
Formulation and Evaluation of Inhalable Porous Tobramycin Powder
Prepared by Spray Drying Technology
Tianxiong Xu
1,a
, Hao Miao
2
, Renjie Li
2
, Daoyin Liu
3
and Zhenbo Tong
1,b*
1
Center for Simulation and Modeling of Particulate Systems, Southeast University-Monash University Joint Research
Institute, Suzhou, Jiangsu, China
2
Department of Chemical Engineering, Monash University, Clayton, Vic, Australia
3
Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, School of Energy and Environment,
Southeast University, Nanjing, Jiangsu Province, China
Keywords:
Inhaled Tobramycin, Porous Particle, Spray Drying.
Abstract:
Inhaled tobramycin is one of the ideal ways to treat cystic fibrosis. The main purpose of this work is to prepare
inhaled tobramycin dry powder by spray drying through reasonable formula design. The properties of the
engineering particles are then characterized to reveal the particle-forming mechanism and structure-activity
relationship. Tobramycin, which exists in the form of sulphate, has higher stability compared with its
preparation alone. Leucine is enriched and crystallized on the surface of particles, which inhibits the
absorption of environmental water by forming a hydrophobic shell and reduces the cohesion between
particles. Compared with the organic solvent, ammonium bicarbonate (AB) as a porogen is less toxic and
more suitable for scale-up. An optimized formulation was obtained when active pharmaceutical ingredient
(API): Leucine: AB = 20: 7: 19. The sample under this formula has a porosity of 78.93 %. The loose and
porous structure brings good flowability (CI index is 24.63 ± 1.17 %) and 40.78 % fine particles fraction
(FPF). The particle deposition performance has been significantly improved. The drug load reaches 74.1 %,
which adapts to the requirement of high-dose delivery of antibiotics.
1 INTRODUCTION
In the research history of pulmonary delivery of
antibiotics, the treatment of cystic fibrosis (CF) with
inhaled tobramycin powder was a classic case. CF
patients were particularly vulnerable to the lung
infections caused by gram-negative bacteria such as
pseudomonas aeruginosa (PA) (
Alhajj, 2022)
.
Patients infected with PA experienced acute lung
deterioration, reflected by worsening respiratory
symptoms and a sharp decline in lung function.
Tobramycin was a well-known fungicide, which was
mainly active against aerobic gram-negative bacilli
and was considered to be more active against PA than
most other aminoglycoside antibiotics (
Elborn,
2022)
.
The first tobramycin inhaled dry powder (TIP,
TOBI) inhalation product was developed by Novartis
and available in the U.S. market since 2013 (Miller,
2015). TIP should be taken twice a day. Each dose
contained 4 capsules, of which contained 200 mg dry
powder (including 112 mg tobramycin) (
Buttini,
2018)
. The number of capsules limited the
improvement of patient compliance. The excipient
used in TIP was DSPC, a human endogenous
substance, which would bring high pharmaceutical
costs. At the same time, the perfluorooctyl bromide
(PFOB) in the formulation could be replaced with a
suitable porogen, which helped avoid some safety
hazards in the production process (explosion-proof in
workshop and equipment) (
Miller, 2017)
.
The purpose of this paper was to explore the
effects of different formulations and process
parameters on the particle microstructure and final
product properties, and to study the correlations
among parameters, microstructure, properties and
performance. In this work, tobramycin inhaled dry
powder was prepared by spray drying, and the
function and particle microstructure of the above
components were verified by several charac-
terizations, and the particle forming mechanism and
structure-activity relationship of tobramycin inhaled
powder were explored.
42
Xu, T., Miao, H., Li, R., Liu, D. and Tong, Z.
Formulation and Evaluation of Inhalable Porous Tobramycin Powder Prepared by Spray Drying Technology.
DOI: 10.5220/0012001200003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 42-46
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

2 MATERIALS AND METHODS
2.1 Materials
United States Pharmacopeia (USP) tobramycin was
purchased from Yuanye Biotechnology Co., Ltd.
