, it is difficult to achieve
CH
4
/N
2
separation. Most common kinds of porous
materials, including zeolite, do not sufficiently
separate CH
4
/N
2
in industrial applications. Also, it is
crucial to separate CH
4
from N
2
, which can reduce
greenhouse gas emissions. The only technique
employed directly to separate the gaseous mixture on
a significant scale is cryogenic distillation. Although
this method produces high purity products, it is
expensive and energy-intensive.
According to the separation mechanism, there are
two categories of adsorbents. Since CH
4
has stronger
adsorption contacts and a greater capacity for
adsorption than N
2
, CH
4
-selective adsorbents are
primarily based on the equilibrium process. N
2
-
selective adsorbents based on kinetic or steric effects
as N
2
can be adsorbed over CH
4
. Because of their
strong chemical stability and unique surface areas,
porous organic frameworks contribute significantly
in the separation of CH
4
and N
2
. For example, when
light metal ions are used to prepare functional
materials, the affinity for the prepared functional
materials can be greatly enhanced for adsorbing CH
4
as compared to the materials without added light
metal ions, resulting in a high selectivity for CH
4
/N
2
Application of Metalâ
˘
A¸SOrganic Frameworks-Based Functional Materials for Gas Separation
123
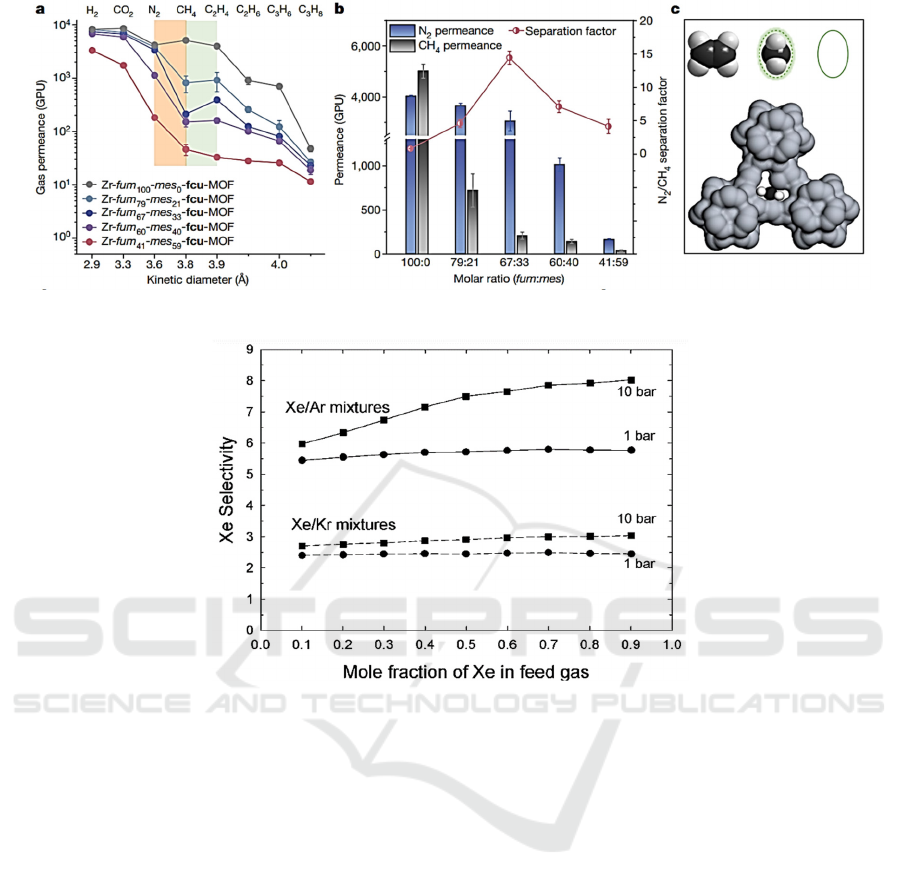
Figure 2: Performance of CH
4
/N
2
separation by using the prepared MOFs-based functional materials (Zhou, 2022).
Figure 3: Influence of mole fraction of Xe in feed gas on Xe selectivity (Greathouse, 2009).
separation. Gas separation may be able to filter
natural gas effectively using membranes (Wu, 2021).
One component of the gas mixture can enter these
membranes’ holes only when desired. However, even
the most sophisticated membranes offer poor
selectivity because CH
4
and N
2
share many physical
and chemical characteristics. Zhou et al. designed a
new MOFs-based membrane material for CH
4
/N
2
separation (Zhou, 2022), as shown in Fig. 2. In this
work, they have fine-tuned the pore structure
characteristics of the prepared membrane materials,
making them exhibit good selectivity for CH
4
/N
2
separation. In addition, the prepared membrane
material still exhibits high CH
4
/N
2
selectivity and N
2
permeability at practical pressures up to 50 bar.
