
Different CO
2
Capture Methods Based on Metal-Organic Frameworks
Bingxun Zhao
*
Ulink Educational Group Shanghai, Shanghai 201615, China
Keywords:
Carbon Capture, Methods, MOFs, Application.
Abstract: The increasing human production activities have led to an increase in carbon dioxide (CO
2
) emissions year
by year, and the CO
2
concentration in the general atmosphere has continued to increase. The increase in the
concentration of CO
2
in the atmospheric environment brings a series of environmental problems, which in
turn destroys the earth’s ecology. How to remove CO
2
from the atmosphere has become the focus of the
current research field. In this research, the advantages and disadvantages of traditional CO
2
capture materials,
and the advantages of MOFs itself were introduced, and followed by the introduction of M
2
(dobdc) which act
as an MOFs type, and the related carbon capacity as well as preparation step, physical and chemical properties
of MOF-801/PEBA with its performance and experimental data for carbon capture. The rht-MOF-7 that
modified by amine and triazine functional groups shows high affinity for CO
2
, and the capture capacity of
MOFs when working with amine and fluorine are also mentioned. And the CAU-1 can achieve a modest CO
2
uptake with a high adsorption due to equal heat. The description of pore size adjustment and active site
distribution effecting carbon capture is also discussed.
1 INTRODUCTION
Industrial production and anthropogenic emissions
have caused a sharp rise in atmospheric carbon
dioxide (CO
2
) levels. The amount of CO
2
in the
atmosphere is expected to continue to grow, and
experts are still unable to address large-scale
emissions. The continued increase in the
concentration of carbon dioxide in the atmosphere has
brought about a series of knock-on effects. For
example, rising CO
2
concentrations lead to global
warming, causing serious damage to the earth’s
ecological environment. Global warming is directly
responsible for different serious problems, such as sea
level rise and food security. In order to avoid further
damage to the earth's ecological environment,
reducing carbon dioxide emissions or removing CO
2
already in the atmosphere has received increasing
attention.
During the daily industrial process, a diverse of
various functional materials have to capture CO
2
being emitted and then through regeneration
(
Sumida, 2012)
. First step needs accurate selectivity
and high affinity between materials and the gas to
make sure CO
2
is completely removed. And the
second step requires abundant energy to break the
binds, let material gets rid of CO
2
. In addition, the
amine method to capture CO
2
holds several
advantages, such as simple operation and high CO
2
removal efficiency. Moreover, they are commercially
available and improvements on the method have been
made over time. For instance, the absorption
performance of the whole method will be further
improved if a mixture of various solvents is used as
the absorption liquid for CO
2
, achieving the best
carbon capture performance. However, the existing
amine method does have various problems and
limitations. Firstly, such capture process is very
energy-consuming, specifically in the regeneration of
absorbents. Due to the high stability of products
formed in the chemisorption process, the backward
reaction, which is the desorption process, is not
favored, therefore high energy is required for
regeneration of the aqueous alkanolamine absorbents.
Furthermore, the corrosive nature of amine solutions
toward the vessels limits the concentration of
alkanolamine species. And it is required to be heated
to a regeneration temperature, leading to great
regeneration energy and derived costs. The amine
method also has limitations like relatively low
stability towards heating and decomposition of amine
overt time, leading to lower performance in
absorption over time.
Although these methods are very effective, there
Zhao, B.
Different CO2 Capture Methods Based on Metal-Organic Frameworks.
DOI: 10.5220/0012002900003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 93-99
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
93

are still some problems. For example, the problem is
that the existing methods for removal CO
2
will lead
to high cost and concerns about the efficiency and
stability of the whole process. In addition, due to the
energy-intensive nature of current processes, new
technologies are required to reduce energy require-
ments. Porous materials have been studied, aiming to
replace aqueous absorbents, as they have the potential
to greatly reduce the energy costs in carbon capture
processes as well as increasing the efficiency of such
processes (Belmabkhout, 2016). Zeolites are
microporous aluminosilicate minerals, which have
high chemical and thermal stability. Most
importantly, as solids, zeolites have much lower heat
capacity as compared to aqueous alkanolamine
solutions, thereby resulting in a lower energy penalty
for regeneration. Moreover, zeolites have a well-
developed structural chemistry, which would lead to
optimized zeolites for carbon capture processes.
