
Glutathione in Mental Disorders: Mechanisms and Therapies
Yawei Liang
School of Materials and Energy, South China Agricultural University, Guangzhou, 510642, China
Keywords:
Glutathione Homeostasis, Oxidative Stress, Glutathione Peroxidase, Glutathione S-Transferase, Mental
Disorders, Therapeutic Potential.
Abstract: In recent decades, topics related to mental diseases have gradually become important, and the treatments for
mental diseases have also attracted attention from increasing number of researchers. Research directions have
been focused on glutathione metabolism and oxidative stress. Appreciable number of reports have suggested
that the content of reduced glutathione in patients with mental diseases, including Alzheimer's disease,
depression, anxiety disorder, autism, bipolar disorder and schizophrenia, is lower than that in normal people,
suggesting that the dysregulation of glutathione redox may be one of leading the pathogeneses of these mental
diseases. Regulation of glutathione metabolism involves many enzymes, such as GPx1 and GST, which have
been comprehensively analyzed in this paper. Additionally, the potential of targeting glutathione metabolism
balance as a breakthrough point for the treatment of psychiatric disorders will be discussed, listing some
reported that have proven therapeutic effects of N-Acetyl-L-cysteine (NAC), minocycline and dimethyl
fumarate (DMF) on mental disorders as well as their further therapeutic potential. The general purpose of this
paper aims to study the recent progress focusing on glutathione metabolism in mental disorders mechanism
and treatment, highlighting that GSH redox problems for mental illness is indispensable and outlook the
potential development of treatments for mental disorders that target glutathione metabolism.
1 INTRODUCTION
Glutathione (GSH), a kind of tripeptide composed of
glutamic acid, cysteine and glycine, exists in
practically every cell inside human bodies.
Glutathione system acts as the most essential
antioxidant defense that maintaining cellular viability
and function. Normally, glutathione in healthy human
body mainly exists in the reduced form, while
Oxidized glutathione (GSSG) takes merely a tiny part
as the inactive state (Tao, 2014). Meanwhile, the
GSH:GSSG ratio in vivo should be within a
reasonable range and defined as a reliable biomarker
of cellular redox homeostasis (Geir Bjørklund, 2020).
Serving as an antioxidant, glutathione has the
function of eliminating free radicals in vivo. Free
radicals are considered as unstable substances that
can cause cell damage, especially when they
accumulate at higher content than antioxidants.
Oxidative stress will be caused when redox
dysregulation of glutathione occurs, meanwhile
reactive oxygen species (ROS) and reactive nitrogen
species (RNS) become overproduced, leading to the
inhibition of the activity of endogenous antioxidant
system, in which the circumstance may engender
over-loading free radicals damaging cell (Javier
Toro-Pérez, 2021). Then a series of psychiatric
disorders, such as bipolar disorder (Lagopoulos,
2013), depression (Kyle, 2014), autism (Xi, 2015),
Alzheimer's disease (Tandra Ghosh, 2012),
obsessive-compulsive disorder, post-traumatic stress
disorder (PTSD), schizophrenia, etc. can be caused.
To maintain the homeostasis of glutathione, impacts
of peroxidase enzymes are indispensable, which will
be discussed in this paper.
Increasing evidence and achievements about
therapies on mental disorders targeting glutathione
pathway are emerging. The function of glutathione
that can influence and regulate DNA methylation and
epigenetics is highlighted, which can cause the cell
and organ perturbations, particularly in the brain
(Geir Bjørklund, 2020). In response to the association
between dysregulation in glutathione metabolism and
bipolar disorder, Murrough et al. have found that
Minocycline can effectively treat symptoms of
bipolar disorder by modulating oxidative stress in
patients with bipolar disorder (Murrough, 2018).
Therefore, taking glutathione metabolism as the
18
Liang, Y.
Glutathione in Mental Disorders: Mechanisms and Therapies.
DOI: 10.5220/0012000800003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 18-25
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

breakthrough point to study the pathogenesis and
treatment of mental diseases has considerable value.
This passage aiming to dig deeper into the value of
glutathione, homeostasis of glutathione and
oxidative, as well as biological enzymes which
regulate glutathione metabolism, including GPx1 and
GST. In addition, the pathogenesis and therapeutic
potential of mental disorders focusing on the
glutathione pathway will also be depicted in detail.
