
Application of Different Materials for Carbon Capture
Rundong Tian
*
Guanghua Cambridge International School, Shanghai 201319, China
Keywords: Carbon Capture, Porous Materials, Application, Sorbents.
Abstract: Human activities have led to increasing CO
2
emissions, mainly coming from factories and plants. The
increasing concentration of carbon dioxide (CO
2
) in the atmospheric environment has become a severe
concern of the world due to the existing and potential consequences. Different carbon capture technologies
have been developed and used to remove CO
2
from the atmosphere. In addition, carbon capture can also solve
certain environmental pollution problems, by directly reducing CO
2
discharge from anthropogenic sources.
The research focuses on carbon capture techniques and corresponding materials (sorbents). The current
situations are stated and the significance of carbon capture is enhanced, while also giving brief introductions
on several sorbents. The amine method, which is conventional and the most frequently used, is discussed first.
Afterwards, porous materials including zeolites, activated carbons (AC) and metal-organic frameworks
(MOFs) are discussed, mainly focusing on the sorption mechanism, advantages, drawbacks and possible
corresponding solutions, specific examples and outlook. These porous materials hold great potential as solid
adsorbents for carbon capture, likely replacing conventional amines in the future. Further improvements and
evaluations will enable porous materials to be used in industrial applications.
1 INTRODUCTION
The issue of global warming has attracted great
attention since last century. Human beings have
already experienced the severe aftermath of global
warming and relevant climate changes, such as sea-
level rising, more frequent extreme weathers,
increased heat and drought and flooding. The
increase in concentration of greenhouse gases like
carbon dioxide (CO
2
) and methane present in
atmosphere contributes to global warming. The
global industrial development has led to increased
CO
2
emissions, mainly from anthropogenic sources
such as burning of fossil fuels. For example, global
CO
2
emissions reached 34.9 Gt in 2021 (Liu, 2022),
leading to a dramatic increase in the concentration of
CO
2
in the atmosphere. Possible solutions to the issue
include replacing conventional energy sources by
cleaner and more sustainable resources as well as
carbon capture and storage processes. The complete
treatment of carbon dioxide includes capturing CO
2
from various sources, regeneration of sorbents,
storage and transport of CO
2
and finally the
conversion of CO
2
into harmless or even valuable
products.
There are three common carbon capture
technologies. The post-combustion carbon capture
belongs to the most studied and applied one, being
considered the most in existing conventional power
units, such as amine method. The amine method is
through the use of aqueous alkanolamine absorbents.
Such chemical absorption carbon capture method has
been used for years in industry and still exists to be
the most recognizable one. Since the absorption
process involves chemical reactions, relatively large
amount of heat is released, where carbamate or
bicarbonate species are formed in the absorption
reactions, depending on the species of alkanolamine
used (Sumida, 2012). The amine method holds
several advantages, such as high process efficiency.
Moreover, they are commercially available and
improvements on the method have been made over
time. However, the existing amine methods do have
various problems and limitations. Such capture
process is very energy-consuming, specifically the
regeneration of absorbents, leading to high energy
requirements and costs. Furthermore, amine
solutions tend to be corrosive against vessels, and
also possess low thermal stability, shown by
decomposition under heating.
112
Tian, R.
Application of Different Materials for Carbon Capture.
DOI: 10.5220/0012003300003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 112-120
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

Due to the energy-intensive nature of current
processes, new technologies are required to reduce
energy requirements. Porous materials have been
studied, aiming to replace aqueous absorbents, as
they have the potential to reduce the energy costs as
well as increasing the efficiency in carbon capture
processes. Zeolites are microporous aluminosilicate
minerals. As solids, zeolites have much lower heat
capacity, thereby leading to lower energy penalty for
regeneration. In addition, zeolites have a well-
developed structural chemistry, which could lead to
optimized zeolites for carbon capture processes.
Moreover, zeolites possess unified micropores,
resulting in rapid adsorption at relatively low
pressures. Zeolites are also relatively cheap to
produce on a large scale which makes them preferred
in industrial applications. However, zeolites’ main
limitation is the hydrophilic nature, so water vapor
would compete with CO
2
for adsorption sites and the
porous materials will get saturated with water vapor
eventually, leading to lower adsorption capacity for
CO
2
over time. They also undergo large enthalpy of
adsorption, which leads to high temperature required
for desorption of guest molecules. Activated carbons
(AC) are porous carbon materials produced from
carbonaceous source materials. Compared to
zeolites, activated carbons are hydrophobic, therefore
the issues from water vapor are not main concerns.
They can be used to lower uptake at low pressure
compared to adsorbents such as zeolites, hence they
are more suitable for high-pressure applications
(Sumida, 2012). Metal-organic frameworks (MOFs)
have been widely studied and engineered for various
applications in recent years, including carbon capture
processes. The MOFs is a kind of porous
coordination polymer, consists of metal clusters and
organic ligands in a crystalline structure. It holds
many advantages that makes it suitable for carbon
capture process, including a precise control over
structures that could be achieved through synthesis
and shaping, high pore volumes and surface areas,
tunable pore sizes by reticular chemistry, as well as
post-synthetic modifications optimizing MOFs for
different industrial environments of carbon capture
processes (Trickett, 2017). However, challenges do
exist for MOFs in various carbon capture processes,
specifically their low mechanical, thermochemical
and hydro-chemical stability, as well as relatively
low density due to the trade-off between pore volume
and density.
