
Cisplatin and Its Intercellular Cytotoxicity: Mechanism Derived from
Different Pathway and Progression Targeting Toxicity Concern
Minhui Liu
1,†
, Yingxuan Yuan
2,*,†
and Xiuqi Zhang
3,†
1
Univeristy of Rochester, NY, U.S.A.
2
NORTHWEST A&F University, Shaanxi, China
3
World Foreign Language Academy, Shanghai, China
†
These authors contributed equally
Keywords: Cisplatin, Mechanism, Resistance, Toxicity, Applications.
Abstract:
Cisplatin is a metal platinum complex with anticancer activity. It is a cell cycle nonspecific antitumor drug
that can produce effective cytotoxicity. It mainly crosslinks with DNA, induces apoptosis, inhibits cell
viability and kills tumor cells, and also has a certain effect on RNA and protein of tumor cells. Cisplatin, as a
kind of classic anticancer drug, plays an important role in clinical chemotherapy. The emergence of cisplatin
resistance is the product of the interaction of multiple mechanisms. In this paper, by consulting the relevant
literature on cisplatin in recent years, the paper summarizes cisplatin's main mechanisms and recent research
progresses.
1 INTRODUCTION
Antineoplastic drugs, specifically against tumor cells,
exert their effects through multiple mechanisms, but
more or less have counter-effects present in each type
of drugs. 5-fluorouracil (5-Fu), a pyrimidine
antagonist, blocks the DNA synthesis by converting
to 5F-dUMP and subsequently prevents tumor cell
growth by inhibiting cell proliferation (Paul, 2021).
However, fluorouracil has a high toxicity in rapidly
growing cells, such as bone marrow and intestinal
epithelium, and it also causes mild leukopenia
(decreasing in white cell counts), thrombocytopenia
(decreasing in blood platelets counts), and central
nerve system (CNS) toxicity that's inversible (Papich,
2016).
Vinblastine, an anticancer drug often used in the
past years binds to microtubules protein tubulin,
which leads to the termination of microtubules
assembly in metaphase and the chromosome splitting
to stop the growth of the cell. Yet, in Zhou's research,
vinblastine causes the death of myocardial cells
through coronary spasm and cardiac arrest. As a
result, heart damage inevitably happened in both vitro
and vivo use of vinblastine (Zhou, 2021). In addition,
vinblastine leads to hyperpigmentation or bluish-
black discoloration over the nose and fingertips along
with darkening of the nail beds without any other
sources of influences (Chakraborti, 2020). Cisplatin
(CP) as a metallic-platinum-containing anticancer
drug targets multiple locations to exert its effects on
the sexual organs on both male and female,
respiration system, pancreas, and breast to block the
growth of the tumor in cells (Santos, 2020), and
platinum based concurrent chemoradiotherapy
becomes a necessary step in cure of locoregionally
advanced nasopharyngeal carcinoma or a head and
neck cancer with a specific geographic distribution
(Zhang, 2019). It generally binds to the mitochondrial
DNA and damages the DNA that triggers the immune
system to haveresponses to the damage (Papich,
2016). CP affects the level of expressed mtDNA
reactive oxygen species (ROS) which disrupts the
equilibrium of oxygen, ATP production, and ROS
which increases the expression of apoptosis caspases
and leads to cell death. Lastly, the release of
chemokines and cytokines from DNA damage
induced by ROS causes inflammation in cell (Papich,
2016; Zhang, 2019) (figure 1).
Despite the number of routes to control the growth
of cancer cells, cisplatin has limitations, so it does not
exhibit its full potential. One limitation is resistance
which the DNA repair system repeals the outsider
80
Liu, M., Yuan, Y. and Zhang, X.
Cisplatin and Its Intercellular Cytotoxicity: Mechanism Derived from Different Pathway and Progression Targeting Toxicity Concern.
