
Evaluation of the Full Dosage Sinovac and Pfizer COVID-19
Vaccination into Adolescent
Agnes Sohilait and Diana Laila Ramatillah
Faculty Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords: Evaluation of Covid-19, Sinovac, Pfizer, Adolescent.
Abstract: Coronavirus Disease 2019 (COVID-19) is an illness caused by Severe acute respiratory syndrome coronavirus
2 (SARS-CoV-2). The purpose of this study was to determine the amount of exposure to Covid-19, to
determine the sociodemographic description of the variables of age, gender, vaccine type, BMI, vaccine side
effects, to determine the relationship between age, gender, BMI, vaccine side effects and exposure to Covid-
19. A prospective cross-sectional study was conducted in this study. This study only involved 600 respondents
aged 11-18 years who had been vaccinated against Sinovac and Pfizer. The results obtained in this study were
the amount of exposure to Covid-19 in Sinovac and Pfizer vaccination participantsin adolescents 6
participants were in Sinovac 4 participants and Pfizer 3 participants. Other influencing factors are the type of
vaccine and side effects with the p-value of each variable <0.05. there is a relationship between the type of
vaccine and vaccine side effects.
1 INTRODUCTION
In 2019, the world was rocked by the COVID-19
pandemic which started in Wuhan, Hubei, People’s
Republic of China. The extent of the virus, which is
classified as SARS, was not previously determined
until finally, a month after it was discovered that
COVID-19 was a highly contagious virus. COVID-
19 can be transmitted mainly through the particles
that come out of the breath when individuals are
within one meter of it(Darwis et al., n.d.).
The virus that causes COVID-19 is called SARS-
CoV-2. Coronavirus is zoonotic (transmitted between
animals and humans). One of the study reported that
SARS was transmitted from civet cats to humans and
MERS from camels to humans. Meanwhile, the
animal that is the source of the transmission of
COVID-19 is still unknown(Darwis et al., n.d.).
Symptoms are usually mild and appear gradually
and some infected individuals may show no signs and
still feel well. According to one study, it was stated
that WHO officially declared COVID-19 a pandemic
on Wednesday, March 11, 2020, and cases have been
steadily increasing since then(Ramatillah et al.,
2021).
According to Arnanda N, Ramatillah DL. SARS-
CoV2 infection and disease can be divided into three
phases: I. asymptomatic, phase with or without
detectable virus; II. milder symptoms, phase with
upper airway involvement; and III. severe, potentially
lethal disease with hypoxia, 'ground glass' infiltrates in
the lungs, and progression to acute respiratory
distress syndrome (ARDS)(Arnanda & Ramatillah,
2022).
There are 3 categories of severity of COVID-19
according to Gee S, Gaughran F, et al : (1) Critical
Covid-19 [Acute respiratory distress syndrome
(ARDS), sepsis, septic shock, or patients requiring
life-sustaining therapy] (2) Severe COVID-19
[SpO2<90 %, had signs of ARDS and pneumonia] (3)
Non-severe COVID-19 [no criteria for severe or
critical signs](Gee et al., 2020).
The COVID-19 epidemic has resulted in a
significant increase in mortality and has thrown the
country into recession. Although the virus spread can
be slowed by physical separation, face coverings,
testing, and tracing, effective vaccines are given to a
large portion of the world’s population to prevent
serious illness and disease and achieve herd immunity
to transmit the virus(Sutardi & Ramatillah, 2022).
According to WHO: Some countries have given
emergency use authorization for mRNA vaccines for
use in the adolescent age group (aged 12-17 years)
BNT162b2 developed by Pfizer. two inactivated
vaccines (Sinovac-CoronaVac and BBIBP-CorV)
250
Sohilait, A. and Ramatillah, D.
Evaluation of the Full Dosage Sinovac and Pfizer COVID-19 Vaccination into Adolescent.
DOI: 10.5220/0011979400003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 250-257
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
and these products were approved by Chinese
authorities for age indication of 3-17 years; although
these vaccine products have received EUL for adults,
they have not received WHO EUL for children(WHO
SAGE ROADMAP FOR PRIORITIZING USE OF
COVID-19 VACCINES, 2022).
2 METHOD
2.1 Design
This research was conducted with a quantitative
approach using a retrospective cross-sectional design
using a questionnaire. The data collection technique
was carried out using a survey method using a google
form which was distributed online to adolescents who
had been vaccinated against complete doses of
Sinovac and Pfizer.
2.2 Population and Sample
Participants in this study were Indonesian people >18
years old who had received the complete dose of
AstraZeneca vaccine with a total of 310 respondents.