The following sections of this research will
discuss current and prospective sorbents for carbon
capture. Specifically, amine-based solvents in
chemical-absorption-based carbon capture process,
zeolites, AC and MOFs in physical-adsorption-based
process and outlook for porous materials in the field
of CO
2
capture are given. And this research will only
concentrate on the CO
2
-capturing step and
regeneration of sorbents, since the regeneration
requirements are essential for determining the
viability of specific sorbents in industrial applications
2 AMINES-BASED SOLVENTS
Aqueous amines are mainly considered for post-
combustion CO
2
capturing, which is the only carbon
capture technique demonstrated at full commercial
scale at present. Amines are conventional solvents,
and still the mostly used in power plants. Different
amines, such as monoethanolamine (MEA),
diethanolamine (DEA), methyl diethanolamine
(MDEA) and ammonia (NH
3
), present different
properties, leading to different performances and
energy requirements in carbon capture processes
(Romeo, 2020). The chemical reaction between
amines and CO
2
is mainly because the lone pair
electrons of nitrogen, which act as Lewis bases, or
nucleophiles. The lone pair electrons attack the
partially-positive carbon atoms in CO
2
. And with
participation of water molecules, carbamate species
are formed while bicarbonate species are formed
when tertiary amines are used. Moreover, the usage
of primary and secondary amines can be used to
cause different kinetics of reactions, as the formation
of carbamate species is generally faster than the
formation of bicarbonate species. Evaluations have
been made for different amines (Romeo, 2020).
Primary amines appeared to possess the greatest
capabilities of capture CO
2
when used singly, since
they possess the highest reaction enthalpies among all
aqueous amines. High reaction enthalpies lead to the
favorable reaction kinetics. Hence a high degree of
CO
2
purity, high efficiency and fast absorption rates
of the capturing process might be achieved. However,
trade-off exists between reaction enthalpies, kinetics
and regeneration penalty, since high reaction
enthalpies would generally lead to high regeneration
temperature and energy requirements. Contrarily,
secondary amines possess less energy penalty as
lower regeneration temperature. Furthermore,
unstable carbamates formed in the reaction would
also lead to lower regeneration energy requirements
since backward reaction would be favored more, and
also lead to less favored absorption kinetics and
lower speed of capturing.
The technology of aqueous amines is mature, and
preferred for industrial applications among other
Application of Different Materials for Carbon Capture
113
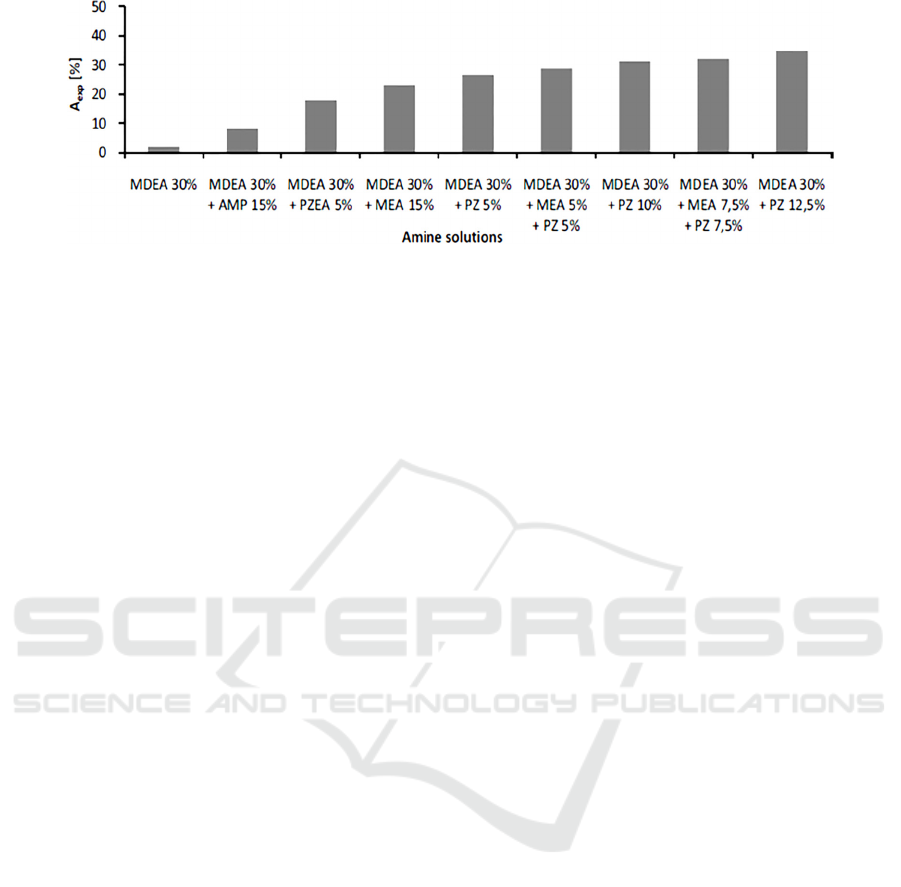
Figure 1: Experimental results of CO
2
absorption using different amine solutions (Dubois, 2011).