DOI: 10.5220/0012002600003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 80-87
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
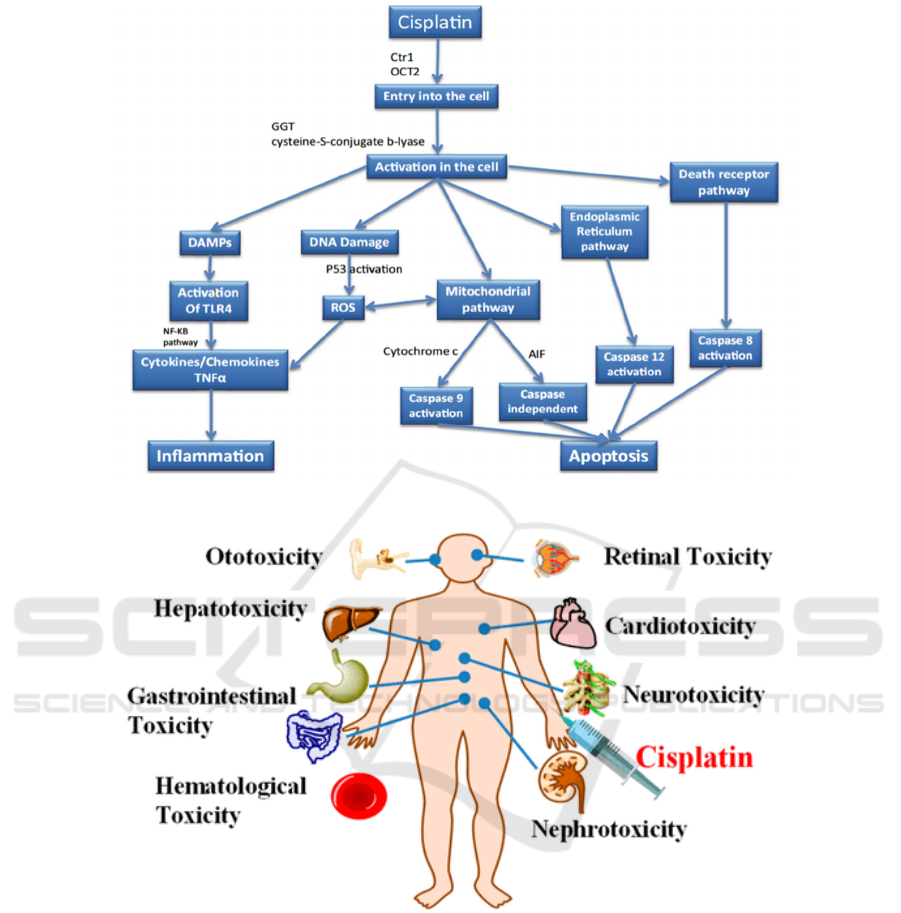
Figure 1: The overall picture of different pathways involved in cisplatin nephrotoxicity (Manohar, 2018).
Figure 2: The variety of toxicities produced after cisplatin is injected (Qi, 2019).
cisplatin and lowers the effectiveness of the drug;
another limitation is the inactivation of the drug by
binding to different proteins which lead to weakened
signaling to apoptosis and pro-survival mechanisms
(Ghosh, 2019); lastly, cisplatin is highly dosage
dependent, and when a higher dose is taken, it leads
the different type of toxicities in the body, and the
nephrotoxicity is the most common one (figure 2).
Those toxicities are not lethal, but they affect the life
quality of the patients through discomfort, and
subsequently reduce the treatment time and the effect
of cisplatin (Qi, 2019). The main focus of this paper
is to introduce different pathways hat are derived
from DNA damage and the production of ROS and
analyze how the resistance occurs.
2 MECHANISM
2.1 DNA Damage and ROS
Cisplatin (CP) contains a platinum atom that can
covalently bind to DNA, but only a small percentage
of CP is directly bound to DNA, and most of the
Cisplatin and Its Intercellular Cytotoxicity: Mechanism Derived from Different Pathway and Progression Targeting Toxicity Concern
81

binding is through histone H1. H1, platinum (pt), and
DNA form a ternary complex. This complex makes
the binding between cisplatin to DNA stronger than
just them alone, and H1 also strengthens the effect of
cisplatin by inhibiting the DNA repair mechanisms
(Cheng, 2019). Recently, another paper has proposed
another mechanism for the binding of CP to DNA
based on dissociative electron transfer (DET), in
which cisplatin accepts electrons from DNA to form
two reactive radicals and DNA adducts (Li, 2019).
Due to the binding, cells activate the repair pathway
to fix the DNA adducts (Kleih, 2019), and two types
of repair are activated, global repair (GR) and
transcription-coupled repair (TCR). Acting on
transcribed strands of active genes to repair the
modified DNA, TCR is initiated by the translocating
RNA polymerases and Escherichia coli RNA
polymerase (RNAP). GR, on the other hand, acts on
the non-transcribed strands of transcribed genes and
is initiated by NER factor UvrA and it removes DNA
damage from the entire genome (Yimit, 2019). If the
DNA adduct is not being fixed by the repairment,
ROS will be produced. ROS is produced during
mitochondrial respiration (Kleih, 2019; Yimit, 2019).
