
Comparative Evaluation of AEFI (Adverse Event Following
Immunization) and Effectiveness of Astrazeneca and Pfizer Booster
Vaccines among Indonesia Citizen
Ayu Novita Sari, Liandhajani and Diana Laila Ramatillah
Faculty Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords: Covid-19, Booster Vaccine, Astrazeneca, Pfizer, AEFI.
Abstract: The booster vaccine was launched amid concerns about the spread of omicron. The booster is effective against
the Omicron variant according to the pseudovirus neutralization test. Complete vaccine plus booster can
provide up to 91% protection from death or other worst risks from Covid-19. This study was conducted to
evaluate the comparison of the effectiveness of the AstraZeneca and Pfizer booster vaccines in the community
in Indonesia. This type of research is observational with cross-sectional. This method is done by direct
observation of the survey data. The most common side effects of receiving the AstraZeneca and Pfizer booster
vaccines are pain at the injection site and pain in the upper arm. This research was conducted using a validated
questionnaire with Cronbach alpha = 0,8. The number of respondents in this study was 600 people, 300
recipients of the AstraZeneca booster vaccine and 300 recipients of the Pfizer booster vaccine. Respondents
in this study were > 18 years. This research requires ethical approval No. 36/KEPK-
UTA45JKT/EC/EXP/07/2022. There is a significant relationsip between socio-demography and AEFI of the
AstraZeneca and Pfizer booster vaccine with p-value <0,05. The effectiveness of the Pfizer booster vaccine
is 98,3% and the AstraZeneca booster vaccine is 97%.
1 INTRODUCTION
At the end of 2019, an infectious disease emerged
which was designated by WHO as "coronavirus
disease (Covid-19)" which originated from the city of
Wuhan, China. The cause of Covid-19 is the SARS-
CoV-2 virus (SanJuan-Reyes et al., 2021). The
chronology of the Covid-19 infection, the first case
occurred in December 2019. On January 2, 2020,
there were 41 confirmed Covid-19 patients. On
January 22, 2020 there were 571 cases of Covid-19.
The Chinese National Committee reported the
first 17 deaths on January 22, 2020 (Ramatillah et al.,
2021). Furthermore, on January 30, 2020, as many as
7734 confirmed Covid-19 in China and 90 other cases
have also been reported from all countries, namely
Taiwan, Thailand, Republic of Korea, UAE, United
States, Philippines, India, Australia, Canada, Finland
and Germany (Ramatillah et al., 2021). The high
death rate is a problem especially in China. On
January 22, 2020, China's National Health
Commission reported 17 deaths (Ramatillah et al.,
2021).
In Indonesia, there were recorded cases of Covid-
19 as of February 17, 2022, namely 63,956 confirmed
cases of Covid-19 including 24,678 active cases,
39,072 confirmed cases recovered and 206 cases died
(Indonesian covid task force, 2022). The fact is that
Indonesian people still do not apply health protocols.
Indonesia launches booster vaccine amid concerns
about the spread of the Omicron variant
(Kompas.com, 2022). Several studies have shown
that antibodies to SARS-CoV-2 gradually decrease
after the second vaccination. Even at 6 months after
vaccination, two-dose mRNA induces long-term
immune memory against the variant SARS-CoV-2
(Seki et al., 2022). Complete vaccination plus booster
can provide protection up to 91% death or other worst
risks from Covid-19 (Kemenkes, 2022). Booster
vaccines are safe for healthy adults aged 18 – 59
years. Vaccines are a way to control outbreaks of
infectious diseases and a way to reduce the risk of
pandemics and epidemics (Seki et al., 2022).
Until 22 February 2022, the number of active
Covid-19 cases was 549,431 people with a total of
37,638 COVID-19 patients being hospitalized,
186
Sari, A., Liandhajani, . and Ramatillah, D.
Comparative Evaluation of AEFI (Adverse Event Following Immunization) and Effectiveness of Astrazeneca and Pfizer Booster Vaccines Among Indonesia Citizen.
DOI: 10.5220/0011978400003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 186-192
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

consisting of 813 in serious condition and 185 in
critical condition (Kemenkes, 2022). Based on an
analysis of the number of 17,871 patients who were
hospitalized in the period from January 21 to
February 22, 2022, the death toll reached 2,489. Most
of the patients who died were not fully vaccinated.
The risk of death of non-comorbid elderly who
received a booster was 0.49%, while the risk of death
of non-comorbid elderly who received two full doses
of the vaccine was 2.9%, while the risk of death in the
elderly without comorbidities who received the full
dose of the vaccine was 22.8% (Kemenkes, 2022).
The number of deaths in the comorbid group that did
not get the complete vaccine was 739 deaths
compared to those who received the booster only 20
deaths (Kemenkes, 2022).