However, zeolites’ main limitation is the hydrophilic
nature, so water vapor would compete with CO
2
for
adsorption sites and the porous materials will get
saturated with water vapor eventually, leading to
lower adsorption capacity for CO
2
over time.
Activated carbons are carbons produced from
carbonaceous source materials, possessing pores.
Compared to zeolites, activated carbons are
hydrophobic, therefore the issues from water vapor
are not main concerns. Activated carbons have high
surface areas and therefore high adsorption capacity.
However, due to the uneven distribution of pores and
the various pore sizes in activated carbons, they are
more suitable for high-pressure gas separation
applications compared to low-pressure carbon
capture processes, such as trace CO
2
capture.
Although both zeolites and activated carbons can be
used to achieve efficient carbon capture, there are
some disadvantages. For zeolites, they are
outstanding in many aspects but it can be easily
saturated by water vapor. For activated carbon, the
pores for activated carbon do not have uniform size,
even though it has high surface area, but the
adsorption capacity in low pressure condition is not
satisfactory.
To this end, the development of new high-
efficiency carbon capture materials has become a
current research focus. Moreover, different types of
carbon capture functional materials have also been
gradually developed, such as metal-organic
frameworks (MOFs). MOFs are a kind of compounds
with one, two- or three-dimensional structures formed
by the coordination of metal ions or clusters with
organic ligands. People use MOFs for gas storage
because of the pores in it. MOFs may help increase
the energy density of the gas stored in the tank,
thereby increasing travel distance or reducing the
space required for the tank, rather than increasing the
pressure in the tank, which results in high tank weight
and compression costs. Compared with the two
porous materials introduced above (zeolites and
activated carbons), the use of MOFs shows great
advantages in carbon capture because they are tunable
in both porosity and chemical functionality. For
example, in the carbon capture process, there are
synergistic effect that can result in rapid and strong
adsorption of CO
2
in the pores, excluding larger and
smaller molecules, make the material has high
selectivity for CO
2
when compared with Reactive
amine-containing materials or complete molecular
sieves. As a result, MOFs are expected to replace
zeolite and activated carbon for carbon capture in the
future.
In general, this research will mainly introduce the
synthesis of several typical MOFs materials and their
performance regulation. On this basis, the carbon
capture performance of these MOFs materials was
further systematically analyzed.
2 MOFS-BASED CO
2
CAPTURE
MOFs materials have been widely used in the
selective separation of gases and show attractive
application prospects, due to its highly coordinatively
unsaturated metal surface sites. One notable example
is the use of the prepared Mg
2
(dobdc) for gas
separation (
Sumida, 2012)
. As the CO
2
molecule
features electronegative O atoms and an
electropositive C atom, any metal site that could
donate or accept electrons would be great adsorption
choices, as shown in Fig. 1. However, many current
materials with such features perform rather average
(Liu, 2019), such as Cu-BTC which had only modest
carbon capture capabilities, due to low isosteric heat
of CO
2
. Even the best adsorption capacity reported,
Mg-MOF-74, was comparable to other sorbents like
zeolites (Liu, 2019). However, strongly basic metals
with unsaturated coordination sites like Mg and Cu
have great potential as building blocks for the
preparation of new MOFs-based functional materials
to capture CO
2
. Increasing the number of open metal
sites allows greater opportunities for gas-MOF
interactions, with metals with multiple oxidation
states yielding some of the best CO
2
adsorption
values (Aniruddha, 2020).