In most situations, the energy is produced in the form
of ATP by mitochondria, and oxygen produced from
the ETC will form water molecules. However, some
oxygen can escape from this process, and ROS are
produced. The overproduction of ROS can cause
oxidative stress which disrupts the equilibrium of
organelles and lowers the ATP synthesis from the
ETC which increases apoptotic factors and decreases
in anti-apoptotic factors which lead to cell death and
other serious issues.
2.2 Mitochondrial Pathway
It is known that mitochondria confer bioenergetic
plasticity to tumor cells, enabling cells to escape
death pathways under stress conditions such as
chemotherapy. To date, we recognize the central role
of mitochondria, and we recognize that mitochondria
are promising pharmacological targets for
overcoming cisplatin resistance. Cisplatin-induced
cytotoxicity is not caused by the heavy metals
themselves, but rather by active metabolites
transformed by high concentrations of cisplatin into
the intracellular environment. Under cisplatin,
mitochondria regulate death receptors by affecting
the expression of tumor suppressor proteins (TNF-)
and then lead to cell mitochondrial dysfunction which
activates AIF, caspases, and other pro-apoptotic
factors to induce tumor cell apoptosis (Volarevic,
2019).
The first pathway is the pro-apoptotic factor
cytochrome activates caspase-9. This is an apoptotic
pattern dependent on proapoptotic factors. This
process is after cisplatin stimulated cells, BH3-
Interacting Domain Death Agonist (Bid), a member
of the Bcl-2 protein family that regulates the
permeability of exterior mitochondrial membranes,
and apoptosis-promoting gene BCL2-Associated X
protein (Bax), a water-soluble-associated protein that
belongs to the rabbit anti-human monoclonal
antibody. Activation of isoproteins induces increased
mitochondrial outer membrane permeability,
interferes with mitochondrial function, and induces
the automatic release of cytochrome c-mitochondria,
which all lead to the activation of Caspase-9 (Bernal-
Barquero, 2019). Meanwhile, the reduction in ATP
synthesis forces the stressed cells to function in a
starvation mode and activate the caspase-induced
apoptosis through the release of caspase-9 mediators
which is an irreversible cell apoptosis process.
Figure 3: Caspase-dependent mechanism in cisplatin-
induced apoptosis.
Although caspase is an important pro-apoptotic
factor in apoptosis, there is increasing evidence for a
caspase-independent mechanism in cisplatin-induced
apoptosis, which is the AIF-induced apoptosis. This
is an apoptotic pathway independent of the caspase
pro-apoptotic factor (Pabla, 2008).
AIF, the universal apoptosis inducer factor, was
the first factor to induce caspase-independent cell
apoptosis in 1999. It is present in the inner
mitochondrial membrane. It differs from the typical
apoptotic process, where AIF transfers from the
mitochondria to the cytoplasm and then enters the
FSB 2022 - The International Conference on Food Science and Biotechnology
82
nucleus leading to nuclear DNA agglutination,
forming 50kb size fragments (Feldman, 2008).
Typically, AIF has oxidoreductase activity and is
involved in mitochondrial ATP generation and redox
reactions. When mitochondrial dysfunction occurs,
mitochondrial membrane permeability increases,
prompting the release of AIF molecules from the
mitochondrial cytoplasm, which then translocated to
the nucleus according to their nuclear localization
sequence and binds to chromosomal DNA. This leads
to DNA condensation and degradation, thereby
generating caspase-independent forms of apoptosis
induction. During cisplatin-induced apoptosis, AIF is
cleaved and activated and mediates caspase-
independent apoptosis signals, working together with
caspase-dependent apoptosis to induce more efficient
apoptosis in a complementary manner (Zheng, 2016).
2.3 Inflammatory Pathway
The ROS produced from the DNA damage causes the
release of chemokines and other cytokines like TNF-
α (Padgett, 2013). Resident kidney cells, such as
mesangial cells, glomerular cells, endothelial cells,
and renal tubular cells, produce TNF-α locally
(Zhang, 2007). Of all these cells, mainly renal tubular
cells contribute to the production (Ramesh, 2006).