(Shanghai, China). 98 % concentrated sulfuric acid
and L-leucine (USP) were purchased from Titan
Technology Co., Ltd. (Shanghai, China). AB (USP)
was purchased from Jizhi Biotechnology Co., Ltd.
(Shanghai, China). Distilled water used in all steps
was produced from a Milli-Q device (~18.2 MΩ cm).
2.2 Preparation of The Feed Solution
and SD Particles
The feed solution composition was shown in Table 1.
So as to avoid the crystallization of naked
tobramycin, a certain amount of tobramycin was
dissolved in the aqueous solution before adding 98 %
concentrated sulfuric acid with a mass ratio of 3 to 1
under ultrasonication to acquire a clear solution. The
ratio was obtained from the commercial formulations
(TIP, TOBI), which had been thoroughly discussed
and reviewed regarding their safety (US Patent,
10744098B2). Leucine was then added to the
precursor formula. The enrichment of leucine on the
particle surface also helped reduce the cohesion
between particles and improved the powder delivery
efficiency. To obtain a particle of low density, e. g. a
porous particle structure similar to Pulmosphere
®
, AB
was eventually added to replace PFOB. In the formula
abbreviation, SD represented for spray drying; the
subscript numbers of TS and L (leucine) represented
the mass ratio between them; the subscript numbers
of AB represented their ratio to API; the last digit
represented the total solid content of the feed solution.
2.3 Methods
2.3.1 Particle Size Distribution
The particle size distribution was determined by laser
diffraction using the Sympatec HELOS system
equipped with the INHALER module (Sympatec
GmbH, Clausthal-Zellerfeld, Germany). The
dispersion pressure of the powders was 4 bars, and the
dispersion agent was compressed air. Before
measurement, each capsule was filled with
approximately 10 mg of sample and subjected to
static elimination. The particle size distribution of the
powder was calculated by applying the Fraunhofer
model preset in the instrument software.
2.3.2 Scanning Electron Microscopy
Scanning electron microscope (SEM, S-4700, Hitachi
High Technologies Corporation, Japan) was used to
characterize the powder morphology. The
accelerating voltage of the SEM was 15 kV and the
magnification of the images was 800–20000 times.
Before observation, the conductive carbon tape was
fixed on the sample preparation table, and an
appropriate amount of sample powder was placed on
the conductive carbon tape, and then loaded into the
ion sputtering coater (MC1000, Hitachi Ltd., Japan).
Finally, the powder was platinized by sputtering to
make the surface sample conductive and avoid charge
build-up during observation in the SEM.
2.3.3 Infrared Spectroscopy
The residual degree of AB was determined by Fourier
transform infrared spectroscopy (FTIR) to evaluate
the decomposition of AB. These spectra were
obtained by a single reflection diamond ATR
(Universal ATR) in a Nicolet IS50 FTIR spectrometer
(Thermo Fisher Scientific Inc.). The FTIR spectra
were obtained at a resolution of 0.09 cm
-1
over a
wavelength range from 400 cm
-1
to 4000 cm
-1
.
2.3.4 Pore Size, Porosity and Density
The porosity and pore size distribution of the sample
were determined with a mercury porosimeter
(AutoPore IV, Micromeritics, US). Under standard
atmospheric pressure conditions, 3ml sample was
placed in a permeameter, which was bonded to a glass
capillary rod. When pressure was applied, the
mercury moved down the capillary rod and filled the
voids in and around the sample. The change in
capacitance due to the loss of mercury in the capillary
rod could be analyzed to determine the porosity and
pore size distribution.
The bulk density (ρ
b
) was measured by measuring
the mass of about 4 mL of particles in a glass cylinder
before being tapped and the tapped density (ρ
t
) was
determined by measuring the volume after tapping the
above-mentioned particles of known mass. The
sample’s flowability was characterized by Carr's
index (CI) calculated by Eq. (1):
𝐶𝐼 =
𝜌
−𝜌
𝜌
∗ 100%
1
2.3.5 Aerodynamic Properties
The aerodynamic characteristics of inhaled
tobramycin particles were studied using the next
generation impactor (NGI) (Copley Science,
Formulation and Evaluation of Inhalable Porous Tobramycin Powder Prepared by Spray Drying Technology
43

Nottingham, UK). The samples were dispersed by the
Twincaps
®
(BrightGene Ltd., Soochow, China) at 60
L/min evaluated by a Critical Flow Controller
(Erweka, Heusenstamm, Germany). A vacuum pump
(Erweka, Heusenstamm, Germany) was used to set
the pressure drop across the whole device at 4 KPa.