For instance, MOF-801 resembles UiO-66 in
terms of its backbone architecture and exhibits a high
affinity for CO
2
as a result of the coordination of
FSB 2022 - The International Conference on Food Science and Biotechnology
94

Figure 1: Application of MOFs materials for CO
2
capture (Liu, 2019).
hydroxyl groups to Zr clusters (Sun, 2019). The
MOF-801/PEBA composite membrane had much
better CO
2
permeability and selectivity. MOF-801
particles are created via a multi-step process. In an
autoclave, fumaric acid and and ZrOCl
2
was
dissolved in a mixture of N, N-dimethylformamide
(DMF) and formic acid (20:7 by volume). The
hydrothermal synthesis was place in an autoclave at
130 °C for 6 hours, after which the white particles
were precipitated and washed at least three times with
DMF and methanol. The cleaned particles underwent
solvent exchange with methanol and three DMF
rinses per day for three days in a row. To create the
activated MOF-801, the solvent exchange particles
were vacuum dried at 150 °C for 12 hours. An
ethanol/water solvent was used to disperse MOF-801
nanoparticles, and the mixture was agitated and
sonicated for two hours. The PEBA was then
combined with the MOF-801 dispersion at 80 °C
while being stirred and refluxed for two hours. A
mixed matrix composite membrane made of MOF-
801/PEBA was created. In order to create a pure
PEBA composite membrane as a control, the PEBA
solution was spin-coated on the PAN support under
the same circumstances. The PTFE petri dish also
develops a thick layer. The films were dried for two
to three days at ambient temperature, the solvent was
then removed and vacuum dried. The weight of MOF
divided by the sum of the weights of MOF and PEBA
was used to calculate the loading of MOF-801 in the
film.
The capacity of mixes is assessed through
measurements of gas permeation. A constant volume
system was used to measure the permeability of pure
gas at 20 °C. Before measuring each gas, the
membrane and permeation system accumulated
overnight. Under a specific pressure, gas is pumped
into the gas reservoir, and the sensor records the
permeability’s change as a function of pressure. The
prepared MOFs was characterized by using SEM,
XRD, FT-IR and TGA, as shown in Fig. 2. The
synthetic MOF-801 octahedral structure had a
homogeneous particle size distribution according to
the SEM images. These tiny nanoparticles make it
easier to create mixed-matrix composite films that are
uniformly thin. XRD was further used to examine the
crystal structure of the produced materials, and the
thermogravimetric analysis (TGA) was used to
examine the thermal stability of the produced
materials. The outcomes demonstrate that at 500 °C,
the synthesized MOF-801 goes through three steps of
mass loss. The first minimal weight loss of the
activated sample occurred prior to 100 °C and was
brought on by the elimination of moisture. The
second weight loss occurred at about 250 °C and was
caused by the evaporation of visitor molecules from
the pores of MOF-801. These molecules included
solvents like DMF and methanol. The final
breakdown points of MOF-801 shows at about 500 °C
after additional heating, proving the substance’s high
thermal stability. Although MOF-801 has a greatly
enhanced affinity and adsorption capacity for CO
2
, it
has a relatively low adsorption capacity for N
2
. The
adsorption capacity of CO
2
grew significantly as the
adsorption operating pressure increased, whereas the
adsorption capacity of N
2
barely changed. This
behaviour suggests that particular CO
2
gas adsorption
sites exist, proving that CO
2
has a greater affinity than
N
2
. The outcomes demonstrate the potential of MOF-
801 as a N
2
-selective CO
2
adsorbent.