TNF-α is essential to the generation of pro-
inflammatory factors and activation of inflammatory
cells. There are two types of TNF-α receptors in a
kidney cell: TNFR1 and TNFR2. Different signaling
cascades are activated when TNF-α binds to these
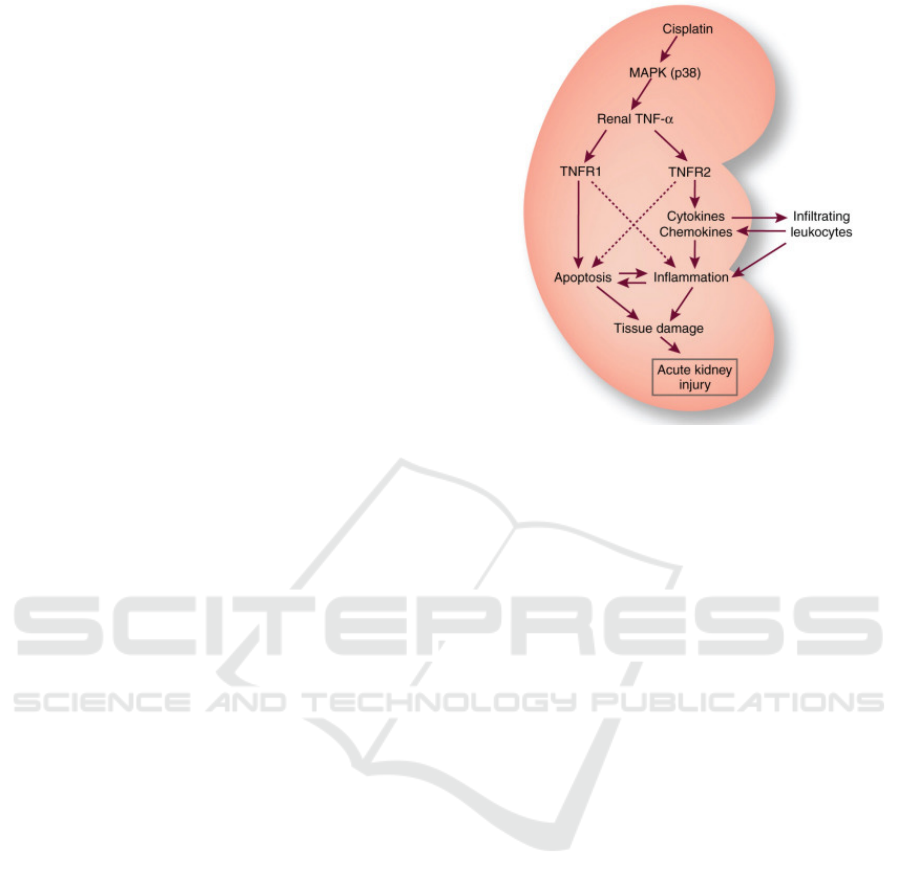
two receptors. The binding of TNF-α toTNFR2 leads
to the inflammatory response (Figure 4). When
membrane-bound TNF-α activates TNFR2, it recruits
TNFR-associated factor 2 (TRAF2), which then
activates NF-κB-inducing kinase (NIK) and
subsequently activates IKK complex (Xiao, 2001).
Originally, an inhibitory protein IκBα associates with
the NF-kappaB dimer, preventing Nf-kappaB from
entering the nucleus. Upon activation of IKK
complex, IKK phosphorylates IκBα at two N-
terminal serines, which triggers ubiquitin-dependent
IκBα degradation in the proteasome, resulting in the
nuclear translocation of NF-κB dimers (Beinke,
2004; Hayden, 2008). NF-κB dimers then drive the
expression of pro-inflammatory genes in innate
immune cells, while also controlling the activation of
inflammatory T cells (Lawrence T, 2009).
Figure 4: TNF-α production and involvement in cisplatin-
induced acute kidney injury (Dong, 2007).
2.4 Autophagy in Kidney
Autophagy is a process in which some damaged
proteins or organelles are wrapped by autophagic
vesicles with double membrane structure and then
sent to lysosomes (animals) or vacuoles (yeast and
plants) for degradation and recycling. Because it
occurs in both physiological and pathological
processes of the body, so far the relevant research has
not fully elucidated the role of autophagy in the
human body. In the past 20 years, autophagy has
always occurred in the research related to cisplatin.
Therefore, autophagy is generally considered to be
the key to promote cell survival and prevent acute
cisplatin nephrotoxicity.
Autophagy protects the kidney from damage
during cisplatin exposure, thereby limiting or
inhibiting tumor growth, but it may also reduce the
efficacy of chemotherapy because of the protection
of cancer cells. Autophagy plays an intrinsic
protective role in renal tubular cells. Under normal
physiological conditions, autophagy in the kidney
usually plays a protective cell model of promoting
growth to maintain the homeostasis of kidney cells.