2.3 Instrument
This study uses a questionnaire distributed through
social media (WhatsApp, Facebook, Instagram, and
Telegram). The number of questionnaires in this
study was 67 questions about nonidentity and
comorbidities. 67 questions were about the side
effects received after the first and second doses of
vaccination in the short and long term, as well as
monitoring the side effects of the vaccine for 1-6
months after being vaccinated. For validating the
questionnaire in this study using a standardized
questionnaire where the Cronbach alpha was
appropriate as in the following literatur(Ramatillah et
al., 2019).
2.4 Selection Criteria
Inclusion Criteria
a. Adolescent boys and girls
b. Adolescents 11-18 years old
c. Adolescents who have been vaccinated with
Sinovac and Pfizer complete doses for a
minimum of 2 months
d. Adolescents who are willing to take partin
researchha
e. Indonesia citizens
Exclusion Criteria
a. Adolescents 11-18 years who have not been
vaccinated at full doses
b. Cancer, Autoimmune, Hepatitis, HIV AIDS
c. He Pregnant women
2.5 Statistical Analysis
The collected results were analyzed using the SPSS
version 25 application. Fisher, Chi-square, and Mann-
Whitney test was used to find the relationship
between risk factors (gender, age, BMI, vaccine
type), and a side effect-valuable of 0.05 was
considered significant
.
2.6 Ethical Approval
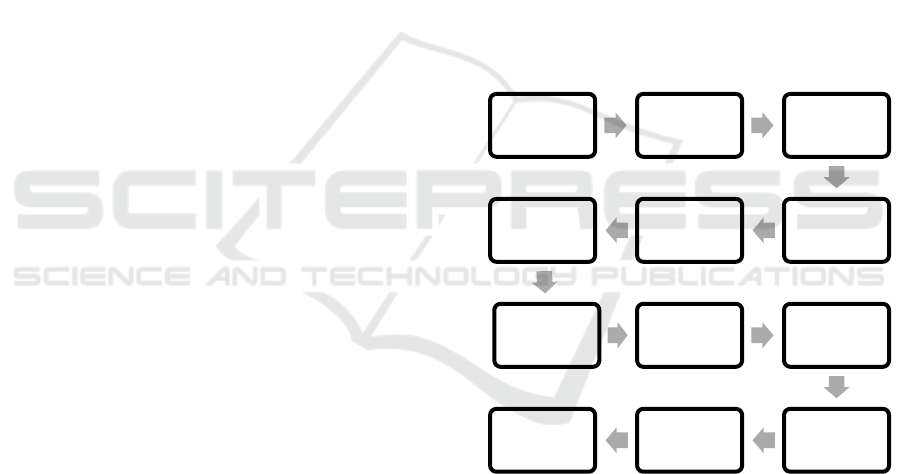
As stated in figure 1 ethical approval was obtained
before conducting the study. Ethical approval comes
from the Health Research Ethics Committee,
University of 17 August 1945 Jakarta, with approval
letter No.49/KEPK-UTA45JKT/EC/EXP/07/2022
Figure 1: Research Framework.
3 RESULT
In this study, 600 participants were used with an
average age of 11-18 years. To get participants in this
study, social media WhatsApp, Telegram and
Instagram were used for distributing questionnaires.
