
Study on Methods of Protoplast Preparation of Aspergillus Niger
Xiangzhen Cui
Department of health caring industry, Shandong Institute of Commerce and Technology, Jinan 250103, China
Keywords:
Aspergillus Niger, Protoplast, Regeneration.
Abstract:
Filamentous fungi are important industrial strains. Aspergillus niger is a safe organism for the production of
proteins, organic acids, and chemicals. In order to improve the efficiency of genetic manipulation of A. niger,
we studied the method of preparing protoplasts and improving the regeneration rate, and established a method
of efficient transformation of protoplasts by exogenous DNA. Studies have shown that the preparation and
regeneration of A. niger protoplasts are affected by many factors, such as mycelial growth state, osmotic
pressure stabilizer, cell wall lyase system, enzymatic hydrolysis time, regeneration medium composition and
so on. The highest efficiency of protoplast release and regeneration was obtained by using the mycelium of
A. niger fresh spores cultured for 13-14 hours, using a mixture of snailase and lyticase, 0.6 M sorbitol as an
osmotic pressure stabilizer, and enzymatic hydrolysis at 32 °C for 2 hours.
1 INTRODUCTION
Filamentous fungi are important industrial strains.
The typical filamentous fungi such as Aspergillus
niger, Aspergillus oryzae, Penicillium chrysogenum,
Trichoderma reesei are widely used in the production
of organic acids, antibiotics, enzymes and proteins.
The filamentous fungi A. niger, A. oryzae and T.
reesei are recognized as safe production strains with
mature fermentation and post-processing technology
(Frisvad JC, 2018). As the host of genetic
engineering, filamentous fungi have unique
advantages different from that of bacteria and yeast.
They can correctly perform various post-translational
processing similar to higher eukaryotes. They have
high protein secretion capacity. They can degrade and
utilize a variety of biopolymers, such as starch,
cellulose, hemicellulose, pectin, xylan and protein, so
that they can use renewable resources such as plant
biomass for culture (Knuf C, 2012).
The commonly used transformation methods of
filamentous fungi are protoplast olyethylene glycol
(PEG) transformation, electrotransformation, and
Agrobacterium-mediated transformation. To obtain
higher transformation efficiency, we must obtain
more and higher quality protoplasts, and more
efficient transformation method.
In this study, we first established a method for the
preparation and regeneration of a large number of
niger protoplasts.
2 MATERIALS AND METHODS
2.1 Strains and Media
A. niger N593 ( ATCC Number : 64973 ) is a uridine-
deficient strain. A. niger were inoculated in yeast
extract peptone dextrose (YPED) plus uridine agar
(peptone 2%, glucose 2%, yeast extract 1%, uridine
0.25%, agar 1.5%, natural pH) and cultured at 30ºC
for 5 days to obtain mature spores. Collect the spores
from the agar with sterile water, fully disperse spores
with oscillator to obtain spore concentration 10
6
/mL.
2 ~ 3 mL of spore suspension was inoculated in a 500
mL flask containing 80 mL of modified Czapek 's
medium (glucose 1 %, citric acid 0.3 %, potassium
dihydrogen phosphate 0.5 %, ammonia nitrate 0.2 %,
magnesium sulfate 0.02 %, yeast extract 0.05 %,
Tween 80 0.1 %, uridine 0.25 %, pH6.5), and cultured
for 13-15 hours on a shaker at 30 ºC and 180 rpm.
2.2 Preparation of Protoplasts
The mycelium liquid of A. niger cultured for 13 ~ 14
h was put into two 50 mL centrifuge tubes,
centrifuged at 6000 rpm for 10 min. The supernatant
was removed, and the mycelium precipitate was
retained. Add 20 mL 0.6 M KCl solution to the
centrifuge tube, fully suspend the precipitate,
centrifuge at 6000 rpm for 10 minutes, and discard
the supernatant. Wash repeatedly 2 times. Then 30
Cui, X.