When the kidney is damaged or in the period of
cisplatin exposure, cell stress will rapidly activate
induced autophagy to protect the kidney. This
autophagy is a form of cytotoxicity that promotes
tumor cell death. In addition, there is an unprotected
form that does not seem to directly affect cell
proliferation or apoptosis (Xu, 2022). When it is
proposed that autophagy may inhibit the sensitivity
of cisplatin or lead to drug resistance, it is necessary
Cisplatin and Its Intercellular Cytotoxicity: Mechanism Derived from Different Pathway and Progression Targeting Toxicity Concern
83

to distinguish the function of autophagy. In addition,
autophagy also plays a role in regulating kidney
repair, renal fibrosis, acute renal injury and other
renal diseases in the kidney.
3 CISPLATIN RESISTANCE
The main disadvantage of cisplatin therapy is its
resistance to cancerous cells. Resistance to cisplatin
varies with the kind of cancer. For example, small
cell lung, testicular, ovarian, head and neck cancers
are highly susceptible to cisplatin, while non-small
cell lung cancers and colorectal cancers are highly
resistant to cisplatin (Ghosh S, 2019). For to solve
this thorny problem, current treatments are taken
from the following perspectives: reducing cellular
drug uptake, reducing drug influx or increasing
efflux, cell thiol detoxification, changing drug
targets, and repairing DNA (Ghosh S, 2019; Zhang,
2018). There are four primary positions for resistance
to cisplatin in the human body as followed.
3.1 Resistance to Drugs When
Circulating Through the Blood
Stream
As cisplatin is injected intravenously, it flows into the
blood before entering cancerous cells. Proteins in the
blood may bind to cisplatin, particularly proteins that
contain groups of thiols, such as human serum
albumin (the carrier of fatty acids in the blood). When
the body needs energy, fat cells will release fatty
acids into the blood, which will be collected by serum
proteins and transported to the place where they are
needed. And cysteine (a common amino acid in an
organism.). Binding of this protein results in the
inactivation of cisplatin.
3.2 Resistance to Drug Influx or
Outflow Through Cell Membranes
A decrease in inflow and an increase in cisplatin
efflux leads to a decrease in drug accumulation in
cancer cells. Fulnos et al. mentioned that the decrease
in cisplatin build-up was due to a decrease in drug
uptake rather than an increase in drug efflux (Wang,
2016). In the human body, extracellular cisplatin
lowers the concentration of Ctr1, so that the dose of
its intracellular cisplatin is significantly reduced,
resulting in drug resistance. In addition, the two
copper carriers, ATP7A and ATP7B, contribute to
the flow of cisplatin from the cells, exacerbating drug
resistance.
3.3 Resistance To Cisplatin Is Present
in The Cytoplasm
By binding to glutathione and metallosulfur proteins
(low-molecular-weight proteins with metal-binding
capacity and high induction properties), the GSH and
cisplatin complex is then excreted using an outlet
pump with gs-coupling. There have been reports
suggesting that GST either aids in this response or
occurs spontaneously (Holzer, 2006).
3.4 Resistance After Cisplatin-DNA
Binding
NER is the most effective way to eliminate DNA
damage to produce resistance to cisplatin. To restore
expression of gene integrity, the NER system
removes damaged nucleotides from the two strands
to resynthesize DNA. Cells overexpressed with the
NER would be far less cisplatin conscious. The MMR
(miss match repair protein) protein is a very essential
protein that is commonly used to repair DNA-
cisplatin injuries. If it can be fixed, those cells can
still survive. In place of this, apoptosis is caused
(Ghosh S, 2019). As a result, drug resistance takes
place.
3.5 Toxicity
Cisplatin induces different levels of nephrotoxicity,
ototoxicity, and neurotoxicity depending on the dose
and the individual difference. The nephrotoxicity is
the most severe one. Cisplatin-induced Acute kidney
injury (AKI) involves proximal tubular injury,
apoptosis, oxidative stress, inflammation, and
vascular injury in the kidneys, which followed by
acute renal failure, chronic kidney disease if the
toxicity remain untreated and the medicines are keep
in using. From Fang's studies, it shows that natural
products have the ability to against oxidants,
inflammatory, and apoptosis, which regulate the
damage caused by cisplatin. As example, ginseng and
pomegranate reduce ROS that's produced from
cisplatin by restoring the antioxidant enzymes, which
decreases the inflammation and subsequently the
level of nephrotoxicity in the body (Fang, 2021).