2 COMPOSITION OF
GLUTATHIONE SYSTEM
2.1 Oxidative Stress and Homeostasis
Oxidative stress of glutathione has been implicated in
the pathogenesis of several neuropsychiatric
disorders, which is considered as a mechanism
linking genetic, immune and external pathogenic
factors in mental disorders (Xi, 2015). Under normal
circumstances, intracellular ROS and antioxidant
capacity are in a dynamic balance (Currenti, 2010).
As an antioxidant produced by human cells,
glutathione has a limited ability to remove active
oxides. When the content of active oxides exceeds the
reducing capacity of glutathione, imbalance between
cellular oxidation and antioxidant effects will occur.
This oxidative stress can hinder or disrupt cell
signaling and affect cell proliferation as well as gene
expression (Betancur, 2011). In addition to
glutathione, glutathione peroxidase (GPX) also acts
as antioxidant effects, which will be discussed in
detail in the following parts.
In order to illustrate glutathione homeostasis
systematically, normal state of glutathione redox and
glutathione metabolism under oxidative stress are
shown in Fig 1 and Fig 2 respectively. Under normal
circumstance, methionine cycling, and sulfur transfer
are the main factors regulating methylation and redox
buffering activities (Xi, 2015). When oxidative stress
occurs, a variety of regulatory mechanisms are
activated to reduce methionine synthase and cysteine
plus dioxygenase activity, as well as to increase
cystathionine synthase activity to attain the purpose
of changing sulfur flux in response to glutathione
synthesis. Low methionine synthase can reduce
methylation, including methylation of dopamine
receptor phospholipids.
In recent decades, increasing number of studies on
neurological diseases have focused on the glutathione
pathway, from which the fact that glutathione and
oxygen can affect the activities of enzymes
controlling genetics and transcription has been
generally accepted (Geir Bjørklund, 2020). The
maintenance of redox homeostasis is of great
significance for the central nervous system (CNS).
Once homeostasis is disrupted, the nervous system
can be harmed. Dysfunction of mitochondrial can be
one of the reasons that elevated levels of oxidative
stress, which was a phenomenon observed in autism
spectrum disorders (ASD) individuals (Xi, 2015). A
study carried out by ZHANG in 2018 had proved that
GSH can restrain the cell damage by oxidative stress
(Zhang, 2018). In this research, MPP+ was applied to
MES 23.5 dopamine neuron cells, which results in
increasing levels of ROS and MDA while CAT
activity declines in cell MES 23.5, inducing abnormal
mitochondrial function as well as oxidative stress to
create models of oxidative damage cells.
Figure 1: Normal redox status of glutathione. Adapted from (Xi, 2015).
Glutathione in Mental Disorders: Mechanisms and Therapies
19
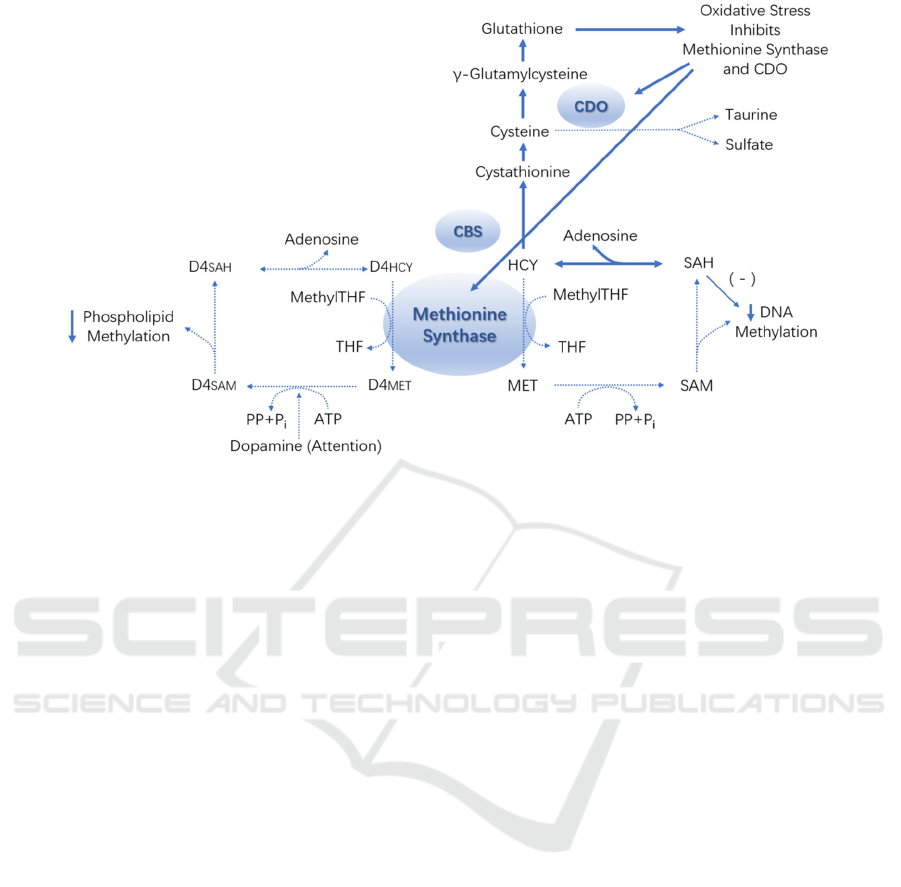
Figure 2: Glutathione metabolism under oxidative stress. Adapted from (Xi, 2015).