Different CO2 Capture Methods Based on Metal-Organic Frameworks
95
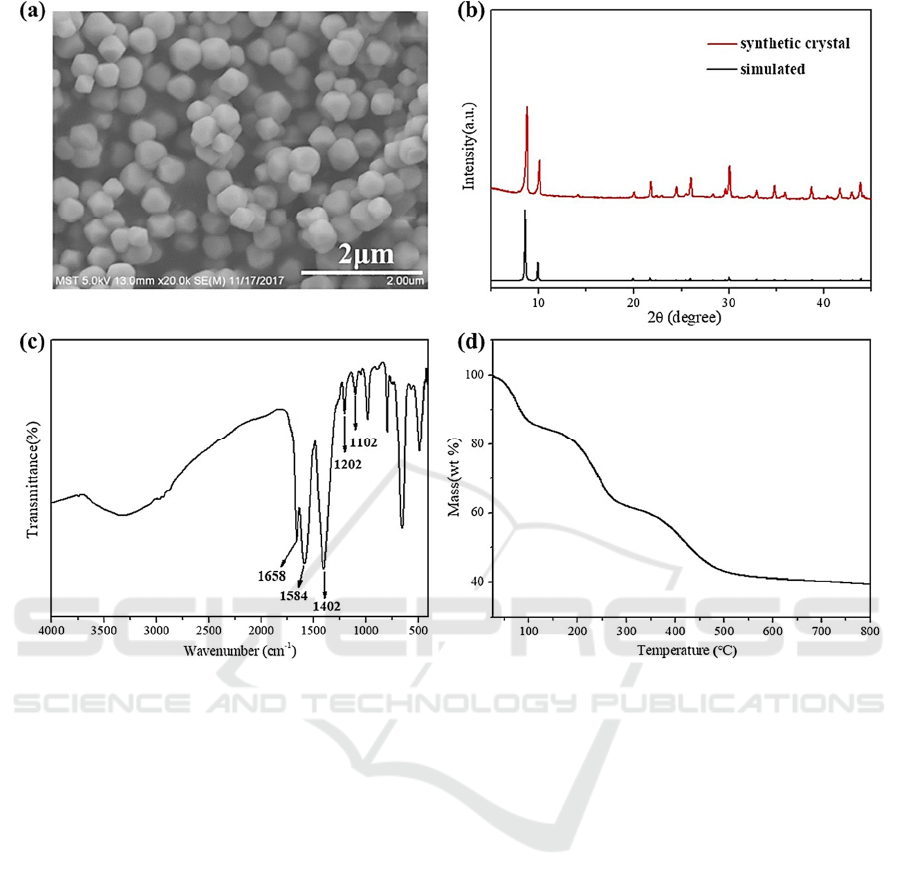
Figure 2: SEM, XRD, FT-IR and TGA characterization of the prepared MOFs materials (Sun, 2019).
If some active functional groups are introduced in
the preparation of MOFs materials, the prepared
MOFs materials can exhibit better carbon capture
effect. For example, the prepared rht-MOF-7
displayed high affinity for CO
2
(Sun, 2019), where
the added functional groups including amine and
triazine functional groups enhanced attraction
towards CO
2
. Enhancement of CO
2
capture can be
achieved with functional groups, where the
introduced different functional groups can donate
electrons. The outer orbital of the N atom in the amine
group has a pair of electrons that can be used for
contribution. Two approaches to tethering amine
units to MOFs to enhance CO
2
capture are achievable,
but amine corrosion is not something that most MOFs
can experience, so the stability of MOFs is
particularly important. For example, it is to attach
amine groups to organic ligands. In the pores of Cu-
BTTri, the N atom in the amine is bound to the C atom
of CO
2
through the strong Coulomb force, and the O
atom of CO
2
is bound to the copper atom of the
unsaturated site (
Sumida, 2012)
. Apparently, the
amine-grafted Cu-BTC could not over-absorb a
satisfactory amount of CO
2
. At higher CO
2
partial
pressure, the amine units blocked the small pores and
reduced the absorption capacity compared with bare
materials. Therefore, some mesoporous MOFs were
used for the modification of amines. A family of fcu-
MOFs that were based on ligands that were rare earth
metals, fluorinated and non-fluorinated and either
included or excluded hetero-functionality was
analyzed. This enabled fine-tuning of the MOFs such
as the fact that earth metals were electron rich and
possessed high charge density that was localized. An
MOF that contains open metal sites but no tetrazolate
and fluoro groups, Y-pek-MOF-1 showed one of the
highest CO
2
volume adsorption till today.
Zn
4
O(BDC)
3
has functionalized ligand analogues by
differing the substitution of linear dicarboxylate
linkers, and are also referred to as IRMOFs, which are
distinguished by different pore sizes and
functionalities (
Sumida, 2012)
.