sorbents, due to high CO
2
affinity possessed by
amines (KUROPKA, 2011). MEA, as a primary
amine, holds merits of high chemical reactivity
against CO
2
as well as high reaction enthalpies,
leading to high absorption rates. While MDEA, as a
tertiary amine, ensures the appreciable absorption
capacity, therefore the combination of the two
different amines leads to excellent performance of
CO
2
capture. Another specific example is KM-CDR
technology, which uses KS-1™ instead of MEA,
achieving higher energy-efficiency (Kadono, 2013).
Moreover, KS-1™ tends to be less corrosive and
more resistant against O
2
degradation compared with
MEA. Recent research has focused on mixing amines
with other solvents, which refers to solvent blends.
By combining types properly, a better absorption
performance can be achieved, specifically producing
solvents with both high absorption rates and
absorption capacities. For instance, a higher
absorption rate is achieved by mixing MEA with a
little PZ (Vega, 2018). In Fig. 1, it is evidently
demonstrated that CO2 absorption increases
significantly by using various solvent blends,
compared to singly using 30 wt.% MDEA, due to
activation phenomenon.
Although the amine method is well-developed
and used in carbon capture plants worldwide, several
drawbacks and challenges have always existed for
aqueous amines, most significantly the energy
penalty of the regeneration process. This issue is
caused by various factors. The chemisorption nature
and high reaction enthalpies lead to high energy
requirements of backward reaction, referring to the
desorption process. The amines are corrosive toward
the vessels, so the concentration of amine species has
to be limited. A lower concentration of amines in the
solution means a larger volume of water, which has
relatively high heat capacities among all liquids. A
larger volume of water is required to be heated to
regenerate the sorbents, causing high energy
requirements and therefore severe energy penalty.
Furthermore, the solutions are unstable toward
heating, therefore the temperature available for full
regeneration is limited, leading to incomplete
desorption of carbon dioxide from the solutions.
Amines will also decompose under heating, leading
to poorer performance over time and shorter life time
of such sorbents.
There are several ways to deal with the drawbacks
and reduce massive energy penalty, including
replacing conventional amines with less corrosive
and more thermally-stable ones, decreasing the
stripper temperature to achieve lower corrosion rate,
as well as discovering ways to increase the
concentration of amines (reducing volume of water)
in solution. NH
3
is considered as a potential
alternative to MEA because of its low-cost, less
corrosive and less likely to degrade under heat, hence
greater stability compared with MEA. Moreover, it
holds relatively high CO
2
absorption capacity which
could lead to better process efficiency. Most
excitingly, NH
3
can react with NO
2
and SO
2
in flue
gas, forming ammonium sulfates and ammonium
nitrates, hence not only removing impurities, but also
directly producing usable and useful fertilizers. This
property of NH
3
is able to greatly reduce the costs in
solvent regeneration, CO
2
compression and storage.
They are far less corrosive compared to conventional
amines like MEA, resulting in green and sustainable
chemistry. Amino-functionalized ionic liquids have
been designed and absorption capacity is highly
enhanced since one amine is able to bind with two
carbon dioxide molecules (Luo, 2019), compared to
the 1:1 or 2:1 stoichiometry of conventional amines.
Overall, different aqueous amines should be
examined multidimensionally before being used in
CO
2
capture processes. Besides absorption capacities
and rates, other key parameters include corrosiveness,
resistance towards O
2
degradation, thermal stability,
tolerance to impurities, recovery in regeneration,
process efficiency and energy efficiency in cycle.
FSB 2022 - The International Conference on Food Science and Biotechnology
114

3 POROUS MATERIALS
Since aqueous absorbents such as amines require high
energy input for regeneration, as the so-called energy
penalty, sorbents with higher energy efficiency are
considered for carbon capture processes. The main
kind of solid adsorbents is porous materials, capturing
CO
2
by either physical adsorption or chemical
adsorption. As solids, porous materials have
significantly lower heat capacity than aqueous
absorbents which involve large volume of water.
Adsorption, in contrast with absorption, refers to the
enrichment of adsorbates (guest molecules) on the
internal or external surface of adsorbents. Hererin,
three common porous materials will be discussed in
the article, zeolites, AC and MOFs, though have
different chemical compositions and structures, all
uptake CO
2
gas molecules by physical adsorption
generally. Key parameters to evaluate solid
adsorbents with best CO
2
capture performance
include adsorption capacities, adsorption kinetics
(rates of adsorption and desorption) and selectivity
towards CO
2
. Moreover, since the solid adsorbents
are to be used in practical applications, other
industrial and engineering evaluations need to be
made. Stability under specific environments and
conditions is highly important, specifically towards
water vapor and moisture as well as other impurities
in gas mixtures. Thermal stability and mechanical
stability also greatly affect the life cycle of adsorbents
and their performance over time, since they might
frequently undergo high temperature and high
pressure. Due to the presence of pores, the density of
porous materials is relatively low, which is not
positive. Low density of sorbents leads to lower
volumetric uptake of gas than expected, hence an
optimized sorbent for industrial application should
have a balanced pore volume and density.