GSH was then applied to cells model, so the activity
of MES 23.5 recovered considerably, indicating that
GSH has the function of antioxidant protection. So,
what contributes in keeping the glutathione
homeostasis? Answer should be enzymes related to
the metabolism of glutathione such as glutathione
peroxidase and glutathione S-transferase.
2.2 GPx1
Glutathione peroxidase (GPx) can be divided into
four major categories, including cytosolic GPx,
plasma GPx, phospholipid hydroperoxide GPx and
gastrointestinal specific GPx. It was recognized that
the first vertebrate protein depends on selenium was
GPx (Little C, 1968). Glutathione peroxidase family
belongs to the antioxidant enzymes, of which GPx1 is
one of the family.
1) Role of GPx1 in mental disorder. Until 1968,
scientists claimed that GPx can lessen various kinds
of organic peroxides, nucleic acids and
hydroperoxides produced by unsaturated fatty acids
(Little C, 1968). Existing as an important antioxidant
enzyme, GPx1 can be found in both mitochondria and
cytoplasm of mammalian cells (Leopold Flohé,
2022). The main function of GPx1 is to oxidate
cellular GSH along with the reduction of hydrogen
peroxide, which can control the thiol redox state
while keeping necessary and harmful states of
oxidants in balance (Diane E. Handy, 2022). One
study by Zorov et al. showed that if dysfunction and
damage occur in mitochondrial membranes or
proteins, production of ATP and uncoupling of
electron transport may happened, which can stimulate
the mitochondria releasing ROS (Zorov D.B, 2014).
In addition, GPx1 also has the potential ability to
regulate the forming of these kinds of extra ROS.
GPx1 is able to protect neurocyte against ROS-related
neurotoxicity (Crack P.J., 2006). When gene
expression of GPx1 dysregulates, the oxidative stress
caused by this case can be related to
neurodegeneration which is a considerable factor of
chronic neurodegenerative diseases like Alzheimer’s
disease (Diane E. Handy, 2022).
Indeed, GPx1 helps to keep glutathione redox
stable and provides neuroprotection. If expression of
GPx1 increases, however, cellular dysfunction and
other diseases can also be stimulated because some
essential reactive oxygen species are removed in this
case (Diane E. Handy, 2022). Superfluous oxidant
like GPx1 can damage cellular DNA, proteins and
lipid. Study by Martini et al. had found that
upregulating GPx1 can mitigate suppression of
ERK1/2 and memory impairment (Martini F, 2019).
The process is illustrated in Figure 3. Interestingly,
the activation of ERK1/2 can also be inhibited when
GPx1 overexpressing, which may possibly be
promoted by ROS production (Diane E. Handy,
2022). These results provide reasonable evidence to
see the connection between GPx1 expression and
psychiatric disorders, but also highlight the
complexities of regulating antioxidant for therapies.