Scientists employ water as a solvent for the
electrochemical synthesis of MOFs membranes for
membrane manufacturing, in which an external
current is used to apply deprotonation. All the films
had an excellent symbiotic layer, a similar crystal
shape, and an ultra-thin thickness of roughly 30 nm
when the conditions were optimized, and the ratios of
the various components were changed (Zhou, 2022).
Additionally, as a demonstration of concept for
reducing the price of membranes, the authors
demonstrate that the same synthetic MOFs film
displays comparable layer thickness and intactness on
low-cost support made of stainless-steel nets
modified with carbon nanotubes. Of course, the
literature has also reported other efficient MOFs-
based functional materials for CH
4
/N
2
separation,
such as zeolites-based MOFs (Wu, 2019).
2.3 Noble Gas Separation
Noble gases have very low conductivity and
reactivity and are therefore favoured in human
industry. Among other things, inert gases can be used
in light bulbs and protective gases. For example,
Krypton can be used in lasers and Xenon can be used
in cosmic rays. A new type of material, MOFs, is
currently valued by scientists because of its high
efficiency, tunable void size, and industrial potential.
Greathouse et al. prepared IRMOF-1 that can
FSB 2022 - The International Conference on Food Science and Biotechnology
124
selectively absorb Xe atoms in Xe/Kr and Xe/Ar
mixtures (Greathouse, 2009), as shown in Fig. 3. In
both their models and their tests, they discovered a
correlation between an increase in the polarizability
of the linker and an improvement in adsorbate
interaction, as well as an improvement in adsorption
selectivity for the Xe/Kr separation. With a specific
cavity width, the IRMOF-1 has a pore capacity that is
equivalent to 74% of its overall volume. Because the
system has a significant amount of empty space, the
adsorbates act as if there were just a single component
present. For instance, slow Xe atoms do not
considerably diffusion of Kr atoms that have
relatively short, which suggests the correlation
relationship influence among the various adsorbates
are relatively minimal, which ultimately results in KP
accuracy that is very high. This comparison
demonstrates that the KP correlation adequately
predicts Xe/Kr mixes’ self-diffusivities under various
situations. The preceding reasoning also applies to
combinations of Xe and Ar in IRMOF-1 (Greathouse,
2009). And the performance of the MOFs in
adsorbing noble gases is also optimized. (Meek,
2012; Gurdal, 2012; Gurdal, 2013).
2.4 Oxygen Separation
Oxygen accounts for approximately 20.9% of the
total composition of air and is one of the main
components for maintaining human metabolism.
Moreover, it is an indispensable substance in various
industries and processes. In the petroleum
purification industry, oxygen has already been
employed for air-in-furnace enrichment, which
provides the convenience of reduced fuel
consumption, increased capacity and better
temperature control. It is used in water treatment for
water purification, as an oxidizer, and in oxyfuel
technology using acetylene for oxyfuel welding and
cutting. However, those low-efficiency methods such
as cryogenic distillation, membrane or zeolite
separation dominate the modern O
2
supply. While
most of these technologies yield high purity and
large-scale finished products, the complexity, high
cost and energy intensity behind them cannot be
ignored. To balance the economics and efficiency of
separation processes, metal-organic frameworks are
naturally one of the promising processes for the future
owing to their porosity and adjustable host/guest
interactions.