Engineering evaluations should also include the costs
in various processes, such as large-scale production,
transportation and regeneration of adsorbents.
3.1 Zeolites
Zeolites belongs to microporous crystalline materials
that consist of silicon, aluminum and oxygen, and the
porous framework can accommodate various cations,
such as Na
+
, Ca
2+
and Li
+
. Zeolites are initially
explored as natural minerals, but have been studied
and manufactured artificially for decades as
adsorbents and catalysts. Zeolites hold a well-
developed surface chemistry, as their porous
structures and chemical compositions can be
precisely altered, leading to different properties for
various industrial applications. Moreover, they are
produced on a large scale commercially and are
relatively cheap among all solid adsorbents.
Both chemical and structural factors determine the
adsorption capacity to zeolites. Because of the
charges on cations and the charges induced by cations
within the structures, a diverse of different gas
molecules can be adsorbed in different amounts.
Zeolites with low and high Si/Al ratios are
categorized into X and Y respectively, while different
Si/Al ratios lead to different adsorption capacities
(Férey, 2008). Adsorption capacities can also be
increased by substitution of cations (Walton, 2006),
resulting in higher charge densities and stronger
attractions. Adsorption capacities of zeolites are
determined by pore structures as well, larger specific
surface area and pore volume will likely promote
adsorption capacities. The CO
2
/N
2
separation
selectivity of zeolites is affected by K/Na ratio, as
CO
2
and N
2
uptake might vary significantly between
low and high K/Na ratios. Furthermore, selectivity
towards CO
2
is also affected by pore size (pore
diameter). Different pore sizes lead to different
diffusion rates and selectivity. Trade-off exists
between diffusion rates and selectivity, as larger pore
sizes generally result in faster diffusion rates while
lower selectivity towards CO
2
, and vice versa.
Zeolites hold advantages including high porosity,
uniform pore size and distribution, and most
significantly high capacities. Good CO
2
capture
performance is achieved by strong interactions and
molecular sieving effect. Moreover, low Si/Al ratio is
preferred due to greater aluminum content, which
leads to stronger basicity, hence better CO
2
capture.
Zeolites are mainly used for low-pressure adsorption
due to their microporous nature which limits the
uptake at high pressure, as the pores will be saturated.
Zeolite 13X has been one of the most studied and
synthesized zeolites, with outstanding adsorption
capacities. Moreover, zeolite 13X can hold high
contents of alkali- and alkali-earth cations such as
sodium cations, which contributes to the strong
interactions with guest molecules, enhancing the
adsorption capacities as well. There have been intense
researches on cation exchange, aiming to improve
adsorption capacities by introducing cations with
higher charge densities into the structure. Cations
with smaller ionic radii and higher ionic charges are
generally preferred. Kongnoo et al. further enhanced
the adsorption capacity of zeolite 13X by acid
activation in the preparation from palm oil mill fly ash
(Kongnoo, 2017), which promoted the adsorption
capacity of zeolite 13X by 22% compared with the
initial (unactivated) zeolite 13X. Type 13X zeolite
Application of Different Materials for Carbon Capture
115
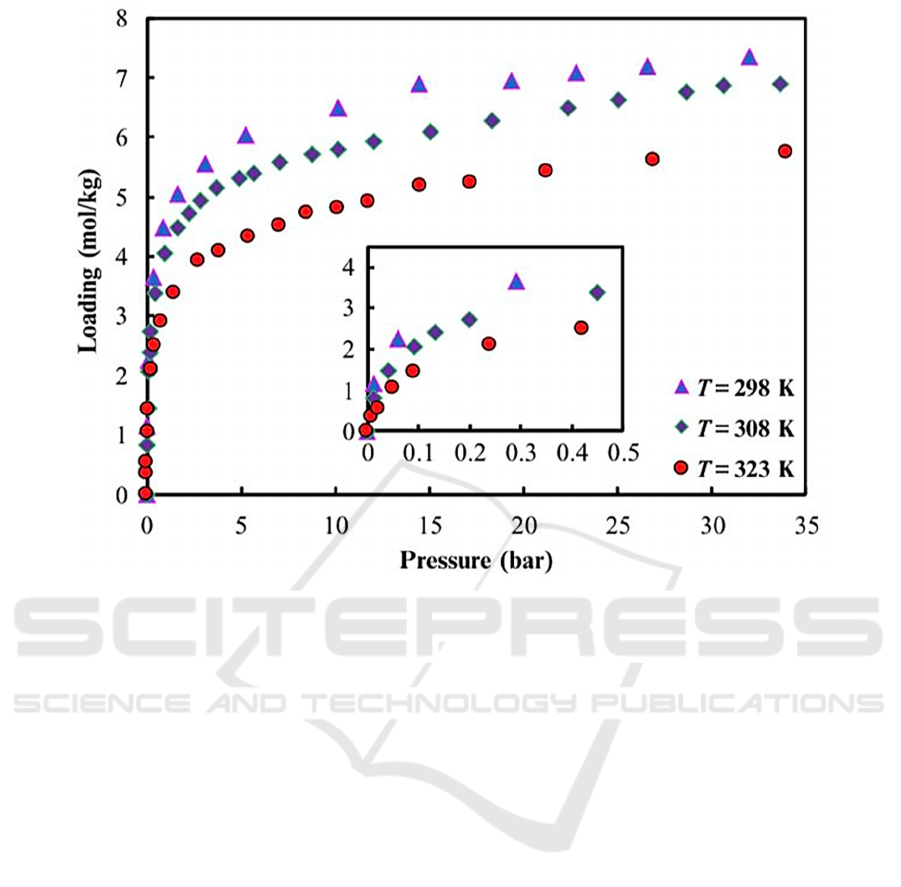
Figure 2: Adsorption isotherm of zeolite 13X for CO
2
(Cavenati, 2004).