Vaccines are a way to control outbreaks of
infectious diseases and a way to reduce the risk of
pandemics and epidemics (Excler et al., 2021). The
occurrence of an adverse reaction to the vaccine
indicates that the vaccine is effective and the
recognition of the disease increases (Francis et al.,
2021). According to the Centers for Disease Control
and Prevention (CDC) and other studies, there are
several reactions that occur after vaccination, namely
injection site symptoms (backache, fatigue, headache,
joint pain, body aches, chills, fever and nausea)
(Francis et al., 2021).
Symptoms of patients infected with SARS-CoV-
2 range from minimal symptoms to heavy breathing
with multiple organ failure. On computerized
tomography (CT) scans, characteristic opacities of the
lung base glass can be seen even in asymptomatic
patients. Symptoms of Covid-19 start from 2-14 days
of exposure, symptoms include dry cough, fever, and
fatigue. In some cases, the symptoms of Covid-19
patients may include pain, nasal congestion, diarrhea,
loss of smell and chills (Al-Awwal et al., 2022).
Vaccines are a way to control outbreaks of
infectious diseases and a way to reduce the risk of
pandemics and epidemics (Excler et al., 2021). The
Pfizer vaccine uses RNA or genetic code to make
body cells produce specific spikes for the coronavirus
(Health AGD, 2022). Astrazeneca-Oxford is an
adenovirus viral vector vaccine (Francis et al., 2021).
The viral vector vaccine uses an adenovirus-based
safe vector that does not cause disease, but can
function as a vector to deliver the genetic material of
the Covid virus to host cells. The host cell creates a
copy of the corona virus protein (spike protein)
making an immune response, producing T-
lymphocytes and antibodies against viral antigens
(spike protein) (Shekhar et al., 2021).
After the first and second doses, the immune
response slowly decreases. The second dose causes a
second, larger immune response, which gradually
declines over time. However, a large number of
memory B cell pools (with higher affinity for antigen
affinity maturation) are left behind, favoring a
broader and faster stage of the immune process
against the same pathogen in the future (Shekhar et
al., 2021).
A third or subsequent dose of the Covid-19
vaccine has the potential to increase titers of
neutralizing antibodies against SARS-CoV-2 and its
variants, particularly in immunocompromised
individuals or individuals with underlying
comorbidities or who are at increased risk of COVID-
19 exposure and transmission. However, it is
imperative to apply appropriate use criteria for the
third or subsequent doses of the Covid-19 vaccine
without jeopardizing global vaccination efforts and
further exacerbating global vaccine inequities
(Shekhar et al., 2021).
2 MATERIALS AND METHODS
2.1 Design
This research uses mixed methods and prospective
cross-sectional study. This research was conducted
for 3 months (May - July). The data
collection technique was carried out using a survey
method using google form which will later be
distributed through social media to all Indonesian
people who have carried out booster vaccinations
with the Astrazeneca and Pfizer booster vaccines
using the convenience sampling method. The method
is carried out by direct observation of the survey data.
The purpose of this survey method is to measure the