Each formulation would be dispersed for 4 s to attain
4 L volume. The specific operation was carried out
according to the weighing method stipulated in the
British Pharmacopoeia. The FPF and mass median
aerodynamic diameter (MMAD) of the sample could
be calculated by the data processing software
CITDAS (Copley Science, Nottingham, UK).
3 RESULTS AND DISCUSSION
3.1 Particle Size, Morphology and
Density
The sample particles were successfully prepared by
spray-drying feed liquids with different formulations
(Table 1). Table 2 summarized the physical properties
of various spray-dried (SD) microparticles.
After adding AB to the precursor solution (Fig. 1), the
morphology of the particles showed that the
sphericity of the particles gradually recovered with
the increase of the amount of AB added. Since the
amount of AB was included in the total solid content,
its decomposition and gas escape did not lead to a
significant change in particle size. Under the same
ratio of TS and leucine, the CI decreased from 29.12
± 0.82 % (SD-TS
1
L
0.35
-AB
1
-0.68) to the lowest 24.63
± 1.17 % (SD-TS
1
L
0.35
-AB
3.5
-0.5), indicating that the
appearance of pores brought about a significant
improvement in fluidity. It should be noted that the
addition of excess AB would lead to obvious
agglomeration behavior of the particles, the particle
size rose to 34.10 ± 1.88 μm, and the CI rose to 28.44
± 2.79 %, indicating that the flowability also had a
certain degree of loss. The inference of this
phenomenon would be analyzed in conjunction with
the pore structure below.
Table 1: Precursor formulation and process parameters for spray drying.
Sample Tobramycin
(10
-2
g/ml)
Sulfate
(10
-2
g/ml)
Leu
(10
-2
g/ml)
AB
(10
-2
g/ml)
Total solid content
(%w/w)
SD-TS
1
L
0.35
-AB
1
-0.5
0.228 0.080 0.108 0.083 0.50
SD-TS
1
L
0.35
-AB
2
-0.5
0.196 0.068 0.093 0.143 0.50
SD-TS
1
L
0.35
-AB
3.5
-0.5
0.161 0.056 0.076 0.206 0.50
SD-TS
1
L
0.35
-AB
5
-0.5
0.137 0.048 0.065 0.250 0.50
Table 2: Physical properties of different formulations.
Sample Bulk density
ρ
b
(g/cm
3
)
Tapped density
ρ
t
(g/cm
3
)
CI
(%)
D
50
(μm)
Porosity
(%)
SD-TS
1
L
0.35
-AB
1
-0.5
0.353±0.002 0.498±0.003 29.12±0.82 8.19±0.28 -
SD-TS
1
L
0.35
-AB
2
-0.5
0.164±0.001 0.224±0.002 26.79±1.08 9.95±0.39 56.75
SD-TS
1
L
0.35
-AB
3.5
-0.5
0.061±0.001 0.081±0.001 24.69±1.53 10.92±0.36 78.93
SD-TS
1
L
0.35
-AB
5
-0.5
0.075±0.002 0.105±0.003 28.57±3.83 34.10±1.88 54.07
Figure 1: SEM images of (a) SD-TS
1
L
0.35
-AB
1
-0.5; (b) SD-TS
1
L
0.35
-AB
2
-0.5; (c) SD-TS
1
L
0.35
-AB
3.5
-0.5; (d) SD-TS
1
L
0.35
-AB
5
-
0.5.