Problem topic
determination
Determination
of research
location
Proposal
drafting process
Get ethical
approval
Questionnaire
draft
Reviewed by 3
experts
Valid 3 experts
Pilot Study 30
sample
Cronbach
<0,6>
Survey for 300
respondents
AnalysisReporting
Evaluation of the Full Dosage Sinovac and Pfizer COVID-19 Vaccination into Adolescent
251
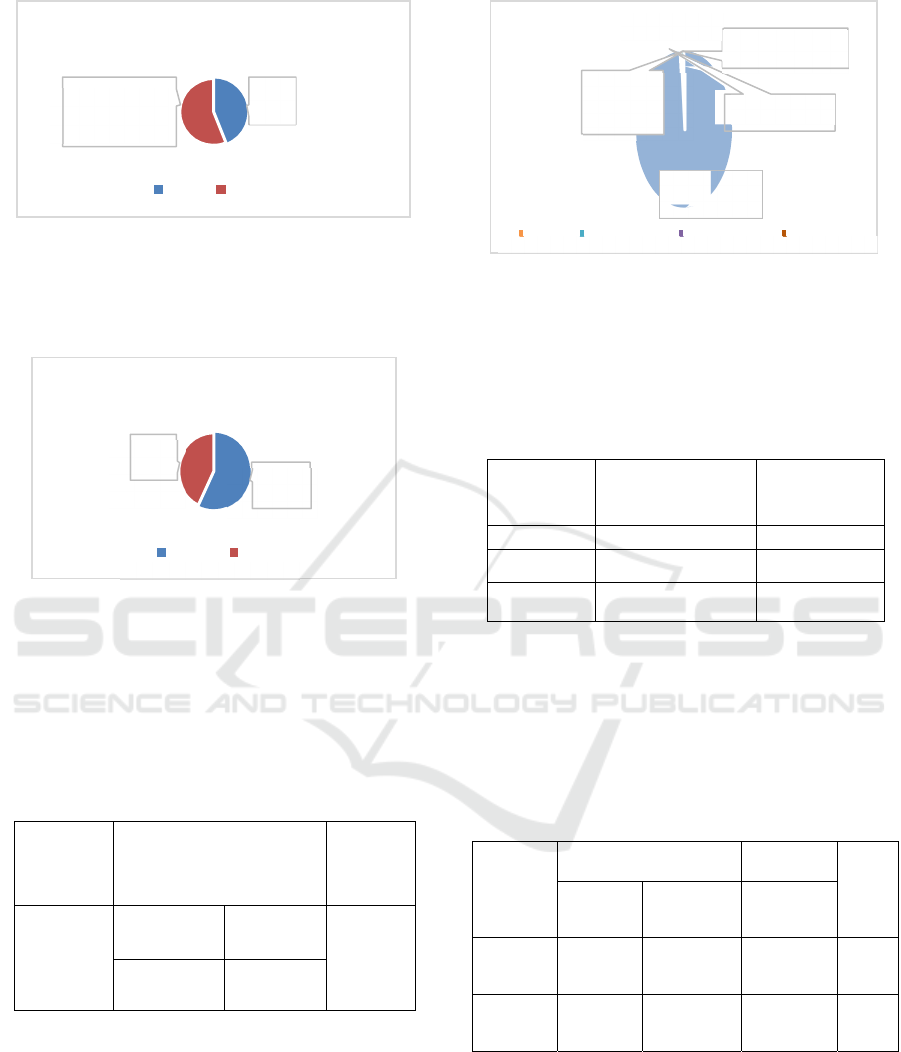
Figure 2: Participants by Gender.
Based on Figure 2, it can be seen that there were 600
participants of different genders, 44% (264) of
participants, and 56% (336) of fee participants.
Figure 3. Participants Affected by COVID- 19.
Based on figure 3, it can be seen from 600 participants
that 57% (4) Sinovac vaccine participants were
affected by Covid-19 and 43% (3) Pfizer vaccine
participants were affecred by Covid-19.
Table 1: Relationship between vaccine types and
comorbidities.
Variable Frequency/Percentage
(%)
P.Value
Asthma
Sinovac
(n=300)
Pfizer
(n=300)
0.414
10/3.33 15/5
*Fisher test, #Chi-
Square
From a total of 600 participants with 300 Simovac
and 300 Pfizer each with a p-value 0.005 of all
participants, there was only astma in the Sinovac
vaccine at 3.33% and the Pfizer vaccine at 5%. As
showtablee 1. There is no significant data so there is
no relationship between vaccine typed and
comorbidity.
Figure 4; Presentation treated from Covid-19.
Figure 4. It can be seen that 99% of those who are not
exposed and those who are self-isolating are 1%, in
hospital/Non-ICU and Hospital/ICU 0%
Table 2. Correlation between vaccine type and exposure to
COVID-19.
Kind of
Vaccine
Exposed to
Covid-
19/Percentage
P-Value
Sinovac
0
Pfize
r
3/1
Total p-
value
0.249
*Fisher test, #Chi-square
It can be seen in table 2. There is no significant data
on the correlation between the type of vaccine and
exposure to COVID-19 with a p-value of
0.249>0.005.
Table 3: Correlation between age, BMI, and vaccine side
effects.