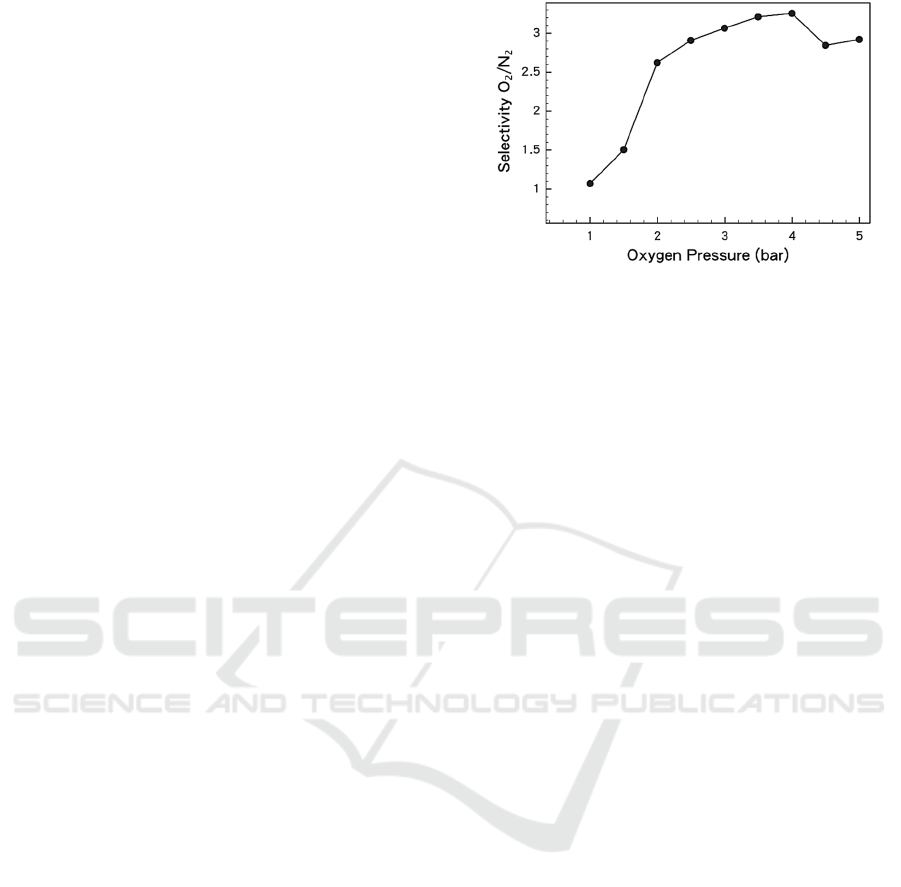
The MOFs-based functional materials are
reported to have different selectivity for mixtures of
oxygen and nitrogen at characteristic temperatures
and pressures, as shown in Fig. 4. At low
Figure 4: The selectively for O
2
/N
2
with the used functional
materials (Wang, 2017).
temperatures, oxygen can be adsorbed more
selectively compared to nitrogen. This
thermodynamic and kinetic selectivity was confirmed
in experiments and structural probes. At typical
temperature regions, the internal energy of oxygen
adsorption amount is one half more than that of
nitrogen, an unprecedented increment in materials
with oxygen selectivity. The opening door process
can be used to explain the positive proportional
change in the coverage and the internal energy of
adsorption of the two gases. One of them is due to the
increasing number of gas molecules during the
opening of the door, or it is because the energy in the
subsequent steps reduces the amount of escape in the
expansion of the process (Wang, 2017).
UIO-66 containing fluorine was found to have
superior oxygen adsorption capacity. In the
experimentally obtained adsorption isotherm results,
highest flourine concentration UiO-66-F100 showed
the remarkable absorptional ability to adsorb more
and more oxygen with increasing pressure. At 40 bar,
each gram of MOFs can even store an astonishing
amount of oxygen-72mg. However, the steric
hindrance of the functional group causes the decrease
of the average available volume of material which
does not affect the superiority of the total adsorption
capacity of this MOFs (compared to the conventional
UIO-66). It is the oxygen/fluorine interaction makes
its exceptional ability, which is different from the
usual functional group/guest adsorption (Piscopo,
2016).
The newly-discovered MOFs “Fe-BTTri” is
decorated with an iron (II) center bound in a
hemoglobin-like environment. Its unique framework
could adjust to iron centers of different spin-height
which made it suitable for studying the various
electronic transitions. The experiment demonstrates
that Fe-BTTri can undergo electronic changes like
those occurring when hemoglobin and oxygen bind,
Application of Metalâ
˘
A¸SOrganic Frameworks-Based Functional Materials for Gas Separation
125

thereby allowing for a much higher O
2
/N
2
selectivity
than other iron-based adsorbents. The oxygen
adsorption result of the MOFs is characterized by a
progressive decrease in its relative adsorption
capacity for oxygen as the pressure increases.
However, the performance is average at high
pressures, in the region of 210 mbar. The adsorption
capacity of the used functional materials can be up to
3.3 mM/g, or about 10 wt% of O
2
, suggesting that the
prepared MOFs-based functional material can be
used as a promising material for O
2
separation (Reed,
2020).
3 CONCLUSION
In conclusion, MOFs-based functional materials have
the potential to be applied in gas separation due to
their unique properties and structure. Through the
research in the previous study, the application of a
diverse of different MOFs-based functional materials
to four different types of gas separations is analyzed.
As for the C
2
H
2
/CO
2
separation, three categories of
MOFs, including light, unpenetrated and
ultramicroporous MOFs, are studied through
experiments such as molecular simulation. Owing to
the data, they can all separate C
2
H
2
and CO
2
molecules effectively. MOFs-based membrane with
fumarate and mesaconate linkers can be utilized in
CH
4
/N
2
separation, which shows an excellent
selectivity on specific gases and is also so energy-
efficient that it can substitute cryogenic distillation.