FSB 2022 - The International Conference on Food Science and Biotechnology
20
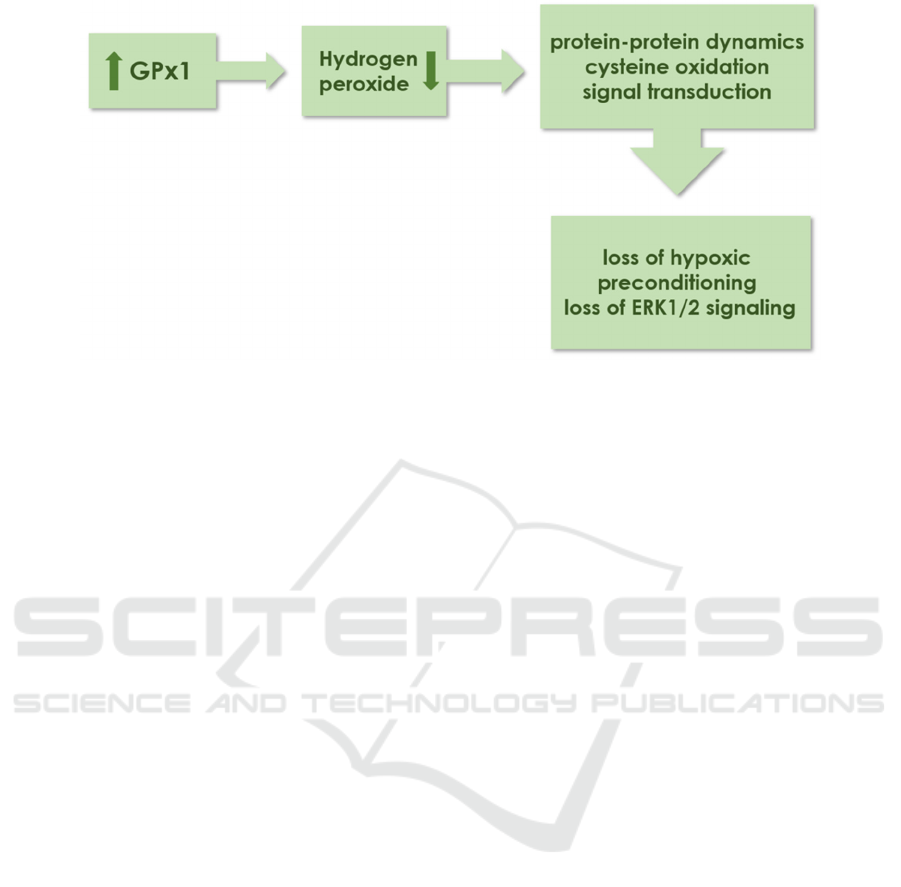
Figure 3: Process illustrating how the increase of GPx1 lead to inhibition of ERK1/2. Adapted from (Diane E. Handy, 2022).
2) Regulation of GPx1 expression. It was claimed
that not all the glutathione peroxidases are
selenoproteins but GPx1 is, which includes the amino
acid selenocysteine at the active site (Floh´e L.,
1973). Selenium deficiency may not result in a
defective GSH regeneration but cause the utilized
impairment (Leopold Flohé, 2022). Another research
by Smesny et al. revealed how omega-3 PUFA affects
the glutathione antioxidant defense system (AODS)
in individuals with ultra-high risk of psychosis
(Stefan Smesny, 2015). Result of this study had
shown the mechanisms underlying clinical
effectiveness, of which the effects were not specific.
Contrarily, it is certain that omega-3 PUFA can
influence the antioxidative effects in AODS,
decreasing the demand of glutathione. However,
there is no absolute association between omega-3
PUFA and GPx1.Therefore, it is reasonable to give an
opinion that GSH levels in vivo can be indirectly
influenced by selenium, also further study about
substances that can affect the production of GPx1 is
needed.
2.3 GST
1)Role of GST in mental disorder. Comparing with
GPx1, GST has different chemical properties and
biological effects. Details are listed in Table 1. With
detoxification, GST mainly helps to promote the
electrophilic groups from endogenous substances or
xenobiotics coupling to the sulfhydryl group of
reduced glutathione, increasing the hydrophobicity of
the combination to cross the cell membrane,
decompose and then be excreted (Sen Ma, 2008). In
this way, GST can remove peroxide as well as
detoxify xenobiotics. However, mechanism of GST
may be affected by GPx. When the activity of GPx
decreases, GST can only eliminate the lipid peroxides
(LPO), losing the ability of detoxification.
As one of the members of family of phase-II
isoenzymes, GST protect human cells from
electrophiles and substances produced by oxidative
stress, playing a critical role in pathogenesis of
Alzheimer’s disease. In 2012, Ghosh et al. digged into
the relationship between GST and Alzheimer’s
disease, indicating that gene deletion of GSTM1(Mu)
and GSTT1(theta) which are belong to GST family,
can decline the expression of related enzymes
(Tandra Ghosh, 2012). Result shown that deletion of
GSTT1 was dramatically related to Alzheimer’s
disease. Probability of people getting Alzheimer’s
disease with gene deletion of GSTT1 was 2.47 times
higher than that of people with positive GSTT1. Also,
study by Spalletta et al. found that patients with
schizophrenia who carry the GSTA1*B allele were
facing to higher risk of damage by oxidative stress
than non-B carriers, displaying severer symptoms of
illusion (Gianfranco Spalletta, 2012).