Study on Methods of Protoplast Preparation of Aspergillus Niger.
DOI: 10.5220/0012001500003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 59-62
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
59
mL 0.6 M KCl solution was used to re-suspend the
precipitate, and different proportions of snailase
(S10083, Yuanye biotech, Shanghai, China) and
lyticase (from Arthrobacter luteus, L2524, Sigma-
Aldrich) (totoal 0.01g) were added. The centrifuge
tube was placed at 32ºC water bath for 1~3 hours,
intermittently shaken slightly and sampled regularly
to monitor protoplasts formed. After enzymatic
hydrolysis, the protoplast fluid was filtered with four
layers of sterile wipe mirror paper. The filtrate was
centrifuged at 4000 rpm for 10 min, and the
supernatant was discarded. The precipitate was gently
washed twice with 0.6 M KCl solution and
centrifuged. The protoplast precipitation was
resuspended in 0.5~2 mL of 0.6 M KCl solution and
placed in an ice bath for later use.
2.3 Regeneration of Protoplasts and
Determination of Regeneration
Rate
Preparation of protoplast regeneration medium
(PRM) (glucose 1%, citric acid 0.3%, potassium
dihydrogen phosphate 0.5%, ammonia nitrate 0.2%,
magnesium sulfate 0.02%, yeast extract 0.025%,
uridine 0.25%, potassium chloride 0.6 mol / L, agar
(upper 0.5 %, lower 2%), pH 6.5). Double-layer plate
coating regeneration: After preparing the regenerated
solid medium (lower layer), the freshly prepared
protoplasts were gently mixed with the cooled
regenerated solid medium (upper layer) and poured
onto the lower regenerated solid medium. cultured at
30 ºC for 3~5 days, the formed colonies were counted
(A); in order to eliminate the error caused by the
mycelial fragments remaining in the prepared
protoplast solution and the colonies formed by the
regrowth of hyphal fragment, the prepared protoplast
solution was spread on the regeneration medium
without osmotic stabilizer, and the number of
regenerated colonies was used as control (B). The
number of protoplasts observed under microscope
was C. Regeneration rate was calculated as follows:
Protoplast regeneration rate = [ (A-B) / C] × 100 %
3 RESULTS AND DISCUSSION
3.1 Effect of Lyase on Protoplast
Formation
In order to obtain a higher protoplast yield, it is
necessary to select the appropriate lyase. Fungal cell
wall composition is complex. It is mainly composed
of four layers, from outside to inside is the amorphous
glucan layer, glycoprotein layer, protein layer, chitin
layer (Free SJ, 2013). Therefore, using mixture of
different lyase is better than using one kind of enzyme
alone. Snailase contains chitinase, cellulase,
pectinase, amylase, protease, etc., often used for yeast
cell wall disruption (Cheng, 2018). lyticase, also
known as N-acetylmural glycan hydrolase, can
destroy the β-1,4 glycosidic bond between N-
acetylmural acid and N-acetylglucosamine in the cell
wall (Tang, 2015).