manufactured by Ceca has gravimetric capacity of
2.05 mol/kg at temperature 298 K and pressure of 0.1
bar (Cavenati, 2004). From Fig. 2, most of the
adsorption process takes place at low pressure as
shown by a steep rise on the curve. Moreover, the
results show that CO
2
adsorption capacity for 13X
zeolite at room temperature (298 K) is significantly
higher than that at elevated temperatures.
The major drawback of zeolites is their
hydrophilic nature which leads to lower adsorption
capacities and selectivity toward CO
2
when moisture
and other impurities are present. Due to the cations
and induced charges within the porous structures of
zeolites, various polar molecules are favorably
adsorbed, including H
2
O, NO
x
, SO
x
and H
2
S, which
compete with CO
2
molecules for adsorption sites.
Moreover, such characteristics also lead to
regeneration penalty, as higher regeneration
temperatures are required in order to remove water
molecules adsorbed in the pores. Besides higher
energy requirements, the recovery of capacities and
other properties after desorption is also an issue, as
some zeolites lose evident adsorption capacities after
regeneration process under high temperature, which
will generate greater costs, since the adsorbents have
to be replaced more frequently. One solution to the
issue is by incorporating amine functional groups into
the mesopores of zeolites. After amine grafting,
interactions with CO
2
molecules tend to be
chemisorption, involving chemical reactions between
CO
2
molecules and amines, hence less affected by
H
2
O molecules. Moreover, adsorption capacity is
enhanced significantly by amine impregnation, and
water molecules can even promote the uptake,
according to experimental data. Overall, evaluations
of zeolites for industrial applications should not only
be conducted under ideal CO
2
/N
2
or CO
2
/CH
4
gas
mixtures, but also include all the substances that
appear in practical working environments, such as
water vapor, moisture and other impurities.
3.2 Activated Carbon
Activated carbon (AC) is another kind of
conventional solid adsorbent, used for gas storage, air
purification, solvent recovery and water purification.
The AC is produced from various carbonaceous
source materials, such as coconut husk, bamboo,
wood and coal, which than undergo physical
FSB 2022 - The International Conference on Food Science and Biotechnology
116
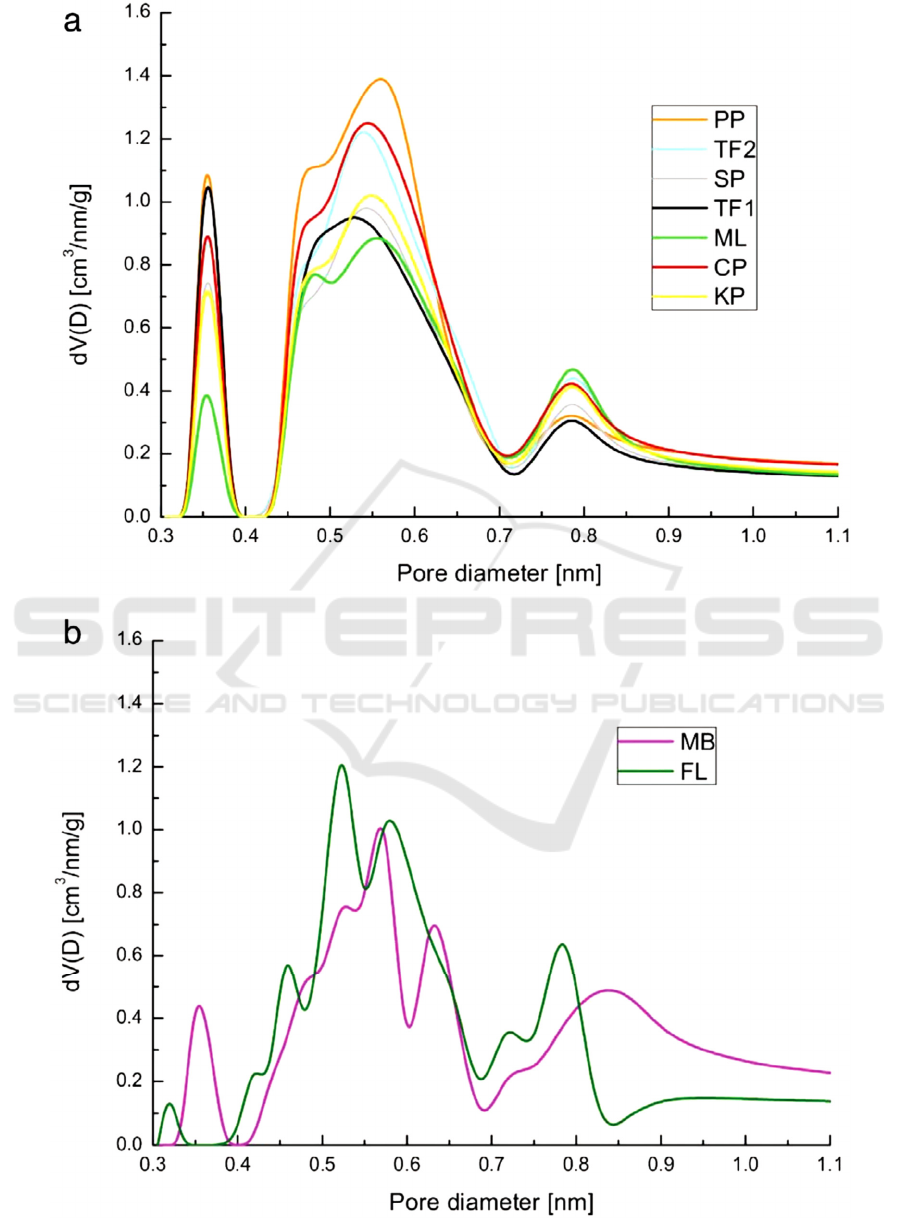
Figure 3: Different pore size distributions of the prepared AC (Serafin, 2017).
Application of Different Materials for Carbon Capture
117

activation and/or chemical activation. Activation
results in high porosity and large surface areas, with
pores of different shapes and sizes. The AC uptake
guest molecules by physisorption, as the interactions
are caused by Van der Waal forces and induced
dipoles. Textural properties, doped nitrogen contents