FSB 2022 - The International Conference on Food Science and Biotechnology
44
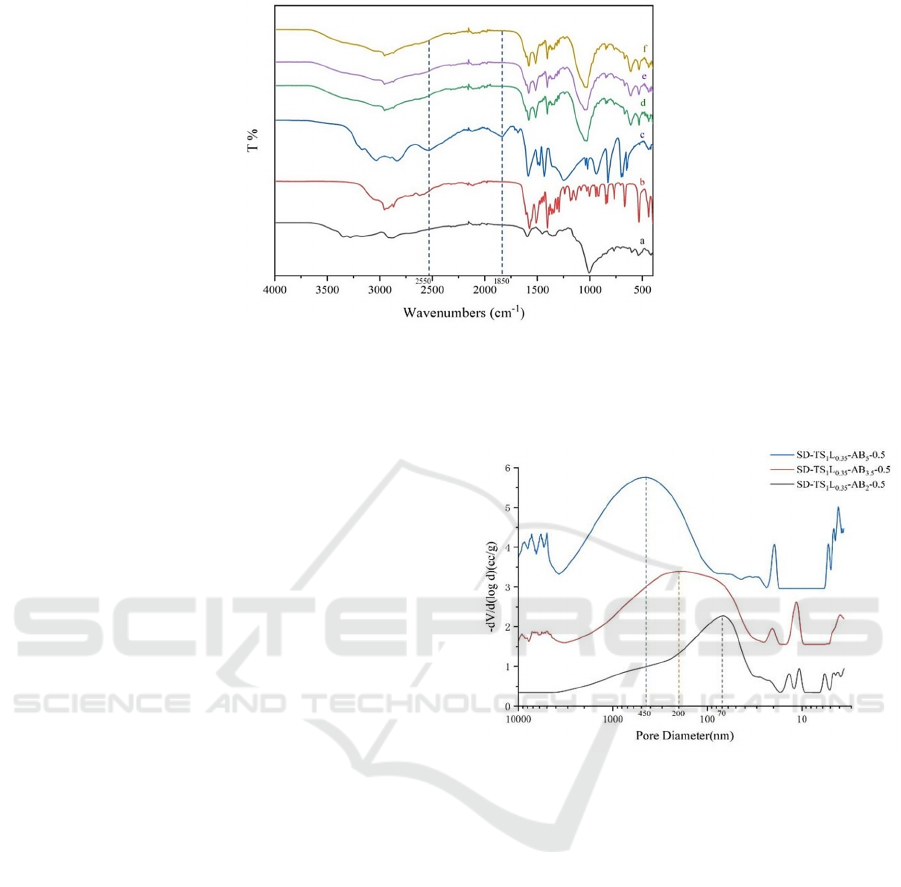
Figure 2: Infrared spectral images of (a) tobramycin sulfate; (b) leucine; (c) ammonium bicarbonate; (d) SD-TS
1
L
0.35
-AB
2
-
0.5; (e) SD-TS
1
L
0.35
-AB
3.5
-0.5; (f) SD-TS
1
L
0.35
-AB
5
-0.5.
3.2
Porous Structure and Residual
Ammonium Bicarbonate
Infrared results for TS, leucine, AB, and samples
were analyzed (Figure 2). Specifically, AB had
unique characteristic peaks near 1850 cm
-1
and 2550
cm
-1
, but these characteristic peaks did not appear in
the infrared image of the sample. This confirmed that
AB has been completely decomposed with negligible
residues.
The mercury intrusion analysis results (Fig. 3)
showed that from sample SD-TS
1
L
0.35
-AB
2
-0.5 to
SD-TS
1
L
0.35
-AB
3.5
-0.5, the porosity increased from
56.75 % to 78.93 %, and the pore size increased from
70 nm to 200 nm. This showed that the decomposition
of AB, gas accumulation and final pore formation
were stable and controllable in the particle formation
process under the current conditions, which was of
great significance for the formation of a feasible
process flow. However, when the addition of AB
continued to increase (SD-TS
1
L
0.35
-AB
5
-0.5), the
pore size expanded to 450 nm, while the porosity was
significantly reduced to 54.07 %. Combining the
results of the SEM images, particle size, and
flowability data for this formulation above, the reason
for this phenomenon was that the relative proportions
of AB and the rest of the solids (TS and leucine) were
too high, while the total solids content of the
precursors was constant. In order to maintain the
particle structure at a substantially constant particle
size, the required solid content in the sample particles
was already insufficient, which led to the collapse of
the basic structure of the particles, and the porosity
that should continue to increase also decreased. The
finally obtained sample particles had serious
agglomeration, increased cohesion, and decreased
dispersibility and fluidity.