output value and to find out how the AEFI and the
effectiveness of the Astrazeneca and Pfizer booster
vaccines in people aged >18 years. The instrument
used in this study was a questionnaire used to collect
research data and was made based on existing
references.
2.2 Instrument
This research was conducted using a validated
questionnaire with Cronbach alpa = 0,8 and it
distributed through social media (WhatsApp,
Facebook, Instagram, Telegram, and Twitter). Data
collection was carried out from May to July 2022. The
data collected were 600 respondents during the study.
Only 600 respondents fulfilled the criteria inclution.
Comparative Evaluation of AEFI (Adverse Event Following Immunization) and Effectiveness of Astrazeneca and Pfizer Booster Vaccines
Among Indonesia Citizen
187
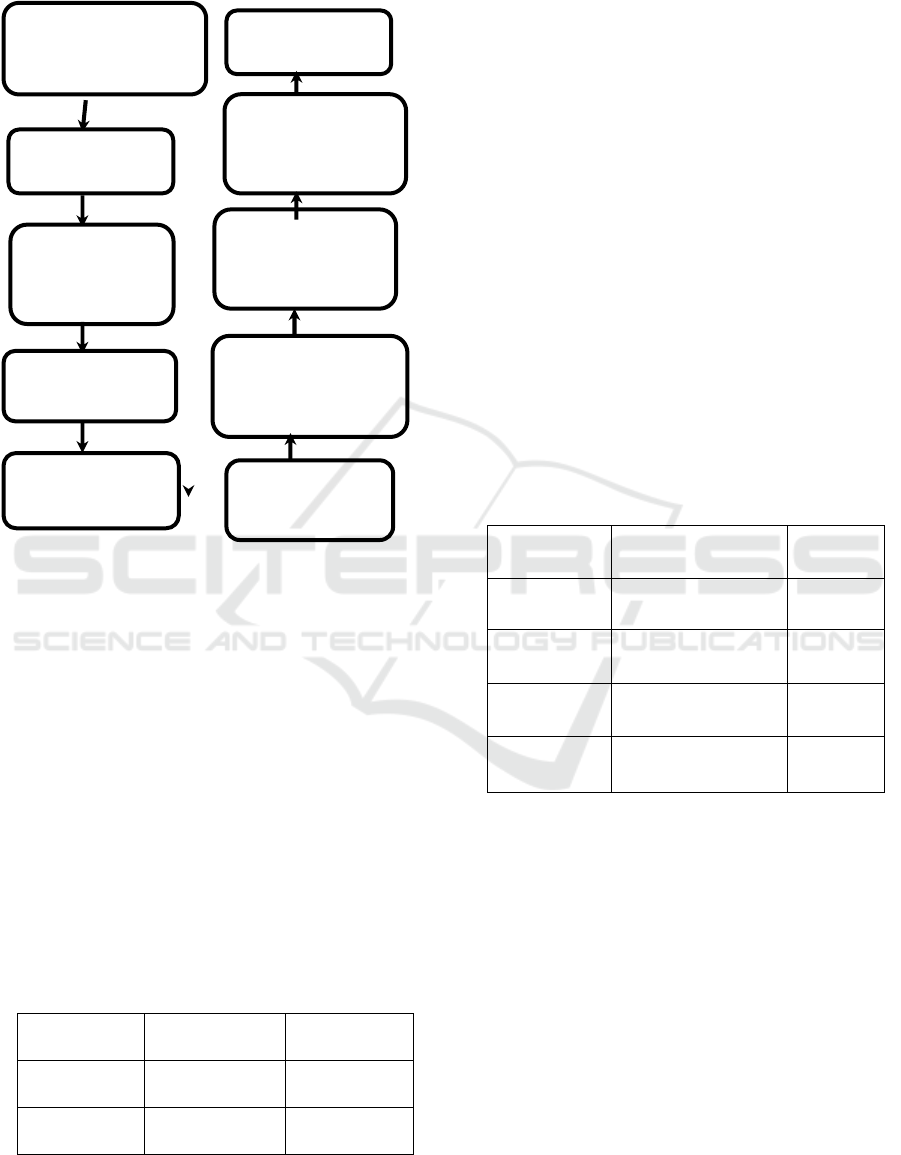
2.3 Statistical Analysis
Figure 1. Research Framework.
3 RESULT AND DISCUSSION
3.1 Result
Based on table 1. It shows that most of the
respondents in this study were female with a
percentage of Astrazeneca vaccine booster recipients
65,3% while men with a percentage of 34,6%. while
women recipients of Pfizer booster vaccine with a
percentage of 68.3% and men with a percentage of
31.6%. The same as the research of Araminda et al.
that reported women were predominant than men it
was found that the majority of respondents were
women than men (Araminda & Ramatillah, 2022).
Table 1: Gender.
Variab
el
Astrazenec
a n=300
Pfizer
n=300
Male 104/34,
6
95/31,6
Female 196/65,
3
205/68,3
As many as 600 respondents who filled out the
questionnaire, the age of 18-25 years old recipients of
the Astrazeneca booster vaccine was 161 people with
a percentage of 53,6%, and 181 people who received
the Pfizer booster vaccine with a percentage of
60,3%. There were 118 people aged 26-40 years who
received the Astrazeneca booster vaccine with a
percentage of 39,3% and 109 people who received the
Pfizer booster vaccine, with a percentage of 36,3%.
Ages 41-50 years were recipients of the Astrazeneca
booster vaccine with a percentage of 4,6% and the
recipients of the Pfizer booster vaccine were 1,3%.
Ages 51 – 67 years are recipients of the Astrazeneca
booster vaccine with a percentage of 3% while the
Pfizer booster vaccine recipients are 2%. the age
between 26-40 years is a productive society. Their
work requires vaccination before they come to the
office. we can see people who have been vaccinated
are around 18-40 years old. Booster vaccine
recipients in Indonesia are over 18 years of age and
over (kemenkes, 2022a). The priority target recipients
of the booster vaccine are the elderly (kemenkes,
2022a).
Table 2. Age.
A
g
e Astrazeneca
n=300
Pfizer
n=300
18
–
25
years
161/53,6% 181/60,3
%
26
–
40
years
118/39,3% 109/36,3
%
41
–
50
years
14/4,6% 4/1,3
%
51
–
67
years
9/3% 6/2%
This research was conducted throughout
Indonesia, namely Java with a percentage of 88.33%,
Sumatera at 7.67%, Kalimantan at 3.00%, Bali at
0.67%, and Sulawesi at 0.33%. The Covid-19 booster
vaccination on August 3, 2022 for the category of
people and the elderly, namely Java 30,062,042 with
a percentage of 31.76%, Bali as much as 1,467,027
with a percentage of 55.72%, Kalimantan 2,152,076
with a percentage of 22.66%, Sulawesi 1,317,983
with a percentage of 12.25% , and Sumatra 7,752,729
with a percentage of 23.83% (kemenkes, 2022b).