FSB 2022 - The International Conference on Food Science and Biotechnology
96
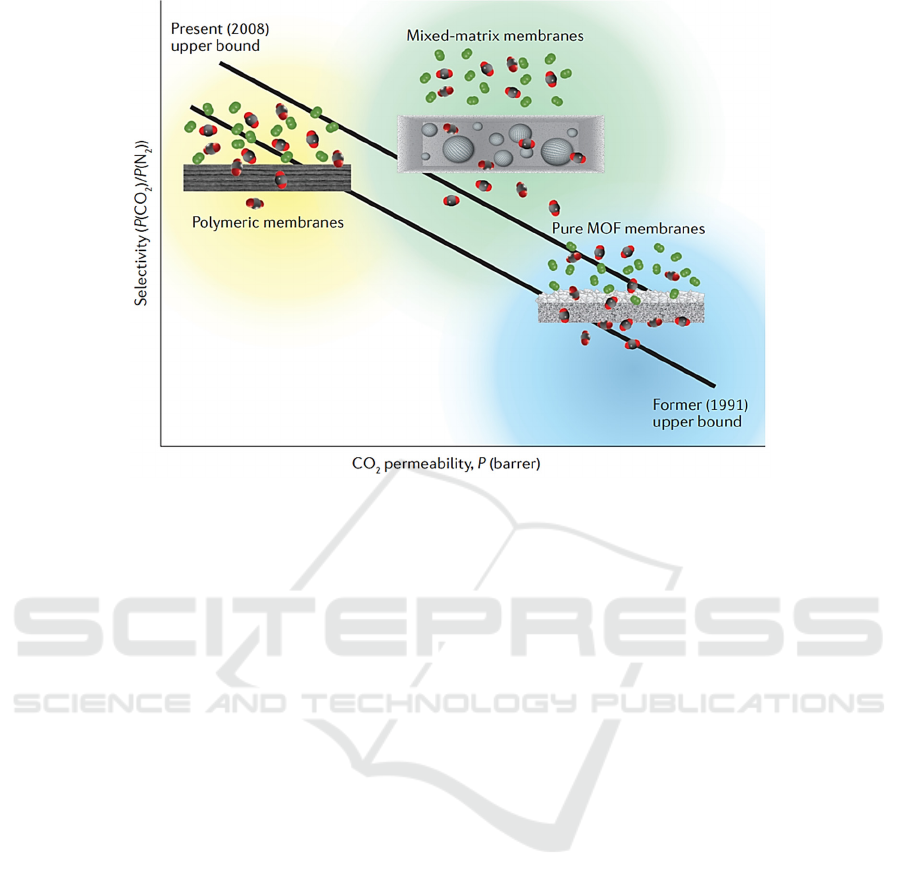
Figure 3: The selectivity and permeability of CO
2
with the prepared MOFs materials (
Trickett, 2017).
The inclusion of heteroatoms, atoms which are not
carbon, usually those which are nucleophilic, serve as
strong interactors with CO
2
. Amines are of great
interest, and one such MOF, CAU-1 achieved a
modest CO
2
uptake with a high isosteric heat of
adsorption (
Trickett, 2017)
. As shown in Fig. 3, the
as-prepared MOFs material exhibits excellent
performance in carbon dioxide selectivity and
permeability. In addition, according to the relevant
research, the CAU-1 was synthesized by
solvothermal method (Zhong, 2021). The samples
were heated to 125 °C in an autoclave for 5 h, cooled
to room temperature, and rinsed three times with
CH
3
OH. The impurities in the channels were then
completely removed by three purifications (5-6 h with
magnetic stirring) with deionized water.