and ultra-microporosity all affect CO
2
capture
performance of AC. Pore characteristics depend on
carbon precursors, preparation (activation)
techniques and further modifications. Fig. 3 shows
that different carbon precursors (biomass here) can be
used to control different pore size distributions within
AC.
The merits of using AC for carbon capture
processes include high adsorption capacities, high
thermal stability as well as high stability in moist
conditions due to high hydrophobicity, well-
developed production technologies leading to
optimized porous structures and surface chemistry.
Moreover, AC generally require mild conditions for
regeneration because of low enthalpy of adsorption,
which is caused by relatively low CO
2
affinity
compared to other solid adsorbents, while also
exhibiting good recovery of adsorption properties
after regeneration, resulting in relatively high process
efficiency. Most importantly, low costs and broad
availability make AC the most frequently considered
solid adsorbent in industry on a commercial scale.
Adsorption capacities and selectivity of AC can
be enhanced by applying various functional groups
into the porous structures. Nitrogen functionalities
are the most common ones introduced into AC,
achieved by heat treatment with gaseous ammonia at
elevated temperatures. Experimental results show
that N-doped AC demonstrate improved CO
2
affinity
hence higher uptake at low pressures, but slightly
lower adsorption capacity. After amination of the
sample AC, adsorption capacity was significantly
increased (Plaza, 2009). Greater CO
2
affinity
improves selectivity towards CO
2
as well. Similar
effects can also be achieved by doping sulfur atoms
(oxidized-S). KOH activation is another technique to
improve AC’s CO
2
adsorption performance.
Adsorption capacities of various ACs are
significantly improved as KOH activation develops
pore network, specifically micropores and
mesopores. A series of chemical reactions take place,
generally favored by high temperatures. After the
reactions, the carbons are washed in order to remove
metallic potassium and other K compounds, resulting
in porous carbon lattices with high microporosity.
Moreover, KOH-activated AC exhibit high recovery
of CO
2
adsorption capacities after multiple
adsorption/desorption cycles.
3.3 Metal-organic Frameworks
Different from zeolite and activated carbon, MOFs is
a novel porous material, mostly developed in the 21st
century, exhibiting exciting properties that are
suitable for a broad range of applications, including
chemical sensing, drug delivery, gas separations, gas
storage and CO
2
capture, owing to MOFs’ tunable
textural properties and surface chemistry. Highly
flexible modifications are available to achieve
optimized MOFs for specific applications, since there
is a great variety of building blocks.