Variable Frequency/
Percentage (%)
P-
value
Overall
(n=600)
Sinovac (n=
300)
Pfizer (n=
300)
Median
age
15.34/2.55 15.78/5.26 14.90/4.96 19.82
Median
BMI
24.72/4.12 27.72/9.24 21.72/7.24 75.70
*Man-Whitney test, #
Kruskal
Wallis test
It can be seen in the table above that there is no
significant difference between age and BMI. The
median total agent can be seen at 2.55%, and the
median Bis MI is 2.12%. For Sinovac median age
5.26%, Sinovac median BMI 9.24%. for Pfizer's
median age of 4.96%, and Pfizer median BMI of
GENDER
Male Female
Female
56%
Mal
e
EXPOSED to COVID-19
Pfizer
43%
Sinovac
57%
Sinovac Pfizer
TREA
Light Weigh
t
(
Self-isolation
)
Currently
(Hospital/Non
ICU) 0%
Heavy
(Hospital/ICU
Neve
r
Ex
p
ose
d
Li
g
ht Wei
g
ht
(
Self-isolation
)
Never
Expose
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
252
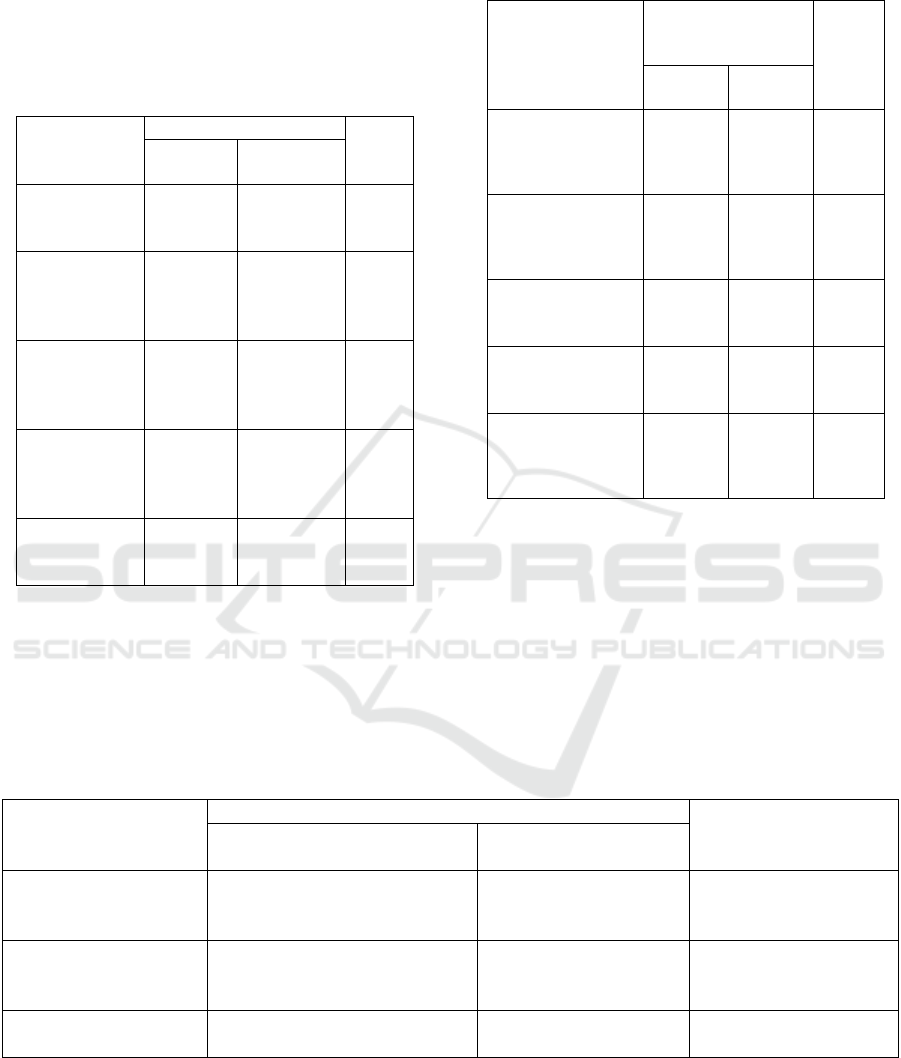
7.24% the P.Value value was significant (<0.005)
while in the media age (1.982) and median BMI
(75.70) there was no correlation between age and
BMI.
Table 4: Correlation between vaccine type and dose of
vaccine side effect.
Variable Frequency/Percentage (%) P-Value
Sinovac =
300
Pfizer n = 300
Side Effects of
Fever After The
1st Vaccination
124/41.3 135/45 0.365
Pain inThe
1st
Vaccination
Injection Area
152/50.6 180/60 0.027
Side Effects of
Coughing After
The 1st
Vaccination
22/7.33 28/9.33 0.460
Experienced
Diarrhea After
The 1st
Vaccination
12/4 19/6.33 0.268
Feel Sleepy
After The 1st
Vaccination
132/44 115/38.3 0.184
*Fisher test, #Chi-square
It can be seen in the table above that the only
significant data was pain in the injection area due to
its p. value (0.027) while the others were not
significant for fever (0.365), cough (0.460), diarrhea
(0.26,8), and drowsiness (0.184).
Table 5: Correlation between vaccine type and dose of
vaccine side effects 2.
Variable
Frequency/perce
ntage
(%)
P-
Value
Sinovac
n=300
Pfizer
n=300
Side Effects of
Fever After
The 2nd
Vaccination
110/36.
6
150/50 0.001
Side Effects of
Coughing After
The 2nd
Vaccination
19/6.33 33/11 0.058
Feeling Dizzy
After The 2nd
Vaccination
65/21.6 74/24.6 0.439
Feel Sleepy After
The 2nd
Vaccination
105/35 83/27.6 0.064
Feel Pain in The
Upper Arm After
The 2
nd
Vaccination
85/28.3 69/23 0.161
*Fisher test, #Chi-square
It can be seen in the table above that the significant
data is only fever (0.001), and the no significant is
cough (0.058), dizziness (0.439), drowsiness (0.064),
Pain in the Upper Arm (0.161).