Cisplatin causes hearing damage that is
irreversible, high frequency hearing loss. Cisplatin
mainly damages the outer sensory hair cells, and it
also could have impacts on the spiral ganglion
neurons (SGNs) in the cochlea. To prevent the harm,
FSB 2022 - The International Conference on Food Science and Biotechnology
84

the main goal is to prevent inflammation, oxidation,
and apoptosis occurring in the cells. Alpha-Lipoic
acid according to research can lower the ROS levels,
and strengthen the hearing ability. In addition,
neurotrophins have also been proposed in the
treatmentof ototoxicity, because hair cells maintain a
healthy SGNs by releasing neurotrophic factors, and
ototoxic damages conduct degeneration of SGNs.
Moreover, because neurotrophins’ receptors are
present in neurons but not in cancer cells, it
maximizes the effect of chemotherapy without
interrupting other treatment on cancer cells (Santos,
2020).
Neurotoxicity in some situations is pherpieral
toxicity which is the loss of proprioception or feeling
of one's position and body parts (Santos, 2020).
Agomelatine, an antioxidant, has been proven on its
ability to prevent cisplatin-induced neurotoxicity in
the mouse hippocampal neuronal cell line by areduce
oxidative stress and inflammation (Cankara, 2021).
Additionally, Cisplatin has been discovered toreduce
serotonin-regulated pharyngeal pumping activity
independent of neurons such as dopamine and
glutamates (Wellenberg, 2021). However, two of
serotonin derivatives, -feruloylserotonin and
coumaroyl serotonin has ability to significantly
reduce the level of ROS by increase the level of
glutathione peroxidase in the kidney, which reduce
the neurotoxicity caused by cisplatin (Park, 2019).
4 RESEARCH AND
APPLICATION OF RELATED
TECHNOLOGIES
At present, there are still a lot of scientific problems
to be solved in this field. In view of the above four
processes, cisplatin resistance in human body has
been treated in recent years. In view of that drug
resistance of cisplatin in the process of blood
circulation, Nanoparticles coupled with platinum
drugs is also one of the effective ways to solve drug
resistance, The combination of nanoparticles and
platinum has the following advantages: they can
carry more platinum compounds and the targeting
drugs to locate cancer cells more accurately, and we
can add some hydrophilic molecules to increase drug
solubility. Thus, this combination to spread in tumor
sites widely. That is not only soars efficiency, but
also declines the toxic and adverse effects of drugs
(Zhu, 2016). When cisplatin develops drug resistance
through the inflow or outflow of cell membrane, we
usually use cisplatin sensitizers, which can improve
the sensitivity to drugs at normal doses, among which
thiazides are typical representatives. It can reduce
cisplatin outflow by inhibiting P-glycoprotein (P-gP)
in drug-resistant KBV20C cells, and increase the
concentration of cisplatin in cells, thus enhancing the
cytotoxicity of cisplatin (Choi, 2014). As a new drug
delivery system, nano-micelles have great potential
advantages in reducing the toxic and side effects of
cisplatin and exerting the best therapeutic effect. For
the inactivation of platinum drugs, for example, PEG-
b-PBEMA micelles loaded with tetravalent platinum
were prepared by combining platinum complexes
with glutathione consumer-quinone methylate by
pharmaceutical means. By improving the efficiency
of platinum uptake, reducing intracellular glutathione
level and reducing platinum inactivation, the cisplatin
resistance of tumor cells can be reversed (Han, 2018).
Cisplatin binds to DNA to produce drug resistance.
At present, it is mainly by inhibiting the expression
of DNA damage repair related proteins to inhibit
DNA damage repair and improve platinum drug
resistance. PolyADPribose polymerase (PARP) is a
kind of DNA damage repair enzyme, and its inhibitor
can play a synergistic role in combination with
platinum drugs by inhibiting DNA damage repair (Li,
2021), which can enhance the anti-tumor activity of
drugs and effectively overcome tumour resistance to
cisplatin.A membrane glycoprotein PD-1, which is
expressed on the surface of various different immune
cells, is paramount in both diseases and adaptive
immune responses. Xiaoguang Liu et al. first
confirmed that PD-1 plays an important role in
regulating cisplatin induced muscle atrophy (Liu,
2022) by regulating autophagy of skeletal muscle.