Another MOFs called IRMOF-1 plays a role in
adsorbing Xe atoms from Kr or Ar mixtures based on
the data of experiments and simulation. Regarding O
2
separation, MOFs-based functional materials like
UIO-66 containing fluorine, Fe-BTTri, and RPM3-
Zn are all practical for oxygen adsorption according
to their unique structural characteristics. Only several
kinds of gas separation are focused on in this report,
but it would be better if more research on MOFs
application could be done so that utility of MOFs can
be achieved to the maximum extent in the industry
and benefit humans.
REFERENCES
Cui, H., Xie, Y., Ye, Y., Shi, Y., Liang, B., & Chen, B.
(2021). An ultramicroporous metal-organic framework
with record high selectivity for inverse CO
2
/C
2
H
2
separation. Bulletin of the Chemical Society of Japan,
94(11), 2698-2701.
Chang, G., Li, B., Wang, H., Hu, T., Bao, Z., & Chen, B.
(2016). Control of interpenetration in a microporous
metal–organic framework for significantly enhanced
C
2
H
2
/CO
2
separation at room temperature. Chemical
Communications, 52(17), 3494-3496.
Fischer, M., Hoffmann, F., & Fröba, M. (2010). New
microporous materials for acetylene storage and
C
2
H
2
/CO
2
separation: insights from molecular
simulations. ChemPhysChem, 11(10), 2220-2229.
Greathouse, J. A., Kinnibrugh, T. L., & Allendorf, M. D.
(2009). Adsorption and separation of noble gases by
IRMOF-1: grand canonical Monte Carlo simulations.
Industrial & Engineering Chemistry Research, 48(7),
3425-3431.
Guo, Z. J., Yu, J., Zhang, Y. Z., Zhang, J., Chen, Y., Wu, Y.,
et al. (2017). Water-Stable In (III)-Based Metal–
Organic Frameworks with Rod-Shaped Secondary
Building Units: Single-Crystal to Single-Crystal
Transformation and Selective Sorption of C
2
H
2
over
CO
2
and CH
4
. Inorganic chemistry, 56(4), 2188-2197.
Gurdal, Y., & Keskin, S. (2012). Atomically detailed
modeling of metal organic frameworks for adsorption,
diffusion, and separation of noble gas mixtures.
Industrial & engineering chemistry research, 51(21),
7373-7382.
Gurdal, Y., & Keskin, S. (2013). Predicting noble gas
separation performance of metal organic frameworks
using theoretical correlations. The Journal of Physical
Chemistry C, 117(10), 5229-5241.
Meek, S. T., Teich-McGoldrick, S. L., Perry, J. J.,
Greathouse, J. A., & Allendorf, M. D. (2012). Effects of
polarizability on the adsorption of noble gases at low
pressures in monohalogenated isoreticular metal–
organic frameworks. The Journal of Physical Chemistry
C, 116(37), 19765-19772.
Piscopo, C. G., Trapani, F., Polyzoidis, A., Schwarzer, M.,
Pace, A., & Loebbecke, S. (2016). Positive effect of the
fluorine moiety on the oxygen storage capacity of UiO-
66 metal–organic frameworks. New Journal of
Chemistry, 40(10), 8220-8224.
Reed, D. A., Xiao, D. J., Jiang, H. Z., Chakarawet, K.,
Oktawiec, J., & Long, J. R. (2020). Biomimetic O 2
adsorption in an iron metal–organic framework for air
separation. Chemical science, 11(6), 1698-1702.
Wang, J., Zhang, Y., Su, Y., Liu, X., Zhang, P., Lin, R.
B., et al. (2022). Fine pore engineering in a series of
isoreticular metal-organic frameworks for efficient
C
2
H
2
/CO
2
separation. Nature communications,
13(1), 1-8.
Wu, Y., & Weckhuysen, B. M. (2021). Separation and
purification of hydrocarbons with porous materials.
Angewandte Chemie International Edition, 60(35),
18930-18949.
Wu, Y., Yuan, D., He, D., Xing, J., Zeng, S., Xu, S., et al.
(2019). Decorated traditional zeolites with subunits of
metal–organic frameworks for CH
4
/N
2
separation.
Angewandte Chemie International Edition, 58(30),
10241-10244.
Wang, C. Y., Wang, L., Belnick, A., Wang, H., Li, J., &
Lueking, A. D. (2017). Oxygen-selective adsorption in
FSB 2022 - The International Conference on Food Science and Biotechnology
126

RPM3-Zn metal organic framework. Chemical
Engineering Science, 165, 122-130.
Zhou, S., Shekhah, O., Ramírez, A., Lyu, P., Abou-Hamad,
E., Jia, J., et al. (2022). Asymmetric pore windows in
MOF membranes for natural gas valorization. Nature,
606(7915), 706-712.
Application of Metalâ
˘
A¸SOrganic Frameworks-Based Functional Materials for Gas Separation
127