2) Factors influence metabolism of GST. Several
factors can affect the metabolism of GST. Activity,
inner concentration, issue localization of GST varies
in different people. Gene expression of GST reveals
polymorphism in people who are not living in the
same geographical area (Buratti F.M, 2021). GST
exists in practically all parts of human body, with
highest levels in the cytoplasm of liver, kidney and
blood, in which the elimination of peroxide and
detoxification of xenobiotics mainly take place.
Certain product of metabolism can also influence
GST expression. In 2021, Crawford et al. found that
3,4-dihydroxyphenylacetaldehyde (DOPAL), an
aldehyde metabolite of dopamine can inhibit the
Glutathione in Mental Disorders: Mechanisms and Therapies
21

Table 1: Comparison between GPx1 and GST.
GPx1 GST
can catalyze the reduction of H
2
O
2
and organic
h
y
dro
g
en
p
eroxide com
p
ounds
cannot decompose H
2
O
2
removing peroxide only removing peroxide and detoxifying
poor thermal stability better thermal stability
activity and function of GST, but 1 mM carnosine can
completely protect GST from DOPAL (Crawford
R.A., 2021).
3 ROLE OF GLUTATHIONE IN
MENTAL DISORDER
3.1 Pathogenesis
At present, a fair amount of research has been done to
probe how glutathione metabolism associates with
psychiatric disorders. However, there is still no clear
evidence to confirm that metabolic disorders of
glutathione can always cause psychiatric problems,
but it is considerable to take glutathione as a
biological indicator to analyze certain disease. Lack
of glutathione and oxidative stress can be considered
as primary symbols of some mental problems, but the
precise and specific pathogenesis concerning
glutathione remains uncertain.
Redox dysregulation act as a non-negligible factor
in autism spectrum disorder (ASD). By affecting
redox environment and redox-independent
mechanisms, glutathione metabolism impacts
multiple pathogenesis of ASD (Geir Bjørklund,
2020). N-methyl-Daspartate receptor, a glutamate
receptor regulated by glutathione may contribute to
glutamate excitotoxicity, in which case that
synergistic and antagonistic interactions of
glutathione and glutamate will lead to neuronal
dysfunction (Geir Bjørklund, 2021). These kinds of
synergistic and antagonistic interactions are likely to
involve transcription factors in immune pathway,
changing neuroinflammatory mechanisms and then
neuronal damage emerges.
Pathogenesis of depression implicates oxidative
stress as an essential mechanism. Lapidus et al. used
proton magnetic resonance spectroscopy (1H MRS)
to obtain the glutathione in vivo level of occipital
cortex of patients with major depressive disorder and
came to a conclusion that anhedonia severity shown
negative relation to occipital glutathione level, which
indicated glutathione and oxidative stress have
similar pathogenesis in anhedonia and major
depressive disorder (Kyle A.B. Lapidus, 2014). This
may increase a support to the importance of oxidative
stress of glutathione in pathogenesis of depression,
which means low level of glutathione becoming
potential symbols in the early state of major
depressive disorder. Also, in vivo level of GST is one
of the biomarkers to show the possibility of suffering
from depression. Savushkina et al. found that people
with depression have less platelet GST and
erythrocyte glutathione reductase than normal people
(Olga Savushkina, 2022). Interestingly, research by
Lagopoulos et al. argued that no decline was found in
baseline GSH concentration of the young with bipolar
disorder, while no prominent result to support
glutathione oxidative stress involved in mania
(Lagopoulos J., 2013). Therefore, further study may
be requested, but existing research has provided
human-oriented information that may contribute to
prospective clinical study and therapies.
3.2 Protective Effects
Disturbances in glutathione in the hippocampus can
lead to some of the mental diseases. A mass of
glutathione is needed in hippocampal neurons in
order to protect and maintain dendrite integrity and
cognitive function. In 2022, Ho et al. emphasized the
interactions of glutathione and copper on physiology
and Alzheimer’s disease (Talia Ho, 2022). It was
indicated that effects of glutathione and copper on
Alzheimer’s disease have been studied separately in
previous researches, of which the fact that these two
substances are actually more effective and regulate
each other when interacting. To give details of
mechanism of the interaction of glutathione and
copper, a simplified illustration by Ho is shown in
Figure 4. It can be observed from the figure that
copper is imported into the cell by copper transporter
1 (Ctr1). Then glutathione chelates the copper almost
immediately. Copper can be stored and passed by
glutathione to different chaperones and
metallothionein that functions as a storage molecule.