The mycelia cultured in modified Czapek 's
medium for 14 h were collected. 0.6 M KCl was used
as osmotic pressure stabilizer. Firstly, the effects of
snailase and lyticase on protoplast formation were
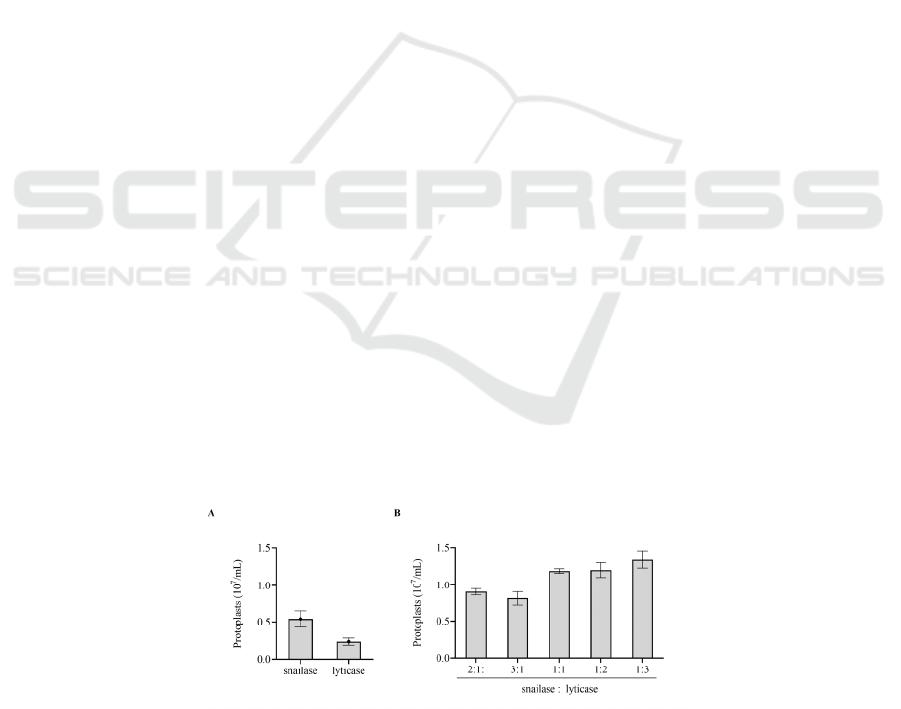
determined. The results showed that the yield of
protoplasts was 5.41 × 106 / mL and 2.32 × 106 / mL
respectively when snailase (0.01 g / 30 mL hyphal
suspend) or lyticase (0.01 g / 30 mL hyphal suspend)
was used alone after 2 hours of enzymolysis (Fig.
1A). When snailase: lyticase was 2:1, 3:1, 1:1, 1:2,
1:3 (total 0.01 g / 30 mL hyphal suspend), the
production of protoplasts was 9.09 × 10
6
/ mL, 8.17 ×
10
6
/ mL, 1.19 × 10
7
/ mL, 1.21 × 10
7
/ mL, 1.35 × 10
7
/ mL, respectively. When snailase: lyticase = 3:1,
protoplasts reached the highest (Fig. 1B).
Figure 1: Effect of cell wall lyase on protoplast formation. (A) The number of protoplast formation when snailase or lyticase
was used alone. (B) Effects of different proportions of snailase and lyticase on protoplast formation. For ease of comparison,
the vertical coordinates of (A) and (B) are consistent.
FSB 2022 - The International Conference on Food Science and Biotechnology
60
3.2
Effect of Osmotic Pressure
Stabilizer on Protoplast Formation
and Regeneration
The type and concentration of osmotic stabilizer is
the key factor to maintain and control the number of
protoplasts. It can not only maintain the osmotic
balance, but also promote the active reaction of lyase.
On the other side, osmotic stabilizer may aggravate
the damage of protoplasts or inhibit the synthesis of
cell wall during regeneration process. The mycelium
was cultured for 14 h in modified Czapek's medium,
0.6 mol / L KCl was used as osmotic stabilizer, and
the ratio of snailase: lyticase = 1:3 was used as mixed
enzyme to prepare protoplasts. The effects of two
sugars (glucose, sorbitol) and two salts (KCl, NaCl)
as osmotic pressure stabilizer on the production and
regeneration of protoplasts were studied.