Reserch (approval of
research and ethical
approval)
Conclusion
Questionnare
deployment using
social media
Questionnare
Questionnare
deployment using
social media
Assement of
questionnaire by
expert
Questionnare valid
and reliable with
cronbach alpha 0,8
Pilot Study (30
respondens
Received up of
questionnare
Validity and
reliability test
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
188
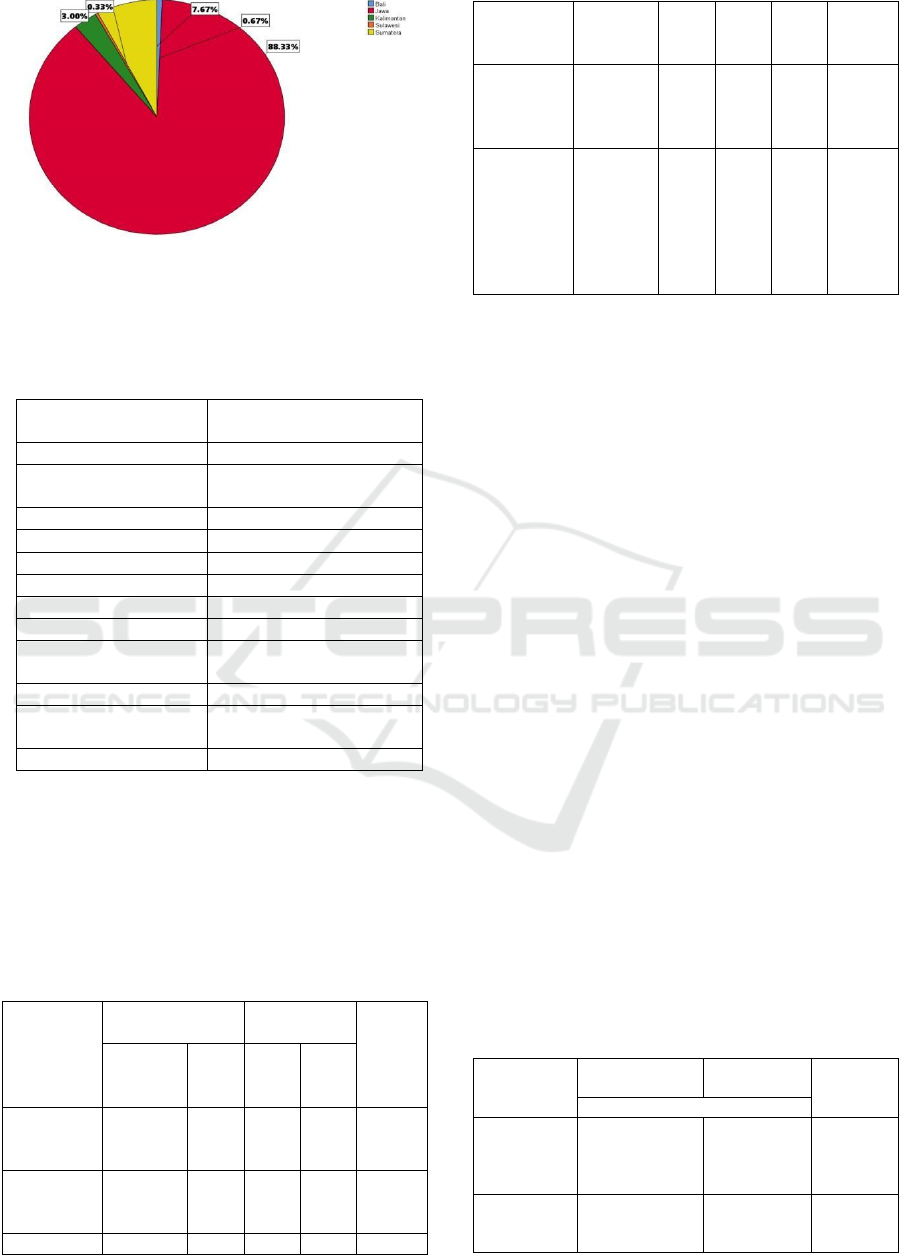
Figure 2. Prevalence Domicile.
3.1.1 Adverse Event Following
Immunization
Table 3: Adverse Event Following Immunization.
Variable Frekuensi/Persenta
g
e
(
n=600
)
Feve
r
266/44,3%
Pain at the injection
site
401/66,8%
Cou
g
h 64/10,6%
Flu 95/15,8%
N
auseous 50/8,3%
Diarrhea 37/6,1%
Dizz
y
214/35,6%
Drowsiness 287/47,3%
Thirst or
deh
y
dration
96/16%
Bleedin
g
4/0,6%
Pain in the upper
ar
m
292/48,6%
Heart
p
roblems 10/1,6%
Based on table 3. the common AEFIs felt by
respondents after the Astrazeneca and Pfizer booster
vaccines were fever 44.3%, pain in the injection area
66.8%, cough 10.6%, flu 15.8%, nausea 8.3%,
diarrhea 6 .1%, dizziness 35%, drowsiness 47.3%,
thirst or dehydration 16%, bleeding 0.6%, pain in the
upper arm 48.6% and heart problems 1.6%.