FSB 2022 - The International Conference on Food Science and Biotechnology
22
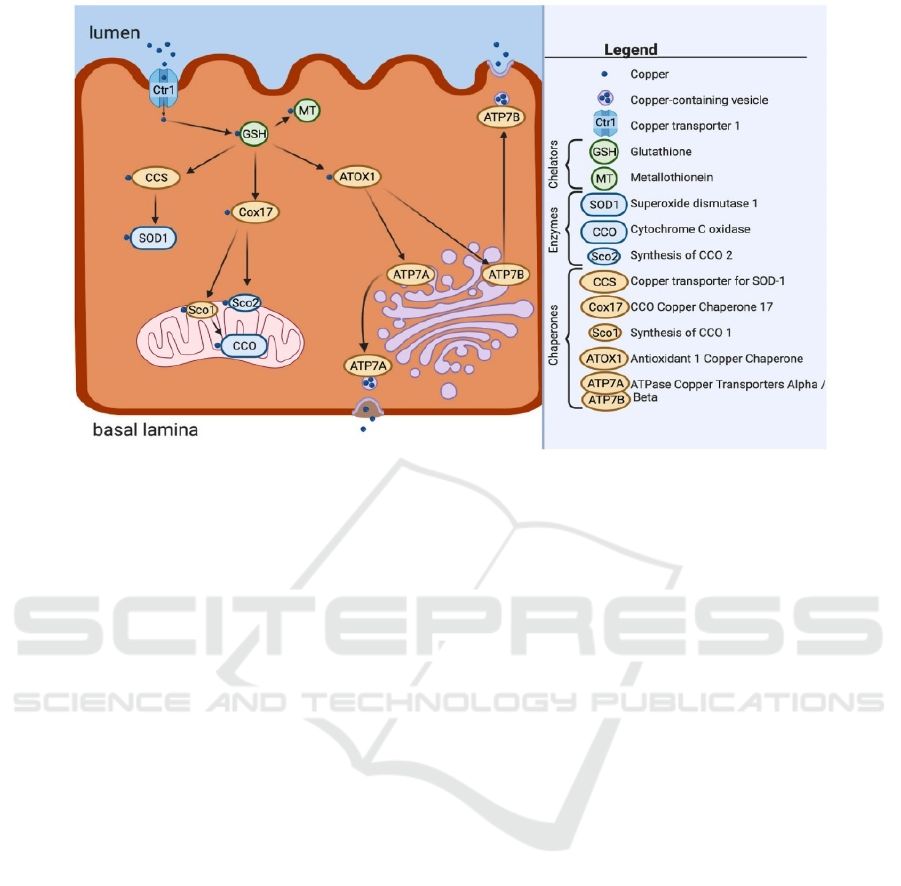
Figure 4: A simplified illustration for the overview of copper importation, utilization, and exportation within the cell.
Referring to (Talia Ho, 2022).
4 THERAPEUTIC POTENTIAL
Although the aforementioned associations of
glutathione homeostasis and oxidative stress with
psychiatric disorders are preliminary results,
targeting glutathione pathway for the treatment of
mental disorders still possess prospective
development. Appling N-Acetyl-L-cysteine (NAC)
as a drug with pharmacological targeting of
glutathione system can be a significant example.
Yang et al. executed clinical studies with
schizophrenics and found that after 8 weeks of
treatment with NAC, prefrontal cortical levels of
glutathione in schizophrenics have been raised (Yang,
2022). As a glutathione precursor, NAC has already
shown adjunctive value in therapies of schizophrenia,
of which the curative effect has been proved to be
apparent (Schiavone S., 2016). In fact, NAC can also
be effective in alleviating depressive symptoms or
anxiety symptoms, but the condition under which
NAC can work is limited. Porcu et al. conducted a 12-
week clinical experiment in 2018, in which patients
were assigned to receive the same daily dose of
placebo or 1.8g NAC (Porcu M., 2018). It has been
proved that only when high sensitivity C-reactive
protein levels exceed 3 mg/L, can the therapeutic
effect of NAC be observed.
Most of the reports about targeting glutathione
pathway to treat psychiatric disorders are by applying
NAC to schizophrenics. There is one sample study
that rarely mentioned, which focused on minocycline
for the treatment of bipolar depression. In 2018,
Murrough et al. treated 20 people with bipolar
disorder with minocycline at a daily dose of 256 mg
(SD: 71 mg) and measuring the cortical glutathione
within a voxel prescribed in the precuneus by proton
magnetic resonance spectroscopy (1H MRS)
(Murrough J.W., 2018). Results by this study proved
that applying minocycline to patients with bipolar
disorder, especially those with high baseline
glutathione levels, is considered as a significant
adjuvant treatment.