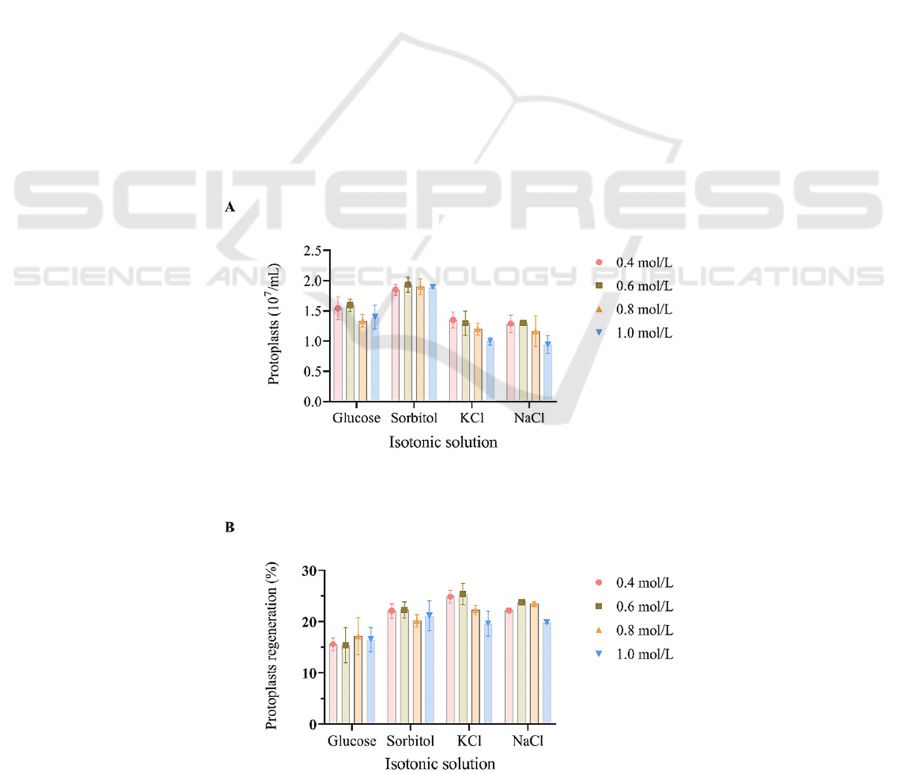
The results showed that the formation of
protoplasts was the highest when sorbitol was used as
a stabilizer. When 0.6 mol / L sorbitol was used, the
production of protoplasts was the highest, reaching
1.93 × 10
7
/ mL. When the concentration of each
osmotic pressure stabilizer was between 0.4~0.8 mol
/ L, it had little effect on the formation of protoplasts.
However, when the concentrations of KCl and NaCl
reached 1.0 mol/L, the protoplast formation was
inhibited (Fig. 2A). The osmotic pressure stabilizers
also had a certain effect on the regeneration of
protoplasts. When 0.6 mol / L KCl was used as the
osmotic pressure stabilizer, the regeneration rate of
protoplasts was the highest, reaching 25.40 % (Fig.
2B). Combining the production and regeneration rate
of protoplasts, the number of regenerated protoplasts
was the highest when 0.6 mol / L sorbitol was used.
3.3 Effect of Lyase Action Time on
Protoplast Formation and
Regeneration
The mycelium was cultured in modified Czapek 's
medium for 14 h. The ratio of snailase: lyticase = 1:3
was used as mixed enzyme to prepare protoplasts. 0.6
mol / L sorbitol was used as osmotic pressure
stabilizer, and protoplasts were prepared at 32 °C.
The process of protoplast release was observed by
regular sampling. The number of protoplast
formation was monitored (Fig. 3). The mycelium
began to release protoplasts after 30 min of enzymatic
hydrolysis.With the extension of enzymolysis time,
the number of protoplasts gradually increased, and
reached the highest after 3 h, reaching 2.55× 10
7
/mL.
Figure 2: Effects of osmotic stabilizers on protoplast formation and regeneration. (A) Protoplast formation. (B) Protoplast
regeneration.