Table 4. Correlation between gender and AEFI.
Variable
Male
(n=199)
Female
(n=401)
p-value
Astrazene
ca
Pfizer Astra
zenec
a
Pfizer
Fever after
booster
vaccine
40/20,1% 34/17
,0%
96/23
,9%
98/24
,4%
0,015*
Sleepy after
booster
vaccine
14/7,0% 25/12
,5%
58/14
,4%
60/14
,9%
0,010*
Diarrhea 4/2,0% 3/1,5 13/3, 22/5, 0,017*
after
booster
vaccine
% 2% 4%
Headache
after
booster
vaccine
11/5,5% 22/11
,0%
44/10
,9%
56/13
,9%
0,022*
Monitoring
after 1
month of
booster
vaccine for
menstrual
p
roblems
0 0 19/4,
7%
11/2,
7%
0,000#
*Fisher Test #Chi Square Test
Table 4. shows that there are more women than men.
AEFI in women is more dominant than men. Such as
fever after booster vaccine in women with
Astrazeneca booster vaccine by 23,9% and Pfizer
recipients by 24,4%, while men with astrazeneca
booster vaccine by 20,1% and Pfizer booster
recipients by 17,0%. women experienced fever after
the Astrazeneca booster vaccine with a percentage of
14,4% and 14,9% of the recipients of Pfizer booster,
while men recipients of Astrazeneca booster with
percentage 7,0% and recipients of Pfizer vaccine
12,5%. 3,2% of female respondents who received
Astrazeneca booster vaccine and 5,4% of the
respondents who received the Pfizer booster vaccine
recipients had diarrhea. While on male recipents are
2,0 of Astrazeneca booster vaccine and 1,5% of Pfizer
booster vaccine recipents. Female respondents who
experienced headache are 10,0% of Astrazeneca
vaccine booster recipents and 13,9% of Pfizer vaccine
booster recipents. While on male recipents the
percentage are 5,5% of Astrazeneca booster vaccine
recipents and 11,0% of Pfizer booster vaccine
recipents. Female respondents who experienced a
change in their menstrual cycle are 4,7% of
Astrazeneca vaccine booster recipents and 2,7% of
Pfizer vaccine booster recipents.
3.1.2 Correlation Between Age and Vaccine
AEFI
Table 5. Correlation Between Age and Vaccine AEFI.
Variable Astrazeneca
n=300
Pfizer
n=300
p-
value
(
median=26,4
)
Cough and
sore throat
after booster
vaccine
113/37,6% 101/33,6%
0,025
*
Fever after
booster
vaccine
155/51,6% 132/44% 0,041
*
Comparative Evaluation of AEFI (Adverse Event Following Immunization) and Effectiveness of Astrazeneca and Pfizer Booster Vaccines
Among Indonesia Citizen
189
Monitorin
g
after 1 month of booster vaccine
Menstrual
p
roblems
19/6,3% 11/3,6% 0,000 #
Tired easil
y
35/11,6% 31/10,3% 0,009 #
Pain in the
arm
40/13,3% 41/13,6% 0,003 #
Thirst or
deh
y
dration
28/9,3% 31/10,3% 0,037 #
Heart
p
roblems
5/1,6% 5/1,6% 0,002 #
*Mann-whitney, #Kruskal Wallis Test
Table 5. shows that the perceived AEFIs include
cough, sore throat, and fever. The average age in this
study was 26.4. From the results of the study, the
AEFI of old age is felt to be less than that of yaoung
age. Meanwhile, the AEFI felt by Astrazeneca
booster vaccine recipients such as cough and sore
throat was 37,6% and fever was 51,6%. while 33,6%
of Pfizer booster vaccines received had cough and
sore throat and 44% had fever.
3.1.3 Correlation Between BMI and Vaccine
AEFI
Table 6. Correlation Between BMI and Vaccine AEFI.
Variable Astrazeneca
n=300
Pfize
r
n=300
P-value
Median = 21
Cough
after
booster
vaccine
36/12% 28/9,3% 0,017*
Diarrhea
after
booster
vaccine
27/9% 10/3,3% 0,027*
Covid-19
infection
after 1
month of
booster
vaccine
9/3% 5/1,6%% 0,024*
Monitoring
after 1
month of
booster
vaccine for
menstrual
p
roblems
19/6,3% 11/3,6% 0,000#
*Mann-whitney Test, #Kruskal Wallis Test
Based on table 6. BMI (Body Mass Index) also affects
AEFI with p-value <0.05. Respondents who received
the Astrazeneca booster vaccine experienced AEFI
cough and diarrhea with a percentage of 12% and 9%
respectively. while the recipients of the pfizer booster
vaccine who experienced cough and diarrhea with a
percentage of 9,3% and 3,3%. Covid-19 infection
after 1 month of Astrazeneca booster vaccine was 3%
and menstrual problem by 6,3%. While 9,3% of the
recipents of Pfizer booster vaccine experienced AEFI
of cough and 3,3% experienced diarrhea.