In table 6 there is no significant data value of
p>0.005 at the efficacy of dose 1, it can be seen that
there is no correlation between the type of vaccine
and the efficacy of the dose 1 vaccine.
Table 6: Correlation between vaccine type and vaccine efficacy dose 1.
Variable
Frequency/ percentage (%)
P-Value
Sinovac
n = 300
Pfizer
n = 300
Loss of Loss and Taste
After The 1st
Vaccination
3/1 0 0.249
Experienced Cough and
Sore Throat After The
1st Vaccination
3/1 0 0.249
Having Head Pain After
The 1st Vaccination
3/1 0 0.249
*Fisher test, #Chi-square test
Evaluation of the Full Dosage Sinovac and Pfizer COVID-19 Vaccination into Adolescent
253

Table 7: Correlation between vaccine type and vaccine efficacy dose 2.
Variable
Frequency/ percentage (%)
P-Value
Sinovac n = 300 Pfizer
n = 300
Loss of Loss and Taste After The 2nd
Vaccination
0
3/1 0.249
Experienced Breathing Difficulty After The
2nd Vaccination
0
3/1 0.249
Experienced Cough and Sore Throat After
The 2n
d
Vaccination
0
3/1 0.249
Experienced Fever After The 2nd Vaccinatio
n
0
3/1 0.249
Having Head Pain After The 2nd Vaccination
0
2/0.66 0.499
*Fisher test, #Chi-square test
In the table above there is no
significant data on the efficacy of
dose 2.
Table 8: Correlation between age and vaccine side effects after 6 months.
Variable Frequency/ percentage (%) P. Value
Age Mean
n= 600 15.34
Have Been Exposed to COVID-19 4-
6 Months Afte
r
Vaccination
1/0.16 0.250
Feel Easy Fatigue 4-6 Months After
Vaccination
1/0.16 0.250
*Man-Whitney test, #Kruskal Wallis test
From the results of the Man-Whitney test regarding
age and side effects for 6 months, the percentage
value for being exposed to COVID-19 4-6 months
after the vaccine was 0.16%, and for easy fatigue, 4-
6 months after vaccination was 0.16% with a P-value
0.250
Table 9: Correlation between body mass index (BMI) and vaccine side effects after 6 months.
Variable
Frequency/Percentage (%)
P.Value
Gender mean
n = 600 24.72
Have Been Exposed to COVID-19 4-6
Months After Vaccination
1/0.16 0.452
Feel Easy Fatigue 4-6 Months After
Vaccination
1/0.16 0.452
*Man-Whitney test, #Kruskal Wallis test
From the results of the Mann-Whitney test regarding
BMI and side effects for 6 months, the p-value of
0.452 is not significant. With a presentation on
exposure to COVID-19 0.16% and easily tired 4-6
months presentation 0.16%.
4 DISCUSSION
This study evaluates exposure to COVID-19 by
Sinovac and Pfizer vaccination participants to
adolescents. The Sinovac vaccine uses dead virus
particles to induce antibody production(Halim, 2021).
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
254

Whereas Pfizer has utilized lipid nanoparticles
(LNPs) with a formulated mRNA vaccine(Sutardi &
Ramatillah, 2022). This study involved 600
participants from 300 Sinovac vaccines and 300
Pfizer vaccines in this study there were 56% female
participants and 44% male participants can be seen in
(figure 2). According to an article, two injections of
the Sinovac coronavac vaccine provide 94%
effectiveness for preventing Covid-19(Kezia &
Ramatillah, 2022). According to research by Klein SL
et al, women are often less likely to receive the
vaccine, but this comparison differs from that of
Indonesian adolescents who receive the
vaccine(Hoffmann et al., 2020). Women after being
vaccinated develop a higher and longer-lasting
protective antibody response, compared to men
However, they experience more frequent and intense
side effects than men(Hoffmann et al., 2020).
The chi-square test in table 1 can be seen that there
is no significant type of vaccine and comorbidity. The
p-value of 0.414 for participants WHO have asthma
is.
Control Center and Disease Prevention China
reported
that cardiovascular disease, hypertension,
diabetes, respiratory disease, and cancer are
associated with an increased risk of death(Ramatillah
& Isnaini, 2021). According to research, the
relationship between asthma and obesity does not
depend on gender, age, and the severity of
asthma(Jay et al., 2012).
One hypothesis of the
possible influence of asthma on
obesity is reduced
energy expenditure due to low levels of physical
activity in children with asthma(Jay et al., 2012).