In addition, targeting the regulation of glutathione
expression is also a potential therapeutic approach.
Nrf2 can control the expression of various antioxidant
enzymes, including those involved in GSH synthesis,
such as GST and GPx1. As early in 2004, Chen et al.
proposed to enhance the antioxidant capacity of GSH
in response to oxidative stress by increasing Nrf2
activity (Chen, 2004). Dimethyl fumarate (DMF), a
fumarate ester, was thought to have an ability to
activate the action of Nrf2 (Linker, 2011). The
principle for this is that DMF can modify the cysteine
residues on Keapl, so that Nrf2 can be stabilized and
transferred to the nucleus to activate ARE targets,
thus reducing oxidative stress. In 2018, El-Fattah
conducted experiments on mice in which DMF was
administered orally at a dose of 25mg/kg 1 h before
each exposure to stress and found that depressive
symptoms due to oxidative stress in mice were
Glutathione in Mental Disorders: Mechanisms and Therapies
23

reduced (Abd El-Fattah, 2018). Although the results
were collected from animal model, this study also
provides us with considerable evidence and potential
that DMF may be beneficial for the treatment of
depression in humans.
Consequently, further study is required to
discover and evaluate novel drug targeting
glutathione pathway as therapy or adjuvant treatment
to various sorts of mental disorders.
5 CONCLUSION
It is undoubted that oxidative stress caused by
glutathione redox dysregulation is involved in the
pathogenesis of a variety of psychiatric diseases.
Studying the metabolic pathway of glutathione and
factors influencing glutathione homeostasis is quite
significant. Targeting the pharmacology of the redox
regulatory system as a therapeutic modality is
believed to be a promising approach for the treatment
of psychiatric disorders. The treatment of mental
disorders centered on glutathione metabolism is not
yet fully developed, but the development of such
topic may show great potential.
REFERENCES
Abd El-Fattah, et al., Resveratrol and dimethyl fumarate
ameliorate depression-like behavior in a rat model of
chronic unpredictable mild stress, Brain Research,
2018, 1701: 227–236.
Betancur C., Etiological heterogeneity in autism spectrum
disorders: more than 100 genetic and genomic disorders
and still counting, Brain Research, 2011, 1380: 42-77.
Buratti F.M., et al., Human variability in glutathione-S-
transferase activities, tissue distribution and major
polymorphic variants: Meta-analysis and implication
for chemical risk assessment, Toxicology Letters, 2021,
337: 78-90, ISSN 0378-4274.
Currenti SA., Understanding and determining the etiology
of autism, Cellular & Molecular Neurobiology, 2010,
30(2): 161-171.
Crack P.J., et al., Lack of glutathione peroxidase-1
exacerbates Abeta-mediated neurotoxicity in cortical
neurons, Journal of Neural Transmission, 113 (2006)
645–657.
Crawford R.A., et al., In vitro inhibition of glutathione-S-
transferase by dopamine and its metabolites, 3,4-
dihydroxyphenylacetaldehyde and 3,4-dihydroxy-
phenylacetic acid, NeuroToxicology, 2021, 86: 85-93,
ISSN 0161-813X.
Chen, X.-L., et al., Induction of Cytoprotective Genes
Through Nrf2 / Antioxidant Response Element
Pathway: A New Therapeutic Approach for the
Treatment of Inflammatory Diseases, Current
Pharmaceutical Design, 2004, 10 (8): 879–891.
Diane E. Handy, et al., The role of glutathione peroxidase-
1 in health and disease, Free Radical Biology and
Medicine, 2022, 188: 146-161, ISSN 0891-5849.
Flohé L., et al., Glutathione peroxidase: a selenoenzyme,
Federation of European Biochemical Societies (FEBS)
Letters, 1973, 32: 132–134.
Geir Bjørklund, et al., The impact of glutathione
metabolism in autism spectrum disorder, Pharma-
cological Research, 2021, 166: 105437, ISSN 1043-
6618.
Geir Bjørklund, et al., The role of glutathione redox
imbalance in autism spectrum disorder: A review, Free
Radical Biology and Medicine, 2020 (60): 149-162,
ISSN 0891-5849.