Figure 3: Images of the pore size distribution of (a) SD-
TS
1
L
0.35
-AB
2
-0.5; (b) SD-TS
1
L
0.35
-AB
3.5
-0.5; (c) SD-
TS
1
L
0.35
-AB
5
-0.5.
3.3
Aerodynamic Performance
In order to obtain particles suitable for inhalation
therapy, proper aerodynamic behavior was required.
In general, the aerodynamic particle size of particles
for inhalation should be between 0.5 and 5 μm. On
this basis, the aerodynamic particle size distribution
of the samples was characterized by NGI.
Preliminary experiments found that under the
existing conditions, when the ratio of API and leucine
was 20:7, the aerodynamic performance of the
formulation was relatively optimal. Based on this
ratio of leucine and TS, AB was added to the
precursor solution and the aerodynamic performance
of the samples were characterized (Fig. 4). From the
formulation of SD-TS
1
L
0.35
-AB
1
-0.5 to SD-TS
1
L
0.35
-
AB
3.5
-0.5, it could be seen that the particle
Formulation and Evaluation of Inhalable Porous Tobramycin Powder Prepared by Spray Drying Technology
45
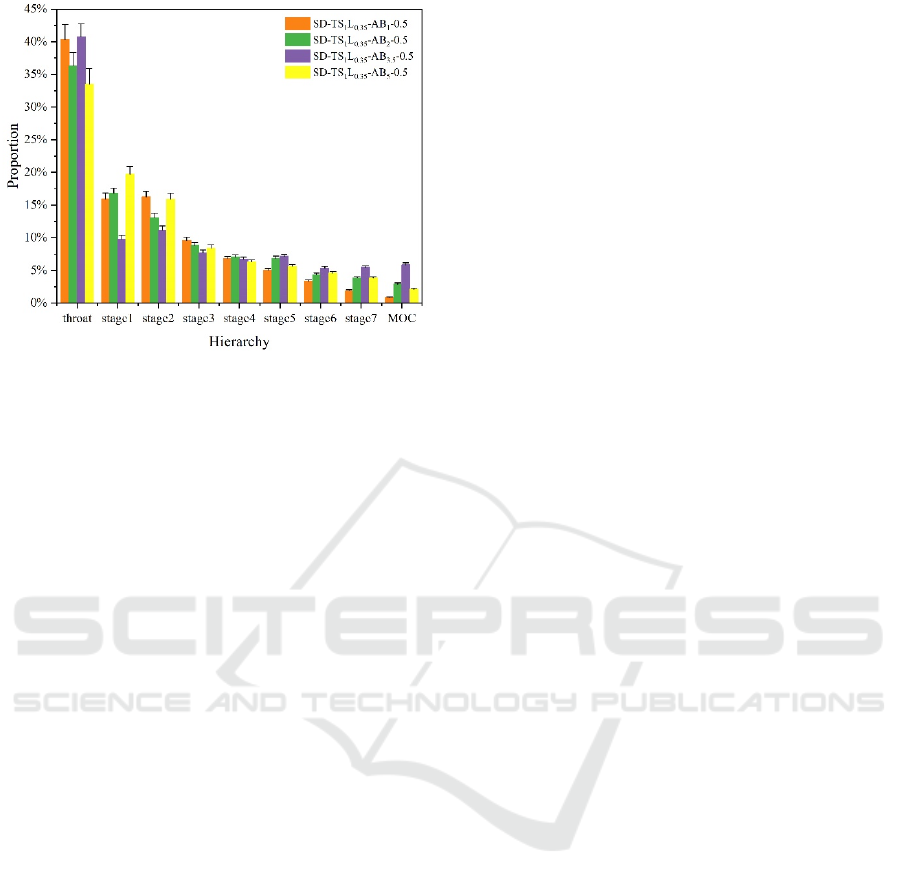
Figure 4: NGI results for different formulations.
