
The Process of Thermal Runaway Is the Reason of Fleischmann-Pons
Effect
Nikolay E. Galushkin
a
, Nataliya N. Yazvinskaya
b
and Dmitriy N. Galushkin
c
Laboratory of Electrochemical and Hydrogen Energy, Don State Technical University,
1 Gagarin Square, Town of Rostov-on-Don, Russia
Keywords: Thermal Runaway, Fleischmann-Pons, Deuterium Accumulation, Deuteride, Battery.
Abstract: In the electrolysis of heavy water, Fleischmann and Pons found the effect of excess power. Then this effect
was discovered by a number of other researchers. They explained this effect of "cold fusion" of deuterium
nuclei. In the electrolysis of heavy water, it is very difficult to obtain the Fleischmann and Pons effect; it
occurs spontaneously and is completely unpredictable. Therefore, a significantly larger number of researchers
were unable to obtain the effect of Fleischmann and Pons. They consider this effect to be a mistake or an
instrumental artifact. In this paper provides recommendations for obtaining the Fleischmann and Pons effect
at will and reliably. Therefore, at present, every researcher can securely obtain the effect of Fleischmann and
Pons and investigate it. The paper proves that the cause of the Fleischmann-Pons effect (of burst type) is the
exothermic reaction of thermal runaway, which is caused by the desorption and recombination of atomic
deuterium accumulated in the electrodes during the electrolysis of the electrolyte. In batteries with aqueous
electrolyte, thermal runaway is due to a similar exothermic reaction. Therefore, the cause of the Fleischmann-
Pons effect is not the "cold fusion" of deuterium nuclei. It is also shown that the cause of the Fleischmann-
Pons effect (of weak type) is the partial recombination of deuterium and oxygen, i.e. in this case, the excess
power is apparent or imaginary.
1 INTRODUCTION
In 1989, when studying the heavy water electrolysis
in cells with palladium cathodes, Fleischmann and
Pons (F&P) discovered the excess power effect
(Fleischmann et al., 1989). This effect appeared
suddenly after prolonged electrolysis of heavy water
and lasted for several hours. In this case, the energy
released by the cell was much greater than the energy
received by the cell from an external power source.
As Fleischmann and Pons did not find any obvious
electrochemical reactions there, they assumed that the
reason for the huge energy release was the deuterons
synthesis nuclear reactions. Subsequently, the nuclear
processes proposed by F&P became known as “cold
fusion”. Studies by Fleischmann and Pons showed
that the excess power effect (or Fleischmann-Pons
effect) occurs randomly and extremely rarely.
a
https://orcid.org/0000-0002-1613-8659
b
https://orcid.org/0000-0001-8147-8599
c
https://orcid.org/0000-0001-8261-6527
Since then, some researchers managed to
reproduce the Fleischmann-Pons (F-P) effect
(Dominguez et al., 2014; Storms, 2007; etc.).
However, much larger is the number of researchers,
who failed to repeat this effect (Lewis et al., 1989;
Williams, 1989; etc.). That is why currently, the
majority of the researchers consider the Fleischmann-
Pons effect to be a result of experimental errors
(Shanahan, 2010).
However, now, the recommendations have been
given (Galushkin et al., 2020) for the F-P effect
reliable obtainment. From now on, any researcher can
reproduce and investigate the F-P effect reliably, even
if he is skeptical about it.
The F-P effect can be of two types.
(Type A). In this case, the power released by the
cell was much greater than the power received by the
cell from an external power source. This type of the
F-P effect occurs only after prolonged (more than
40
Galushkin, N., Yazvinskaya, N. and Galushkin, D.
The Process of Thermal Runaway Is the Reason of Fleischmann-Pons Effect.
DOI: 10.5220/0011891800003536
In Proceedings of the 3rd International Symposium on Water, Ecology and Environment (ISWEE 2022), pages 40-45
ISBN: 978-989-758-639-2; ISSN: 2975-9439
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

three months) electrolysis of the electrolyte and it
lasts for several hours.
(Type B). In this case, the power released by the
cell was just slightly exceeds power received by the
cell from an external power source. This type of the
F-P effect occurs after several days of electrolyte
electrolysis and it can last for many days.
Some skeptics “explained” the F-P effect (type B)
by experimental errors including calibration errors
(Shanahan, 2010).
The F-P (type A) effect is very difficult to obtain,
so the skeptics just rejected the existence of this effect
(Lewis et al., 1989; Shanahan, 2010; etc.).
But currently, using the recommendations given
in (Galushkin et al., 2020), the F-P (type A) effect can
be reproduced reliably and purposely.
In this paper, the F-P effect is analyzed and
recommendations for its reliable reproduction are
given.
2 FLEISCHMANN-PONS EFFECT
MECHANISM ANALYSIS
2.1 Mechanism of Fleischmann-Pons
Effect (Type A)
As it was proved in the paper (Galushkin et al., 2020),
the (type A) effect occurs according to the following
scenario.