3.1.4 Relationship between Vaccine Types
and AEFI
Table 7. Relationship between vaccine types and AEFI.
Variable Astrazeneca
n=300
Pfizer
n=300
P-value
Flu after
booster
vaccine
57/19% 38/12% 0,044*
Diarrhea
after
booster
vaccine
25/8,3% 12/4% 0,040*
Skin rash
after
booster
vaccine
46/15,3% 27/9% 0,024*
*Mann-whitney
Table 7. show that the AEFI of Astrazeneca booster
vaccine such as fluwhit percentage of 19%, diarrhea
8,3%, and skin rashes 15,3%. Meanwhile AEFI of
Pfizer booster vaccine such as flu whit a percentage
of 12%, diarrhea 4%, and skin rashes 4,5%.
3.1.5 Effectiveness of Astrazeneca and
Pfizer Booster Vaccines Based on
Exposure to Covid-19 After Booster
Vaccination
Figure 3. Exposure to covid-19.
From the results of the study, it is known that the
effectiveness of the vaccine is seen from the number
of people exposed to Covid. Among total 600
respondent, 300 respondents recipients booster
vaccine and 300 recipients of Pfizer booster vaccine.
After the Astrazeneca booster vaccine, 9 respondent
were exposed to Covid-19 and 5 respondents who
received the Pfizer booster vaccine were exposed to
Covi-19 after vaccination.
0
5
10
Astrazeneca Pfizer
exposed to
covid
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
190
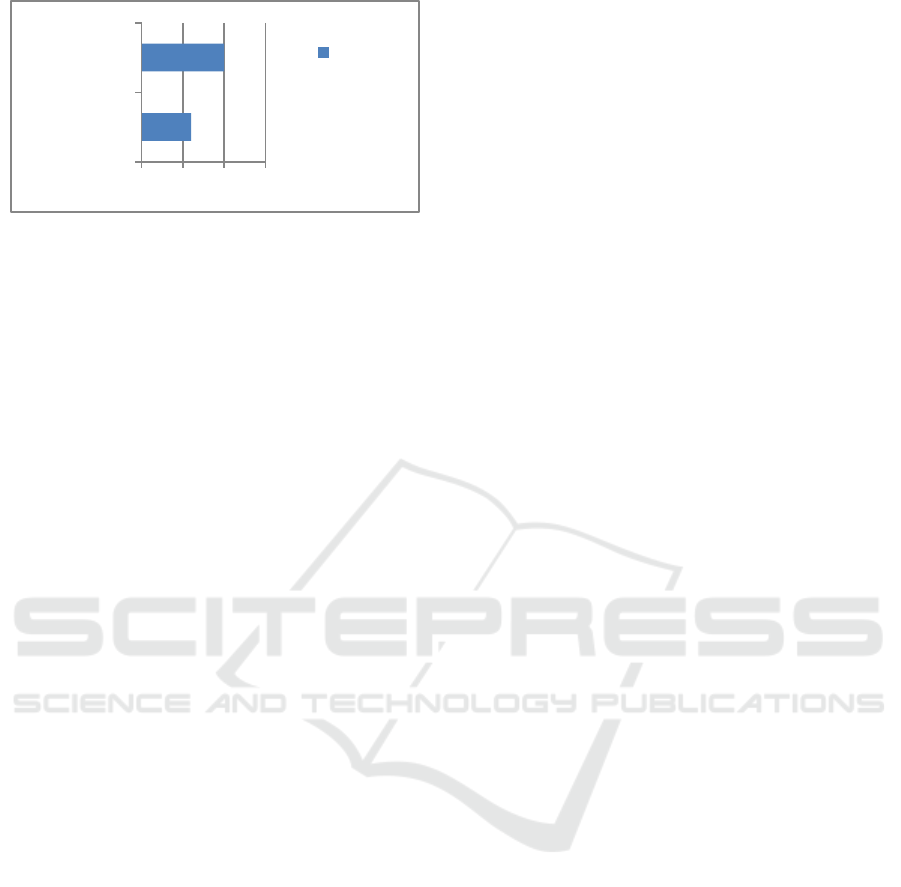
Figure 4. Effectiveness vaccine.
From the results of this study, the effectiveness of
the Pfizer booster vaccine was 98,3% and the
Astrazeneca booster vaccine was 97%.
3.2 Discussion
Based on the results of this study, similar to that of
David Hillus et al., it was found that the most
frequently felt AEFIs were pain at the injection site
and tenderness. Local reactions are usually mild to
moderate. No major differences were observed in the
frequency or severity of local reactions after the
primary or booster vaccine, except of a slightly higher
frequency of local reactions after the heterologous
Astrazeneca – Pfizer booster vaccination compared
with the homologous Pfizer booster vaccine. The
frequency of local reactions was lower after
homologous Astrazeneca booster vaccination than
after heterologous Astrazeneca– Pfizer vaccination
(Hillus et al., 2021).