Comorbidities besides demographic differences and
lifestyle changes are one of the most important
determinants that cause various disease
manifestations(Mitra et al., 2020). The association of
body mass index (BMI) from adolescence to
adulthood with obesity-related diseases in young
adults has not been fully delineated(Tirosh et al.,
2011). Disease in neonates, infants, and children has
also been reported to be significantly milder than in
their adult counterparts(Singhal, 2020). An increase
in BMI in childhood and adolescence may be closely
related to a higher incidence of coronary heart disease
and type 2 diabetes mellitus in young
adults(Nogueira-de-Almeida et al., 2020). Efforts
to
prevent the spread of COVID-19 will result in more
weight gain and a higher BMI, leading to growth
differences from conventional seasonal variations in
children(Han et al., 2021).
The percentage of respondents exposed to COVID-
19
was more exposed 57% of Sinovac vaccine
recipients than 43% of Pfizer vaccine recipients
(figure 3). According to research, There is no evidence
from this national survey data that men are more
likely to be affected than women(Ramatillah et al.,
2022). Initial S-Ab and N-Ab antibody responses to
the mRNA vaccine (Pfizer) were significantly higher
than those elicited by the inactivated virus vaccine
(Sinovac)(Lau et al., 2022). According to the severity
of symptoms, patients can be classified as light,
heavy, and critical types(He et al., 2020). In the
severity of the study, 99% never had exposure to
COVID-19, and 1% There were mild symptoms (self-
isolation) and moderate symptoms (Hospital/Non-
ICU) and severe (Hospital/ICU) 0% (figure 4). Based
on evidence that efficacy and immunogenicity are as
high as (or higher than) in older individuals with rare
serious side effects(Held et al., 2021). Judging from
the results of the study in table 2 there is a correlation
between vaccine type and exposure to COVID-19
which has a p value>0, 005 which means that there is
no significant difference between the type of vaccine
and exposure to COVID-19. Observational studies
show that vaccination is associated with reduced
COVID-19- related hospitalizations, intensive care
unit admissions, and mortality in adolescents(Held et
al., 2021).
As shown in Table 2. Gender and age showed a p-
value > 0.005, which means that gender and age were
not correlated with vaccine side effects. The lack of
age-related changes in the sex-differential effects of
vaccines suggests that genetic or other factors may be
involved(Ciarambino et al., 2021). Gender is
considered in clinical trials for Covid-19 vaccine
development to include analysis of efficacy, vaccine
response, and adverse reactions(Ciarambino et al.,
2021). Finally, we found that gender influenced
vaccine acceptance, with adolescent boys showing a
higher desire to be vaccinated. Previous studies on
gender and acceptance of the COVID-19 vaccine are
inconclusive As summarized by Bono et al., several
studies support lower vaccination willingness by
women, possibly due to their higher fear of side
effects(Humer et al., 2021).
Based on Tables 3&4 it can be seen that there is a
significant correlation in injection site pain where the
presentation rate of Pfizer is greater than Sinovac.
Pfizer 60% and Sinovac 50.6% different from the
second vaccine, a significant correlation occurs in
fever, the second dose of the Pfizer vaccine has higher
side effects than Sinovac. As can be seen in table 5
the presentation value of Pfizer is 50% and Sinovac
36.6%. For vaccine 1 side effects, fever, cough,
diarrhea, and drowsiness are not correlated with side
effects, p-value >0.005, while for side effects of
vaccine 2 coughs, dizziness, and drowsiness were not
correlated with side effects, p-value >0.005.
Evaluation of the Full Dosage Sinovac and Pfizer COVID-19 Vaccination into Adolescent
255

According to Riad, A., et al. Side effects tend to be
more pronounced with the second dose, especially for
those who receive the PfizerBioNTech vaccine.
Health workers in Turkey report that younger women
and individuals are more likely to report vaccine-
related side effects(Elnaem et al., 2021). Reported
side effects include fever, sore throat, myalgia, eye
muscle pain, loss of smell or taste, shortness of
breath, headache, numbness, palpitations, and
gastrointestinal symptoms. Women report more side
effects than men. This type of side effect after
receiving the vaccine was common and consistent
with other studies that found myalgia, headache,
gastrointestinal symptoms, and fever among the
frequently reported side effects(Alghamdi et al.,
2021). Side effects are
more common after the full
vaccination dose. mRNA-
1273 vaccine, and in
participants with younger age,
female gender,
previous COVID-19, Asian race, early
pregnancy, and
marijuana use. Older age, black or
African American
race, social status higher subjective
well-being, asthma,
and anemia were associated with a lower likelihood of
reporting side(Beatty et al., 2021). Mild severity
ofside effects (AE), both local and systemic, with a
frequency of and lower severity in the older group
(>65 years old) (Oyebanji et al., 2021)
5 CONCLUSIONS
This study found that for side effects there was a
significant correlation where Pfizer had higher side
effects than Sinovac, the side effects that occurred
with these two vaccines were a pain in the vaccination
area and fever. No significant side effects were found
with either of these two vaccines. The variable that
affects side effects and efficacy is the type of vaccine.