SiFSiX features ultra-micropores, whose size can
be precisely fine-tuned by trying different
combinations of organic ligands and metal cations in
the overall structure. For example, SiFSiX-3-M was
made from MSiF6 and features a uniform distribution
of adsorptive sites due to its one-dimension channel
which is tailored for CO
2
capture. Isostructural
analogues of these MOFs also show phenomenal CO
2
capture capabilities. CALF-20 provides nanosized
pores which form weak but preferential binding to
CO
2
over water. It prevents the issue of contaminants
ruining the entire process by inhibiting the formation
of hydrogen bonds to water, preventing water from
adsorbing onto the material and blocking CO
2
adsorption. The key highlights of CALF-20 is that it
features a massive surface area, displays selective
CO
2
physisorption at high capacities and has low
energy requirement (Ozin, 2022). Due to its tailored
one-dimensional channels and uniformly distributed
adsorption sites, SiFSiX-3-Cu exhibits a CO
2
capture
capacity of 1.24 mmol/g. When using NbOF clusters,
the obtained NbOFFIVE-1-Ni is more attractive to
CO
2
than SiFSIX-3-Cu because the attraction of
negatively charged fluoride anions and positively
charged carbon atoms can form a one-dimensional
chain to attract CO
2
. In addition, the results show that
the small pores in it allow the MOFs to possess a
strong CO
2
capture capability. Therefore, when CO
2
passes through the small hole, special deformation
occurs, which slows down the gas propagation. The
uniform distribution of fluoride anions also helps
SiFSIX-3-Cu and NiOFFive-1-Ni achieve strong
ability when capture. The high affinity of Florine ions
attracts C cations in CO
2
, increasing the mutual
adsorption and reaction rates (Bhatt, 2016). As shown
in Fig. 4, compared with other porous materials, the
prepared MOF materials also showed excellent
performance in CO
2
absorption. Of course, in order to
improve the CO
2
adsorption capacity of the MOFs
material, the open metal sites of the MOFs material
can also be regulated (Montoro, 2012).
3 CONCLUSION
This research introduces the synthesis methods of
Different CO2 Capture Methods Based on Metal-Organic Frameworks
97
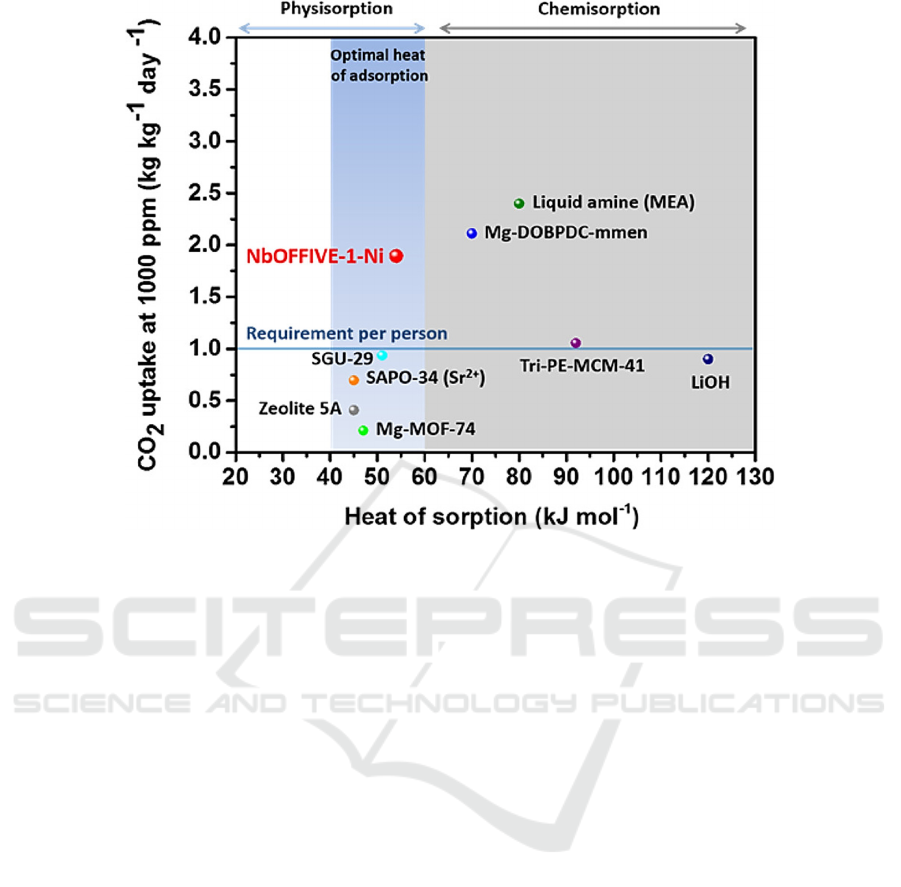
Figure 4: Performance of the heat of adsorption-CO
2
uptake with different porous materials (Bhatt, 2016)
different MOFs materials and their performance in
carbon capture. To further improve the carbon
capture efficiency of MOFs materials, different
preparation strategies were used, such as surface
modification. In the future, as more and more solid
sorbents, especially porous materials, are researched
and produced, key parameters need to be evaluated to
discover the optimal solid sorbent carbon capture
process. First, the adsorption capacity of the solid
adsorbent needs to be high. Second, the adsorption
kinetics should favor the separation of CO
2
from
other gaseous components such as nitrogen, oxygen,
and carbon monoxide. The adsorption rate should
also be relatively high, resulting in higher industrial