AEFI and vaccine effectiveness were not related
because there was no significance between the two
variables. In other studies, there is no evidence that
AEFIs affect the effectiveness of vaccines.
Gender has a p-value <0.05 which means it has a
significant relationship with AEFI. According to
research, because of psychological factors, AEFI in
women in the form of pain is higher than in men
(Alghamdi et al., 2021). According to another study,
women produce antibody titers after vaccination and
have a stronger immune system so they experience
higher AEFI (Iguacel et al., 2021). Monitoring after 1
month after the booster vaccine found menstrual
cycle problems in women with a percentage of 7.4%.
According to research conducted by Muhaid, after 1
week of vaccination, symptoms of changes in the
menstrual cycle appear, then menstruation returns to
normal after 2 months of vaccination (Muhaidat et al.,
2022).
This is in accordance with previous research
where the AEFI of the elderly is less pronounced than
the younger age. Elderly AEFI is less pronounced
than younger people because immune cells are aging
combined with T cell depletion as atrophy
exacerbates the loss of new pathogens or vaccines and
age-related immunity (Kezia & Ramatillah, 2022).
According to research by Zare et al, AEFI is
highest in people with a BMI above 25 (Zare et al.,
2020). From the results of the study, it was found that
BMI and AEFI were significant in the form of
coughing and diarrhea, this might occur because most
of the respondents had a BMI less than 25.
The results showed that the AEFI of the
Astrazeneca booster vaccine was more dominant than
the Pfizer booster vaccine. Respondents felt that their
skin rash was due to hypersensitivity to vaccine
ingredients or the active ingredients of a vaccine
(Cebeci & Kartal, 2021).
From the results of this study, the effectiveness of
the Pfizer booster vaccine was 98,3% and the
Astrazeneca booster vaccine was 97%. Another study
reported that ChAdox1-S COVID-19 booster
vaccination againt the Omicron and Delta variants in
Englandhad the effectivity 82,3% (64,2 to 91,3%)
(Kirsebom et al., 2022).
4 CONCLUSIONS
From the result of this study it can be observed that
the effectiveness of the Pfizer booster vaccine is
98,3% and the Astrazeneca booster vaccine is 97%.
The AEFI in the Astrazeneca booster vaccine was
higher than the pfizer booster vaccine. Most of those
participants are from Java and this study female more
predominant than male. The AEFI felt by the
respondents were fever, pain at the ijection site,
diarrhea, dizzinezz, drowsiness and pain above the
arm. AEFI in female more than male such as fever,
drowsiness, diarrhea, headaches, and menstrual
problems. AEFI on BMI such as cough and diarrhea.
Astrazeneca booster vaccine AEFI are more felt than
Pfizer booster vaccine recipients.
REFERENCES
Al-Awwal, N., Dweik, F., Mahdi, S., El-Dweik, M., &
Anderson, S. H. (2022). A Review of SARS-CoV-2
Disease (COVID-19): Pandemic in Our Time.
Pathogens, 11(3), 368.
https://doi.org/10.3390/pathogens11030368
Alghamdi, A., Ibrahim, A., Almutairi, R., Joseph, M., &
Alghamdi, G. (2021). A cross-sectional survey of side
effects after COVID-19 vaccination in Saudi Arabia :
male versus female outcomes. August.
285 290 295 300
Astrazeneca
Pfizer
Efficcacy
Vaccine
Comparative Evaluation of AEFI (Adverse Event Following Immunization) and Effectiveness of Astrazeneca and Pfizer Booster Vaccines
Among Indonesia Citizen
191

https://doi.org/10.51847/bCwca2qGfP
Araminda, G. N., & Ramatillah, D. L. (2022). Original
article evaluation comparison between astrazeneca and
moderna vaccine ’ s side effects and efficacy among
indonesia society based on sociodemography. 14(2).
Cebeci, F., & Kartal, I. (2021). Petechial skin rash
associated with coronavac vaccination: First cutaneous
side effect report before phase 3 results. European
Journal of Hospital Pharmacy, 0(0), 1–2.
https://doi.org/10.1136/ejhpharm-2021-002794
Excler, J. L., Saville, M., Berkley, S., & Kim, J. H. (2021).
Vaccine development for emerging infectious diseases.
Nature Medicine, 27(4), 591–600.
https://doi.org/10.1038/s41591-021-01301-0
Francis, A. I., Ghany, S., Gilkes, T., & Umakanthan, S.