REFERENCES
Alghamdi, A., Ibrahim, A., Almutairi, R., Joseph, M.,
Alghamdi, G., & Alhamza, A. (2021). A cross-sectional
survey of side effects after COVID-19 vaccination in
Saudi Arabia: male versus female outcomes. Journal of
Advanced Pharmacy Education and Research, 11(2),
51–56. https://doi.org/10.51847/BCWCA2QGFP
Arnanda, N., & Ramatillah, D. L. (2022). Systematic
Review: Evaluation of Cytokine Storm Treatment From
Covid 19 Patient Base on Clinical Trial. International
Journal of Applied Pharmaceutics, 14(Special Issue 2),
5–9. https://doi.org/10.22159/ijap.2022.v14s2.44739
Beatty, A. L., Peyser, N. D., Butcher, X. E., Cocohoba, J.
M., Lin, F., Olgin, J. E., Mark, J., & Marcus, G. M.
(2021). Analysis of COVID-19 Vaccine Types and Side
Effects After Vaccination. 4(December), 1–13.
https://doi.org/10.1001/jamanetworkopen.2021.40364(
reprinted)22
Ciarambino, T., Barbagelata, E., Corbi, G., Ambrosino, I.,
Politi, C., Lavalle, F., Ruggieri, A., & Moretti, A. M.
(2021). Gender differences in vaccine therapy: where
are we in Covid-19 pandemic? Monaldi Archives for
Chest Disease, 91(4), 2–4.
https://doi.org/10.4081/monaldi.2021.1669
Darwis, I., Rukmi, R., & Perdani, W. (n.d.). Increased
Knowledge of Health Workers Regarding Corona
Disease Virus Disease ( COVID ) 19 in Adult Patients.
126–130.
Elnaem, M. H., Mohd Taufek, N. H., Ab Rahman, N. S.,
Mohd Nazar, N. I., Zin, C. S., Nuffer, W., & Turner, C.
J. (2021). Covid-19 vaccination attitudes, perceptions,
and side effect experiences in malaysia: Do age, gender,
and vaccine type matter? Vaccines, 9(10), 1–15.
https://doi.org/10.3390/vaccines9101156
Gee, S., Gaughran, F., MacCabe, J., Shergill, S., Whiskey,
E., & Taylor, D. (2020). Management of clozapine
treatment during the COVID-19 pandemic. Therapeutic
Advances in Psychopharmacology, 10(2),
204512532092816.
https://doi.org/10.1177/2045125320928167
Halim, M. (2021). COVID-19 Vaccination Efficacy and
Safety Literature Review. Journal of Immunology and
Allergy, 3(February). https://doi.org/10.37191/mapsci-
2582-4333-3(1)-058
Han, J. A., Chung, Y. E., Chung, I. H., Hong, Y. H., &
Chung, S. (2021). Impact of the COVID-19 pandemic
on seasonal variations in childhood and adolescent
growth: Experience of pediatric endocrine clinics.
Children, 8(5).
https://doi.org/10.3390/children8050404
He, F., Deng, Y., & Li, W. (2020). Coronavirus disease
2019: What we know? Journal of Medical Virology,
92(7), 719–725. https://doi.org/10.1002/jmv.25766
Held, J., Esse, J., Tascilar, K., Steininger, P., Schober, K.,
Irrgang, P., Alsalameh, R., Tenbusch, M., Seggewies,
C., & Bogdan, C. (2021). Reactogenicity correlates
only weakly with humoral immunogenicity after
COVID-19 vaccination with BNT162b2 mRNA
(Comirnaty®). Vaccines, 9(10), 1–12.
https://doi.org/10.3390/vaccines9101063
Hoffmann, M., Kleine-Weber, H., Krueger, N., Mueller, M.
A., Drosten, C., & Poehlmann, S. (2020). The novel
coronavirus 2019 (2019-nCoV) uses the SARS-
coronavirus receptor ACE2 and the cellular protease
TMPRSS2 for entry into target cells. BioRxiv, 2019,
2020.01.31.929042.
https://doi.org/10.1101/2020.01.31.929042
Humer, E., Jesser, A., Plener, P. L., Probst, T., & Pieh, C.
(2021). Education level and COVID-19 vaccination
willingness in adolescents. European Child and
Adolescent Psychiatry, 0123456789, 19–21.
https://doi.org/10.1007/s00787-021-01878-4
Jay, M., Wijetunga, N. A., Stepney, C., Dorsey, K., Chua,
D. M., & Bruzzese, J. M. (2012). The relationship
between asthma and obesity in urban early adolescents.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
256

Pediatric, Allergy, Immunology, and Pulmonology,
25(3), 159–167. https://doi.org/10.1089/ped.2012.0145
Kezia, V., & Ramatillah, D. L. (2022). Intensive
Monitroing of Sinovac Vaccine for Safety and Efficacy
Among Indonesian Population. International Journal
of Applied Pharmaceutics, 14(Special issue 2), 44–48.
https://doi.org/10.22159/ijap.2022.v14s2.44748
Lau, C. S., Oh, M. L. H., Phua, S. K., Liang, Y. L., Li, Y.,
Huo, J., Huang, Y., Zhang, B., Xu, S., & Aw, T. C.