5 CONCLUSION
Cisplatin nephrotoxicity is a complex process caused
by multiple factors. Therefore, it is crucial to
understand how the several pathways combined to
cause cell dysfunction. This review focuses on two of
the pathways: the mitochondrial pathway and the
inflammatory pathway (figure 1), both originated
from DNA damage. In the mitochondrial pathway,
caspase-independent mechanism triggered by AIF
leads to apoptosis. In the inflammatory pathway,
ROS triggers the release of cytokines and
chemokines which leads to inflammation. These
contributes to the cisplatin intercellular cytotoxicity.
A fascinating area for future study is to compare the
different pathways to determine if there's generality
in these mechanisms. This may give new insight into
renoprotection during cisplatin treatment.
Cisplatin and Its Intercellular Cytotoxicity: Mechanism Derived from Different Pathway and Progression Targeting Toxicity Concern
85

REFERENCES
Bernal-Barquero, C. E., Vázquez-Zapién, G. J., et.al.
(2019). Review of alterations in gene expression and
apoptotic pathways caused in nephrotoxicity induced
by cisplatin. Revisión de las alteraciones en la
expresión génica y vías apoptóticas provocadas en la
nefrotoxicidad inducida por cisplatino. Nefrologia,
39(4), 362-371.
Beinke, S., & Ley, S. C. (2004). Functions of NF-kappaB1
and NF-kappaB2 in immune cell biology. The
Biochemical journal, 382(Pt 2), 393-409.
Cankara, F.N., Günaydın, C., et.al. Agomelatine confers
neuroprotection against cisplatin-induced hippocampal
neurotoxicity. Metabolic Brain Disease 36, 339-349
(2021).
Chakraborti, A., & Sahi, P. K. (2020). Vinblastine-induced
acral hyperpigmentation. Indian Pediatrics, 57(6), 581-
582.
Choi, A. R., Kim, J. H., et.al. (2014). Thioridazine
specifically sensitizes drug-resistant cancer cells
through highly increase in apoptosis and P-gp
inhibition. Tumour biology, 35(10), 9831–9838.
Dong, Z., & Atherton, S. S. (2007). Tumor necrosis factor-
alpha in cisplatin nephrotoxicity: a homebred foe.
Kidney international, 72(1), 5-7.
Fang, C. Y., Lou, D. Y., et.al. (2021). Natural products:
potential treatments for cisplatin-induced
nephrotoxicity. Acta pharmacologica Sinica, 42(12),
1951-1969.
Feldman, D. R., Bosl, G. J., et.al. (2008). Medical treatment
of advanced testicular cancer. JAMA, 299(6), 672-684.
Ghosh S. (2019). Cisplatin: The first metal based anticancer
drug. Bioorganic Chemistry, 88, 102925.
Hayden, M. S., & Ghosh, S. (2008). Shared principles in
NF-kappaB signaling. Cell, 132(3), 344-362.
Han, Y., Yin, W., et.al. (2018). Intracellular glutathione-
depleting polymeric micelles for cisplatin prodrug
delivery to overcome cisplatin resistance of cancers.
Journal of controlled release, 273, 30-39.
Holzer, A. K., Manorek, G. H.,et.al. (2006). Contribution
of the major copper influx transporter CTR1 to the
cellular accumulation of cisplatin, carboplatin, and
oxaliplatin. Molecular pharmacology, 70(4), 1390-
1394.
Kleih M, Böpple K, et.al. (2019). Direct impact of cisplatin
on mitochondria induces ROS production that dictates
cell fate of ovarian cancer cells. Cell Death &
Disease,10(11):851.
Li, Z., Zilberman, et.al. (2019). Electrochemical methods
for probing DNA damage mechanisms and designing
cisplatin-based combination chemotherapy.
BioTechniques, 66(3), 135-142.
Lawrence T. (2009). The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harbor
Perspectives in Biology, 1(6), a001651.
Li, H., Wang, C., et.al. (2021). PARP1 Inhibitor Combined
With Oxaliplatin Efficiently Suppresses Oxaliplatin
Resistance in Gastric Cancer-Derived Organoids via
Homologous Recombination and the Base Excision
Repair Pathway. Frontiers in cell and developmental
biology, 9, 719192.
Liu, X., Xu, M., et.al. (2022). PD-1 Alleviates Cisplatin-
Induced Muscle Atrophy by Regulating Inflammation
and Oxidative Stress. Antioxidants (Basel,
Switzerland), 11(9), 1839.