distribution of each level had a tendency to gradually
aggregate to higher levels. The deposition amount of
the formula SD-TS
1
L
0.35
-AB
3.5
-0.5 at stage 6, stage 7,
and MOC was significantly higher than that of other
formulas, indicating that the pores left by AB in the
sample particles could significantly improve the
aerodynamic performance of samples. The MMAD
of the SD-TS
1
L
0.35
-AB
3.5
-0.5 formula had dropped to
about 2.4 μm, and the FPF had increased to 40.78 %.
However, for the SD-TS
1
L
0.35
-AB
5
-0.5, the FPF did
not increase further but decreased to 34.07 %, and the
MMAD also increased to 2.8 μm, which was
consistent with the characterization results of SEM
images, particle size and particle density. At this time,
the agglomeration between particles was intensified,
the fluidity and dispersion of the powder were also
deteriorated, and the unsatisfactory particle structure
eventually led to the decline of aerodynamic
performance. Eventually, the formulation of SD-
TS
1
L
0.35
-AB
3.5
-0.5 was optimized.
4 CONCLUSION
This work explored the heat-mass coupling process in
the spray drying process by adjusting the formulation
and parameters, and finally achieved a FPF of
40.78 % in the optimal formulation. The low-density,
loose and porous particle structure in the expected
target was verified by multiple characterization
results such as SEM images and porosity. This
structure provided particles with better dispersibility
and flowability and smaller aerodynamic size,
making them suitable for efficient pulmonary
delivery. AB residues in the final product were also
substantially absent as evidenced by infrared
spectroscopy. When the preferred ratio of API to
leucine is 20: 7, leucine was enriched on the outer
surface of the particles to a certain extent. When the
ratio of non-porous components to AB was 10: 7, the
porous particles had higher porosity and lower
density, resulting in lower aerodynamic particle size.
The final optimized spray drying formulation was
SD-TS
1
L
0.35
-AB
3.5
-0.5. The particles prepared under
this formula had large geometric size, loose porosity,
good deposition performance and high stability.
Inhalation of particles was both the core idea of this
article and the starting point for continuous
improvement in formulation design.
ACKNOWLEDGEMENTS
The authors are grateful to the National Key R&D
Project of China (2021YFB1715500) for the financial
support of this work.
REFERENCES
Alhajj, N., O'Reilly, N. J., Cathcart, H. (2022)
Developing Ciprofloxacin Dry Powder for
Inhalation: A Story of Challenges and Rational
Design in the Treatment of Cystic Fibrosis Lung
Infection.
International Journal of Pharmaceutics.
613
: 121388.
Elborn, J. S., Blasi, F., Haworth, C. S., Ballmann, M.,
Tiddens, H. A., Murris-Espin, M., Chalmers, J. D.,
Cantin, A. M. (2022) Bronchiectasis and inhaled
tobramycin: A literature review. Respiratory
medicine. 192: 106728.
Buttini, F., Balducci, A. G., Colombo, G., Sonvico, F.,
Montanari, S., Pisi, G., Rossi, A., Colombo, P. (2018)
Dose administration maneuvers and patient care in
tobramycin dry powder inhalation therapy.
International journal of pharmaceutics.
548: 182-191.
Miller, D. P., Tan, T., Tarara, T. E., Nakamura, J.,
Malcolmson, R. J., Weers, J. G. (2015) Physical
characterization of tobramycin inhalation powder:
I
.
Rational design of a stable engineered-particle
formulation for delivery to the lungs
. Molecular
pharmaceutics
. 12 (8): 2582-2593.
Miller, D. P., Tan, T., Nakamura, J., Malcolmson, R. J.,
Tarara, T. E., Weers, J. G. (2017) Physical
characterization of tobramycin inhalation powder: II.
State diagram of an amorphous engineered particle
formulation.
Molecular pharmaceutics
. 14 (6):
1950-1960.
FSB 2022 - The International Conference on Food Science and Biotechnology
46