The adsorption performance of MOFs can be
improved by various modification techniques. For
example, surface modification for MOFs will
polarize the surfaces and lead to larger initial isosteric
heats, as adsorption of CO
2
tends to be
chemisorption. Enhanced adsorption and higher
affinity lead to higher selectivity towards CO
2
, as
well as greater capacity at low pressures. Moreover,
N
2
adsorption will decrease at all pressures due to
lower surface areas as the amine functionalities
occupy empty spaces, while smaller pore sizes also
result in decreased CO
2
uptake in relatively high CO
2
partial pressures, hence amine grafting is more
suitable for MOFs used for post-combustion CO
2
capture processes. Amine incorporation also
possesses other advantages. Generally, solid
adsorbents such as zeolites are concerned for their
hydrophilicity as well as other impurities competing
for adsorption sites. However, various amine-grafted
MOFs are not affected and even showed better
performance in the presence of water vapor. For
instance, moisture actually promotes CO
2
adsorption
capacity of TEPA-grafted Mg
2
(dobdc), due to
complex mechanisms. The results show that CO
2
is
preferably adsorbed to the grafted amine groups
under humid conditions, resulting in greater
adsorption capacity (McDonald, 2015). Furthermore,
amine-appended materials exhibit better CO
2
capture
performance with mixture gas. Amine groups can
also be incorporated onto organic ligands by click
reaction, enhancing the basic property within the
porous structures. Generally, amine binding with
metal clusters leads to greater improvement on
capacity while amine binding with ligands is more
stable. Amine-grafted MOFs should undergo
breakthrough experiments and dynamic cycling in
order to examine recovery over
adsorption/desorption cycles as well as the
relationship between amine loading and amine
efficiency.
FSB 2022 - The International Conference on Food Science and Biotechnology
118
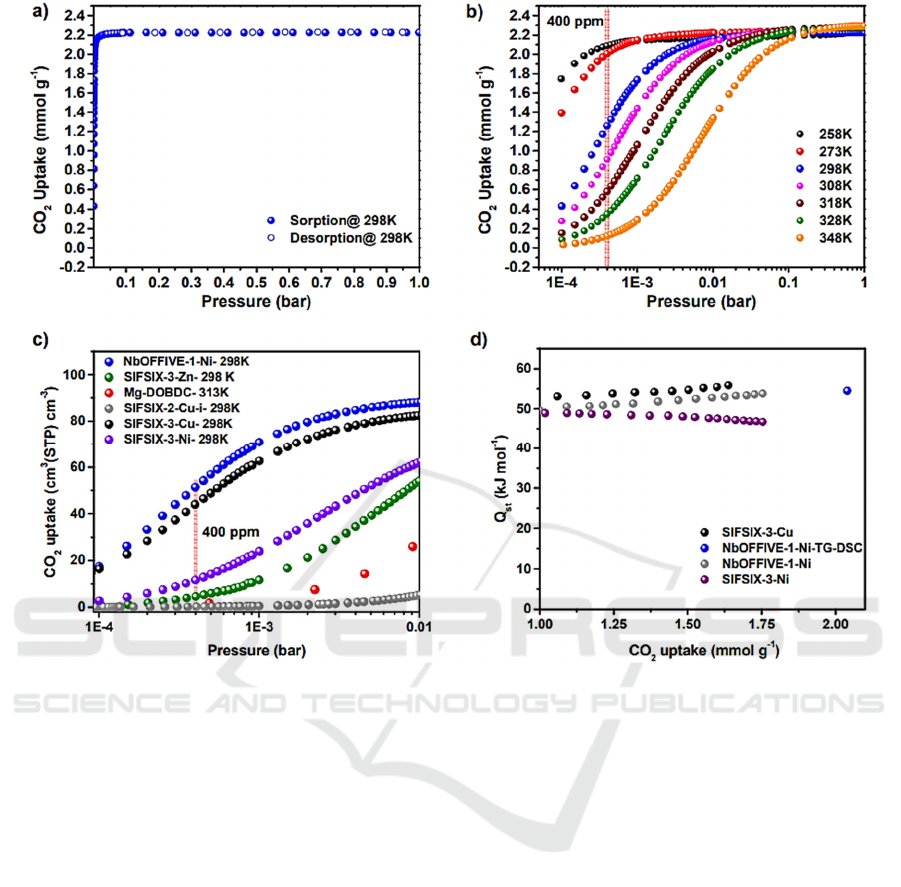
Figure 4: CO
2
adsorption isotherm, uptake and heat of adsorption for the prepared materials (Bhatt, 2016).
Adsorption performance of MOFs can also be
promoted by activating open metal sites, which would
lead to higher charge density and more favorable CO
2
uptake. Open metal sites are achieved either by
desolvation, specifically removing solvent molecules
in vacuum, or inserting metal cations into the porous
structures. Due to the presence of unsaturated
coordinate metal sites, CO
2
molecules transfer
electrons to them, leading to stronger interactions.
Pore size adjustment and distribution of adsorptive
sites also play a key role in improving CO
2
adsorption. Homogenous distribution of adsorption
sites is required in order to achieve identical
interaction strengths, so that high selectivity towards
CO
2
is maintained throughout wide pressure ranges.
Favorable uptake across great pressure range leads to
greater working capacity, which can lower capture
costs. SIFSIX-3-Cu exhibits ultra-microporous
structures and uniform distribution of adsorptive sites
(Bhatt, 2016), with a 54 kJ/mol Qst value leading to
high CO
2
uptake at low pressures, as shown in Fig. 4.
By using NbOF clusters, greater adsorption capacity
(1.3 mmol/g) is achieved, because of the high charge
densities within the one-dimension microchannels
accommodating CO
2
molecules, and the pore size is
smaller than the kinetic diameter of CO
2
molecule,
leading to slow gas diffusion and enhanced
interactions.