Gianfranco Spalletta, et al., Glutathione S-transferase alpha
1 risk polymorphism and increased bilateral thalamus
mean diffusivity in schizophrenia, Psychiatry Research:
Neuroimaging, 2012, 203 (2–3): 180-183, ISSN 0925-
4927.
Javier Toro-Pérez, et al., Contribution of oxidative stress in
the mechanisms of postoperative complications and
multiple organ dysfunction syndrome, Redox Report.
2021, 26(1):35-44.
Kyle A.B. Lapidus, et al., In vivo
1
H MRS study of potential
associations between glutathione, oxidative stress and
anhedonia in major depressive disorder, Neuroscience
Letters, 2014 (569): 74-79, ISSN 0304-3940.
Lagopoulos J., et al., In vivo glutathione levels in young
persons with bipolar disorder: A magnetic resonance
spectroscopy study, Journal of Psychiatric Research,
2013, 47 (3): 412-417, ISSN 0022-3956.
Little C., et al., An intracellular GSH-peroxidase with a
lipid peroxide substrate, Biochemical and Biophysical
Research Communications, 1968, 31(2): 145-150.
Leopold Flohé, et al., The glutathione peroxidase family:
Discoveries and mechanism, Free Radical Biology and
Medicine, 2022, 187: 113-122, ISSN 0891-5849.
Linker, et al., Fumaric acid esters exert neuroprotective
effects in neuroinflammation via activation of the Nrf2
antioxidant pathway, Brain, 2011, 134 (3): 678–692.
Murrough J.W., et al., A pilot study of minocycline for the
treatment of bipolar depression: Effects on cortical
glutathione and oxidative stress in vivo, Journal of
Affective Disorders, 2018, 230: 56-64, ISSN 0165-0327.
Martini F., et al., A multifunctional compound ebselen
reverses memory impairment, apoptosis and oxidative
stress in a mouse model of sporadic Alzheimer’s
disease, Journal of Psychiatric Research, 2019, 109:
107–117.
Olga Savushkina, et al., Activity of energy, glutamate, and
glutathione metabolism enzymes in blood cells of
elderly patients with depression, The European Journal
of Psychiatry, 2022, ISSN 0213-6163.
Porcu M., et al., Effects of adjunctive N-acetylcysteine on
depressive symptoms: modulation by baseline high-
sensitivity C-reactive protein, Psychiatry Research,
2018, 263: 268–274.
Qianqian Xi, Effects of glutathione related factors and
FSB 2022 - The International Conference on Food Science and Biotechnology
24

oxidative stress on autism in children, Tianjin Medical
University, 2015.
Stefan Smesny, et al., Effects of omega-3 PUFA on the
vitamin E and glutathione antioxidant defense system
in individuals at ultra-high risk of psychosis,
Prostaglandins, Leukotrienes and Essential Fatty Acids,
2015, 101: 15-21, ISSN 0952-3278.
Sen Ma, Research progress of glutathione peroxidase and
glutathione transferase, Progress in Veterinary
Medicine, 2008, 29(10): 53-56.
Schiavone S., et al., Pharmacological targeting of redox
regulation systems as new therapeutic approach for
psychiatric disorders: A literature overview,
Pharmacological Research, 2016, 107: 195-204, ISSN
1043-6618.
Tao Dai, et al., Research Progress of Clinical Application
of Reduced Glutathione, Journal of Chengde Medical
College, 2014, 31 (05): 432-435.
Tandra Ghosh, et al., A preliminary study on the influence
of glutathione S transferase T1 (GSTT1) as a risk factor
for late onset Alzheimer's disease in North Indian
population, Asian Journal of Psychiatry, 2012, 5 (2):
160-163, ISSN 1876-2018.
Talia Ho, et al., Do glutathione and copper interact to
modify Alzheimer's disease pathogenesis?, Free
Radical Biology and Medicine, 2022, 181: 180-196,
ISSN 0891-5849.
Yang Y.S., et al., N-Acetylcysteine effects on glutathione
and glutamate in schizophrenia: A preliminary MRS
study, Psychiatry Research: Neuroimaging, 2022, 325:
111515, ISSN 0925-4927.
Yuhan Zhang, et al., Protective effect of reduced
glutathione on oxidative damage of dopaminergic nerve
cells, Journal of Neuroanatomy, 2018, 34(3): 393-398.
Zorov D.B., et al., Mitochondrial reactive oxygen species
(ROS) and ROS-induced ROS release, Physiological
Reviews, 2014, 94: 909–950.
Glutathione in Mental Disorders: Mechanisms and Therapies
25