(2021). Review of covid-19 vaccine subtypes, efficacy
and geographical distributions. Postgraduate Medical
Journal, 1–6. https://doi.org/10.1136/postgradmedj-
2021-140654
Health AGD. (2022). Comirnaty (Pfizer). Health.Gov.Au.
https://www.health.gov.au/initiatives-and-
programs/covid-19-vaccines/approved-vaccines/pfizer
Hillus, D., Schwarz, T., Tober-Lau, P., Vanshylla, K.,
Hastor, H., Thibeault, C., Jentzsch, S., Helbig, E. T.,
Lippert, L. J., Tscheak, P., Schmidt, M. L., Riege, J.,
Solarek, A., von Kalle, C., Dang-Heine, C., Gruell, H.,
Kopankiewicz, P., Suttorp, N., Drosten, C., … Sander,
L. E. (2021). Safety, reactogenicity, and
immunogenicity of homologous and heterologous
prime-boost immunisation with chadox1 ncov-19 and
BNT162b2: a prospective cohort study. The Lancet
Respiratory Medicine, 9(11), 1255–1265.
https://doi.org/10.1016/S2213-2600(21)00357-X
Iguacel, I., Maldonado, A. L., Ruiz-Cabello, A. L., Casaus,
M., Moreno, L. A., & Martínez-Jarreta, B. (2021).
Association between covid-19 vaccine side effects and
body mass index in Spain. Vaccines, 9(11), 1–12.
https://doi.org/10.3390/vaccines9111321
Indonesian covid task force. (2022). Peta Sebaran Covid
19. Covid19.Co.Id. https://covid19.go.id/peta-sebaran
kemenkes. (2022a). Vaksin Booster Segera Dimulai, Cek
Tiket Vaksinasi di PeduliLindungi. Kemkes.Go.Id.
https://www.kemkes.go.id/article/view/22011200001/
vaksin-booster-segera-dimulai-cek-tiket-vaksinasi-di-
pedulilindungi.html
kemenkes. (2022b). Vaksinasi COVID-19 Berdasarkan
Provinsi dan Kabupaten/ Kota. Kemkes.Go.Id.
https://vaksin.kemkes.go.id/#/detail_data
Kemenkes. (2022). Vaksinasi Booster dapat Memberikan
Perlindungan Hingga 91% dari Risiko Terburuk covid-
19. Kemkes.Go.Id.
Kezia, V., & Ramatillah, D. L. (2022). Original article
intensive monitroing of sinovac vaccine for safety and
efficacy among indonesian population. 14(2), 44–48.
Kirsebom, F., Andrews, N., Sachdeva, R., Stowe, J.,
Ramsay, M., & Bernal, J. L. (2022).
Effectiveness of
chadox1-S COVID-19 Booster Vaccination against the
Omicron and Delta variants in England. MedRxiv,
2022.04.29.22274483.
http://medrxiv.org/content/early/2022/05/01/2022.04.2
9.22274483.abstract
Kompas.com. (2022). Indonesia Rolls Out Booster Shots,
amid Omicron Concerns. Www.Kompas.Com.
https://go.kompas.com/read/2022/01/12/192934374/in
donesia-rolls-out-booster-shots-amid-omicron-
concerns?page=1
Muhaidat, N., Alshrouf, M. A., Al-nazer, M. W., & Al-ani,
A. (2022). Menstrual Symptoms After COVID-19
Vaccine : A Cross-Sectional Investigation in the MENA
Region. February, 395–404.
Ramatillah, D. L., Alam, H. F., Hamid Ipadeola, M., Azhar,
S., Sulaiman, S., Lukas, S., Jusnita, N., & Ramadhani,
D. (2021). Public Knowledge about COVID-19 and Its
Impact on the Psychic Condition of Indonesian Society.
48(8).
SanJuan-Reyes, S., Gómez-Oliván, L. M., & Islas-Flores,
H. (2021). Covid-19 in the environment. Chemosphere,
263.
https://doi.org/10.1016/j.chemosphere.2020.127973
Seki, Y., Yoshihara, Y., Nojima, K., Momose, H., Fukush,
S., Moriyama, S., Wagatsuma, A., Numata, N., Sasak,
K., Kuzuok, T., Yato, Y., Takahashi, Y., Maeda, K.,
Suzuki, T., Mizukami, T., & Hamaguchi, I. (2022).
Safety and immunogenicity of Pfizer/BioNTech SARS-
CoV-2 mRNA third booster vaccine against SARS-CoV-
2 Omicron variant in Japanese healthcare workers.
Medrxiv.
https://doi.org/https://doi.org/10.1101/2022.01.20.222
69587
Shekhar, R., Garg, I., Pal, S., Kottewar, S., & Sheikh, A. B.
(2021). COVID-19 vaccine booster: To boost or not to
boost. Infectious Disease Reports, 13(4), 924–929.
https://doi.org/10.3390/idr13040084
Zare, H., Rezapour, H., Mahmoodzadeh, S., & Fereidouni,
M. (2020). Since January 2020 Elsevier has created a
covid-19 resource centre with free information in
english and mandarin on the novel coronavirus covid-
19 . the covid-19 resource centre is hosted on Elsevier
Connect , the company ’ s public news and
information
. January.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
192