Study on Methods of Protoplast Preparation of Aspergillus Niger
61
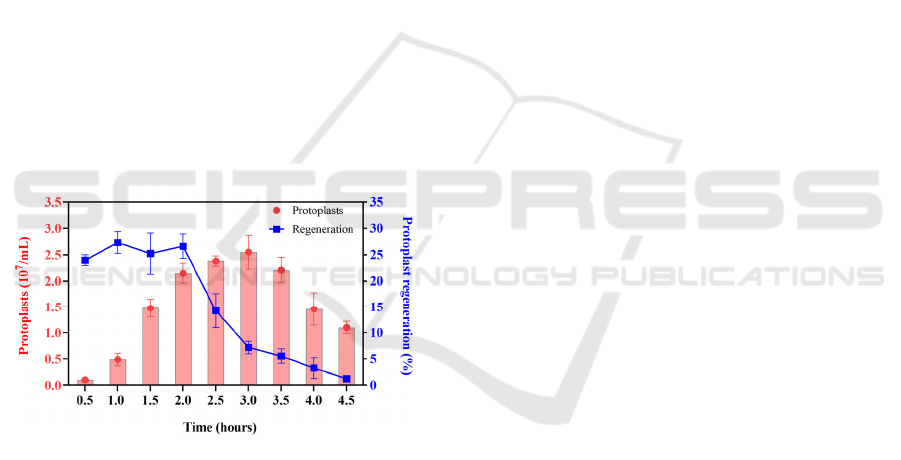
Continue to extend the enzymolysis time, the number
of protoplasts did not continue to increase, but
decreased slightly (Fig. 3). Protoplast is not stable
due to the lack of cell wall. In addition, lysing enzyme
will damage the plasma membrane system of
protoplast to some extent, so the length of
enzymolysis time will seriously affect the
regeneration of protoplast. The enzymolysis time is
short, and the protoplast has not been fully released;
with the extension of enzymolysis time, the number
of protoplasts increased greatly, and the regeneration
rate increased continuously. Continue to extend the
enzymolysis time, the regeneration rate will decline
although the total number of protoplasts will increase.
When the enzymolysis time was 3 h, although the
protoplast production reached the highest, the
regeneration rate decreased significantly, only 27.1 %
of the regeneration rate when the enzymolysis time
was 2 h (Fig. 3). When the enzymolysis time was 4 h,
the regeneration rate decreased to nearly 0. This may
be due to the lack of cell wall in the formed
protoplasts. Therefore, considering the results of
protoplast production and regeneration, the
enzymolysis time should not exceed 2 h. After
enzymolysis, the lysate should be removed as soon as
possible.
Figure 3: Effect of lysing time on protoplast formation and
regeneration.
4 CONCLUSIONS
In this study, the method of preparation and
regeneration of protoplasts and the method of
efficient transformation of A. niger protoplasts by
exogenous DNA were established. The study
provides technical support for improving the
efficiency of gene targeting in A. niger and helps
realizing the genetic engineering with A. niger as cell
factory in future.
REFERENCES
Cheng T, Xu X, Zhang W, Chen L, Liu T. (2018) Protoplast
preparation from enriched flagellates and resting cells
of Haematococcus pluvialis. J Appl Microbiol.
124(2):469-479.
Frisvad JC, Møller LLH, Larsen TO, Kumar R, Arnau J.
(2018) Safety of the fungal workhorses of industrial
biotechnology: update on the mycotoxin and secondary
metabolite potential of Aspergillus niger, Aspergillus
oryzae, and Trichoderma reesei. Appl Microbiol
Biotechnol. 102(22):9481-9515.
Free SJ. (2013) Fungal cell wall organization and
biosynthesis. Adv Genet. 81:33-82.
Knuf C, Nielsen J. (2012) Aspergilli: systems biology and
industrial applications. Biotechnol J. 7(9):1147-1155.
Tang SY, Yi P, Soffe R, Nahavandi S, Shukla R,
Khoshmanesh K. (2015) Using dielectrophoresis to
study the dynamic response of single budding yeast
cells to Lyticase. Anal Bioanal Chem. 407(12):3437-
3448.
FSB 2022 - The International Conference on Food Science and Biotechnology
62