Major drawbacks of MOFs include low
mechanical stability and low density, which can be
solved by monolith production and help from
machine learning to make deterministic predictions
for materials’ performance. Monoliths are produced
through the mild drying of wet gel produced from the
gelation of primary particles, leading to robust,
densely packed MOFs (Tian, 2018). Compared with
MOF powders and pellets which contain glue or
binders that take up great volume leading to poor
volumetric performance and low mechanical
stability, MOF monoliths exhibit great energy density
as well as high stability, hence maximized
performance. Machine learning constructs neural
networks from database to automatically sort out
MOFs fulfilling specific requirements efficiently and
effectively, as the trade-off between density and pore
Application of Different Materials for Carbon Capture
119

volume can be balanced computationally, and
predictions can be further translated into applications.
4 CONCLUSION
Overall, this research discusses four carbon capture
techniques and relevant materials, specifically
aqueous amines for chemical absorption, zeolites, AC
and MOFs, which are porous materials, for physical
adsorption. The amine method is the mostly used one
in industry nowadays, generally for post-combustion
capture. However, more plants and factories are now
considering to replace aqueous amines with porous
materials. Intense researches have been conducted in
recent decades, which greatly improve properties of
several porous materials for carbon capture
applications. More and more suitable porous
materials have appeared, exhibiting optimized
characteristics. If porous materials are to be used
industrial applications, engineering evaluations are
required and the scaling up of production is also an
issue. In the near future, mature porous materials will
be able to replace present aqueous amines, which will
greatly reduce regeneration costs and save energy.
REFERENCES
Bhatt, P. M., et al. (2016). A fine-tuned fluorinated MOF
addresses the needs for trace CO
2
removal and air
capture using physisorption. Journal of the American
Chemical Society, 138(29), 9301-9307.
Cavenati, S., Grande, C. A., & Rodrigues, A. E. (2004).
Adsorption equilibrium of methane, carbon dioxide,
and nitrogen on zeolite 13X at high pressures. Journal
of Chemical & Engineering Data, 49(4), 1095-1101.
Dubois, L., & Thomas, D. (2011). Carbon dioxide
absorption into aqueous amine based solvents:
modeling and absorption tests. Energy Procedia, 4,
1353-1360.
Férey, G. (2008). Hybrid porous solids: past, present,
future. Chemical Society Reviews, 37(1), 191-214.
KUROPKA, J. (2011). Możliwości Ograniczania Emisji
Ditlenku Węgla ze Spalin Energetycznych. Korea, 1, 70.
Kadono, K., et al. (2013). New energy efficient processes
and newly developed absorbents for flue gas CO
2
capture. Energy Procedia, 37, 1785-1792.
Kongnoo, A., Tontisirin, S., Worathanakul, P., &
Phalakornkule, C. (2017). Surface characteristics and
CO
2
adsorption capacities of acid-activated zeolite 13X
prepared from palm oil mill fly ash. Fuel, 193, 385-394.
Liu, Z., Deng, Z., Davis, S. J., Giron, C., & Ciais, P.
(2022). Monitoring global carbon emissions in 2021.
Nature Reviews Earth & Environment, 3(4), 217-
219.
Luo, X. Y., et al. (2019). Designing amino‐based ionic
liquids for improved carbon capture: One amine binds
two CO
2
. AIChE Journal, 65(1), 230-238.
McDonald, T. M., et al. (2015). Cooperative insertion of
CO
2
in diamine-appended metal-organic frameworks.
Nature, 519(7543), 303-308.
Plaza, M. G., et al. (2009). Development of low-cost
biomass-based adsorbents for postcombustion CO2
capture. Fuel, 88(12), 2442-2447.
Romeo, L. M., Minguell, D., Shirmohammadi, R., &
Andrés, J. M. (2020). Comparative analysis of the
efficiency penalty in power plants of different amine-
based solvents for CO
2
capture. Industrial & Engineering
Chemistry Research, 59(21), 10082-10092.
Sumida, K., et al. (2012). Carbon dioxide capture in metal–
organic frameworks. Chemical reviews, 112(2), 724-781.
Serafin, J., Narkiewicz, U., Morawski, A. W., Wróbel, R. J.,
& Michalkiewicz, B. (2017). Highly microporous
activated carbons from biomass for CO
2
capture and
effective micropores at different conditions. Journal of
CO
2
Utilization, 18, 73-79.
Tian, T., et al. (2018). A sol-gel monolithic metal-organic
framework with enhanced methane uptake. Nature
Materials, 17(2), 174-179.
Trickett, C. A., et al. (2017). The chemistry of metal-
organic frameworks for CO
2
capture, regeneration and
conversion. Nature Reviews Materials, 2(8), 1-16.
Vega, F., Cano, M., Camino, S., Fernández, L. M. G.,
Portillo, E., & Navarrete, B. (2018). Solvents for carbon
dioxide capture. Carbon dioxide chemistry, capture and
oil recovery, 142-163.
Walton, K. S., Abney, M. B., & LeVan, M. D. (2006). CO
2
adsorption in Y and X zeolites modified by alkali metal
cation exchange. Microporous and Mesoporous
Materials, 91(1-3), 78-84.
FSB 2022 - The International Conference on Food Science and Biotechnology
120