(2022). Kinetics of the Neutralizing and Spike SARS-
CoV-2 Antibodies following the Sinovac Inactivated
Virus Vaccine Compared to the Pfizer mRNA Vaccine
in Singapore. Antibodies, 11(2), 38.
https://doi.org/10.3390/antib11020038
Mitra, P., Suri, S., Goyal, T., Misra, R., Singh, K., Garg, M.
K., Misra, S., & Sharma, P. (2020). Association of
Comorbidities with Coronavirus Disease 2019: A
Review. Annals of the National Academy of Medical
Sciences (India), 56(02), 102–111.
https://doi.org/10.1055/s-0040-1714159
Nogueira-de-Almeida, C. A., Del Ciampo, L. A., Ferraz, I.
S., Del Ciampo, I. R. L., Contini, A. A., & Ued, F. da
V. (2020). COVID-19 and obesity in childhood and
adolescence: a clinical review. Jornal de Pediatria,
96(5), 546–558.
https://doi.org/10.1016/j.jped.2020.07.001
Oyebanji, O. A., Wilson, B., Keresztesy, D., Carias, L.,
Wilk, D., Payne, M., Aung, H., Denis, K. S., Lam, E.
C., Rowley, C. F., Berry, S. D., Cameron, C. M.,
Cameron, M. J., Schmader, K. E., Balazs, A. B., King,
C. L., Canaday, D. H., & Gravenstein, S. (2021). Does
a lack of vaccine side effects correlate with reduced
BNT162b2 mRNA vaccine response among healthcare
workers and nursing home residents? Aging Clinical
and Experimental Research, 33(11), 3151–3160.
https://doi.org/10.1007/s40520-021-01987-9
Ramatillah, D. L., Azhar, S., Sulaiman, S., & Khan, A. H.
(2019). Original Article Quality of Life among Patients
Undergoing Haemodialysis in Jakarta , Indonesia
Patients During the Period of Observation Based.
19(2), 29–37.
Ramatillah, D. L., Gan, S. H., Pratiwy, I., Sulaiman, S. A.
S., Jaber, A. A. S., Jusnita, N., Lukas, S., & Bakar, U.
A. (2022). Impact of cytokine storm on severity of
COVID-19 disease in a private hospital in West Jakarta
prior to vaccination. PLoS ONE, 17(1 1), 1–14.
https://doi.org/10.1371/journal.pone.0262438
Ramatillah, D. L., Gan, S. H., Sulaiman, S. A. S., Puja, D.,
Abubakar, U., Jaber, A. A. S., Lukas, S., & Jusnita, N.
(2021). Evaluation of treatment outcome for pneumonia
among pre-vaccinated covid-19 patients with/without
comorbidity in a public hospital in Bengkulu,
Indonesia. Vaccines, 9(12), 1–9.
https://doi.org/10.3390/vaccines9121411
Ramatillah, D. L., & Isnaini, S. (2021). Treatment profiles
and clinical outcomes of COVID-19 patients at private
hospital in Jakarta. PLoS ONE, 16
(4 April), 1–11.
https://doi.org/10.1371/journal.pone.0250147
Singhal, T. (2020). A Review of Coronavirus Disease-2019
(COVID-19). Indian Journal of Pediatrics, 87(4), 281–
286. https://doi.org/10.1007/s12098-020-03263-6
Sutardi, A. Q. I., & Ramatillah, D. L. (2022). Evaluation
Comparison Between Sinovac and Pfizer Vaccine
Among Indonesian Children and Teenager Under 18
Years Old. International Journal of Applied
Pharmaceutics, 14(Special issue 2), 22–30.
https://doi.org/10.22159/ijap.2022.v14s2.44745
Tirosh, A., Shai, I., Afek, A., Dubnov-Raz, G., Ayalon, N.,
Gordon, B., Derazne, E., Tzur, D., Shamis, A., Vinker,
S., & Rudich, A. (2011). Adolescent BMI Trajectory
and Risk of Diabetes Versus Coronary Disease. Survey
of Anesthesiology, 55(6), 296–297.
https://doi.org/10.1097/01.sa.0000407026.86152.a1
Who Sage Roadmap for Prioritizing Use of COVID-19
Vaccines. (2022). January.
Evaluation of the Full Dosage Sinovac and Pfizer COVID-19 Vaccination into Adolescent
257