Luyu Qi, Qun Luo, et.al. (2019). Chemical research in
toxicology 32,8:1469-1486
Lanjun Cheng, Chan Li, et.al. (2019). Cisplatin reacts with
histone H1 and the adduct forms a ternary complex
with DNA, Metallomics, 11,3:556-564
Manohar, S., & Leung, N. (2018). Cisplatin nephrotoxicity:
a review of the literature. Journal of nephrology, 31,1,
15-25
Paul, W., Flint, MD. (2021). Cummings otolaryngology:
head and neck surgery. Elsevier Inc. 18, 260-268.e2
Papich, M. (2016). Sauders handbook of veterinary drugs.
Pabla, N., & Dong, Z. (2008). Cisplatin nephrotoxicity:
mechanisms and renoprotective strategies. Kidney
international, 73(9), 994-1007.
Padgett, L. E., Broniowska, K. A., et.al. (2013). The role of
reactive oxygen species and proinflammatory
cytokines in type 1 diabetes pathogenesis. Annals of the
New York Academy of Sciences, 1281(1), 16-35.
Park, C. H., Lee, A. Y., et.al. (2019). Protective Effects of
Serotonin and its Derivatives, N-Feruloylserotonin and
N-(p-Coumaroyl) Serotonin, Against Cisplatin-
Induced Renal Damage in Mice. The American journal
of Chinese medicine, 47(2), 369-383.
Ramesh, G., & Brian Reeves, W. (2006). Cisplatin
increases TNF-alpha mRNA stability in kidney
proximal tubule cells. Renal Failure, 28(7), 583-592.
Santos, N., Ferreira, R. S., et.al. (2020). Overview of
cisplatin-induced neurotoxicity and ototoxicity, and the
protective agents. Food and Chemical Toxicology, 136,
111079.
Santos, N., Ferreira, R. S., et.al. (2020). Overview of
cisplatin-induced neurotoxicity and ototoxicity, and the
protective agents. Food and chemical toxicology, 136,
111079.
Volarevic, V., Djokovic, B., et.al. (2019). Molecular
mechanisms of cisplatin-induced nephrotoxicity: a
balance on the knife edge between renoprotection and
tumor toxicity. Journal of biomedical science, 26(1),
25.
Wang Langli, Li Na, et.al (2016). Research progress of
first-line chemotherapy drugs for non-small cell lung
cancer Chinese pharmacy, 27 (5), 4
Wellenberg, A., Brinkmann, V., et.al. (2021). Cisplatin-
induced neurotoxicity involves the disruption of
serotonergic neurotransmission. Pharmacological
research, 174, 105921.
Xiao, G., Harhaj, E. W., et.al. (2001). NF-kappaB-inducing
kinase regulates the processing of NF-kappaB2 p100.
Molecular Cell, 7(2), 401-409.
Xu, J., & Gewirtz, D. A. (2022). Is Autophagy Always a
Barrier to Cisplatin Therapy? Biomolecules, 12(3), 463.
Yimit, A., Adebali, O., et.al. Differential damage and repair
of DNA-adducts induced by anti-cancer drug cisplatin
across mouse organs. Nature Communications, 10, 309
FSB 2022 - The International Conference on Food Science and Biotechnology
86

(2019).
Zhang, Y., Chen, L., et.al. (2019). Gemcitabine and
cisplatin induction chemotherapy in nasopharyngeal
carcinoma. New England Journal of Medicine,
381(12), 1124-1135.
Zhang, B., Ramesh, G., et.al. (2007). Cisplatin-induced
nephrotoxicity is mediated by tumor necrosis factor-
alpha produced by renal parenchymal cells. Kidney
International, 72(1), 37-44.
Zhang Bicheng, & Zhu Bo (2018). Current status and
future of chemotherapy for advanced non-small cell
lung cancer Medical Guide, 37 (5), 5
Zheng, J. H., Viacava Follis, A., et.al. (2016). Discoveries
and controversies in BCL-2 protein-mediated
apoptosis. The FEBS journal, 283(14), 2690-2700.
Zhou, H., Liu, L., et.al. (2021). RIP1/RIP3/MLKL-
mediated necroptosis contributes to vinblastine-
induced myocardial damage. Molecular and Cellular
Biochemistry, 476(2), 1233-1243.
Zhu, H., Luo, H., et.al. (2016). Molecular mechanisms of
cisplatin resistance in cervical cancer. Drug design,
development and therapy, 10, 1885-1895.
Cisplatin and Its Intercellular Cytotoxicity: Mechanism Derived from Different Pathway and Progression Targeting Toxicity Concern
87