efficiency. In addition, the adsorption should have
high selectivity to CO
2
, which not only can capture a
higher proportion of CO
2
, but also ensure the high
purity of the captured CO
2
. From the perspective of
energy demand and cost, the regeneration conditions
should be mild, so the temperature and energy
required for the regeneration of solid sorbents are
relatively low, which is advantageous compared to
traditional aqueous sorbents. However, there is a
trade-off between the working capacity and the
regeneration requirement, as the higher the adsorption
enthalpy, the more favorable the adsorption of CO
2
,
but also the higher heat required for the desorption
process. In addition, the solid adsorbent should have
high stability, especially against steam and other
impurities such as NO
x
and H
2
S. Therefore, if the
material is to be used in real industrial applications,
life cycle and performance analysis should not be
performed under N
2
/CO
2
mixed conditions, but more
engineering evaluation should be performed in a
dynamic environment. Finally, when it comes to
industrial applications, it is always important to scale
up the yield of various porous materials, as well as the
cost per process.
REFERENCES
Aniruddha, R., Sreedhar, I., & Reddy, B. M. (2020) MOFs
in carbon capture-past, present and future. Journal of
CO
2
Utilization, 42, 101297.
Belmabkhout, Y., Guillerm, V., & Eddaoudi, M. (2016)
Low concentration CO
2
capture using physical
adsorbents: are metal-organic frameworks becoming
the new benchmark materials? Chemical Engineering
Journal, 296, 386-397.
Bhatt, P. M. et al. (2016) A Fine-Tuned Fluorinated MOF
Addresses the Needs for Trace CO
2
Removal and Air
Capture Using Physisorption. J. Am. Chem. Soc. 138,
9301-9307.
Jiajia Sun, Qianqian Li, Guining Chen, Jingui Duan,
Gongping Liu, Wanqin Jin. (2019) MOF-801
incorporated PEBA mixed-matrix composite
membranes for CO
2
capture. Separation and
Purification Technology, 217, 229-239.
FSB 2022 - The International Conference on Food Science and Biotechnology
98

Liu, J., Wei, Y., & Zhao, Y. (2019) Trace carbon dioxide
capture by metal-organic frameworks. ACS Sustainable
Chem. Eng. 7 (1), 82-93.
Montoro, C. et al. (2012) Functionalisation of MOF Open
Metal Sites with Pendant Amines for CO
2
Capture. J.
Mater. Chem. 22, 10155-10158.
Ozin, Geoffrey. “Calf-20: A Carbon Capture Success
Story.” Advanced Science News, January 26, 2022.
https://www.advancedsciencenews.com/calf-20-a-carb
on-capture-success-story/.
Sumida, K., et al. (2012) Carbon dioxide capture in
metal–organic frameworks. Chemical reviews,
112(2), 724-781.
Trickett, C., Helal, A., Al-Maythalony, B. et al. (2017)
The chemistry of metal–organic frameworks for CO
2
capture, regeneration and conversion. Nat Rev
Mater, 2, 17045.
Zhong X, Liang W, Wang H, et al. (2021) Aluminum-based
metal-organic frameworks (CAU-1) highly efficient
UO
2
2+
and TcO
4
−
ions immobilization from aqueous
solution. Journal of Hazardous Materials, 407: 124729.
Different CO2 Capture Methods Based on Metal-Organic Frameworks
99