The reasons, which brings a cell gradually to the
(type A) effect, is accumulation processes, and there
are two types of the accumulations.
Firstly, during long-term electrolysis of the
electrolyte (more than three months), large amounts
of deuterium accumulate in the electrodes of the cell
(Galushkin et al., 2020).
Secondly, this is deposits accumulation on the
cathode.
As long as the deuterium accumulates in the
electrodes, in parallel with the electrolyte
decomposition reactions
2D
2
O+2e
−
→ D
2
+ 2OD
−
(cathode), (1)
2OD
−
→ 1/2O
2
+ D
2
O + 2e
−
(anode), (2)
the electrochemical reactions of thermal runaway
occur (Galushkin et al., 2020; Galushkin et al., 2015):
D
2
O + D
ads
+ e
−
→ D
2
↑+OD
−
(cathode), (3)
D
ads
+ OD
−
→ D
2
O + e
−
(anode). (4)
The overall reaction looks as follows:
D
ads cat
+ D
ads an
→ D
2
↑. (5)
For thermal runaway reactions (3,4), the rate-
limiting step is the step of metal-deuterides
disintegration (Galushkin et al., 2015).
MeD
x
→ Me + D
x
. (6)
With due account of the deuterides decomposition
reaction (6), the atomic deuterium recombination
reaction (5) is a reaction with the heat release of 442.4
kJ/mol(D
2
), i.e. this is a powerful exothermic reaction
(Galushkin et al., 2020) and (7). While the
combustion reaction of deuterium in oxygen has a
heat release of only 295 kJ/mol(D
2
) (Greenwood et
al., 1997).
The enthalpy of exothermic thermal runaway
reactions (3,4) ΔH
T
differs from the enthalpy of the
exothermic reactions of the free deuterium atoms
recombination ΔH
fex
=-443.32 kJ/mol(D
2
) (Luo, 2007)
by the value of the endothermic enthalpy of the
deuterides decomposition ΔH
d
. In the deuterides
accumulated in metals with micro-defects (as in the
F-P effect (Galushkin et al., 2020)), the deuterium
atoms are bounded less strongly than in metals
without micro-defects. Our experiments (similar to
those from (Sakamoto et al., 1996)) showed that in
metal-ceramic electrodes with a large number of
dislocations, the enthalpy of decomposition of the
PdD
x
and PtD
x
is approximately equal to ΔH
d
= 0.92
kJ/mol(D
2
). Hence, the enthalpy of the exothermic
reactions (3,4) is equal to
ΔH
T
= ΔH
fex
+ΔH
d
=-443.32+0.92=-442.4 kJ/mol (D
2
)
(7)
At the room temperature (25°C) and the current of
64 mA cm
−2
(Table 1) (Galushkin et al., 2020), the
deuterides (6) do not decompose at all (Galushkin et
al., 2015). So the contribution of the reactions of the
thermal runaway (3,4) to the total current of the
electrolyte decomposition will be negligible
(Galushkin et al., 2015).
It should be noted that if in the cell electrodes, the
maximum possible amount of deuterium is
accumulated, this does not mean necessarily that the
Fleischmann-Pons (type A) effect will occur.
In the papers (Storms,
2007; Galushkin et al.,
2020), it was proved that for the Fleischmann-Pons
(type A) effect occurrence, the other mandatory
condition is the accumulation of a large amount of
deposits on the cathode surface.
The deposits are the highly destroyed crystal
structures. In a metal, any defects of its crystal lattice
are traps for deuterium because they reduce the
The Process of Thermal Runaway Is the Reason of Fleischmann-Pons Effect
41
deuterium atoms energy as compared to these atoms
location in the normal interstice. Consequently,
defects in the crystal structure of the electrodes
facilitate the absorption of deuterium and its
penetration into metals. The points of deposits on the
cathode represent the most severely destroyed crystal
structures. Therefore, the activation energy of
sorption/desorption of deuterium at the points of
deposits is the lowest.
The deuterides decomposition reaction (6) is the
limiting step (Galushkin et al., 2015) for the reactions
of the thermal runaway (3,4). That is why, in the
deposits locations, the intensity of the reactions (3,4)
will be much higher than in locations without
deposits. In its turn, the intensity growth of the
exothermic reactions (3,4) will result in even greater
heat-up of the same spot, which leads to even higher
speed of the deuterides decomposition (6), and so on.
This way, in the deposits spot, a sharp increase will
occur in the intensity of the thermal runaway
reactions (3,4). As a consequence, there will be a
release of the total amount of the atomic deuterium
stored in this spot; it will be observed as a burst with
a high energy release.
The heating-up of the cathode at the burst point
results in heating-up of deuterides in nearby deposits,
which leads to a burst occurrence in these deposits,
too, and so on. This is how the Fleischmann-Pons
(type A) effect develops (Fig.1 and Fig. 3 in
(Galushkin et al., 2020)).
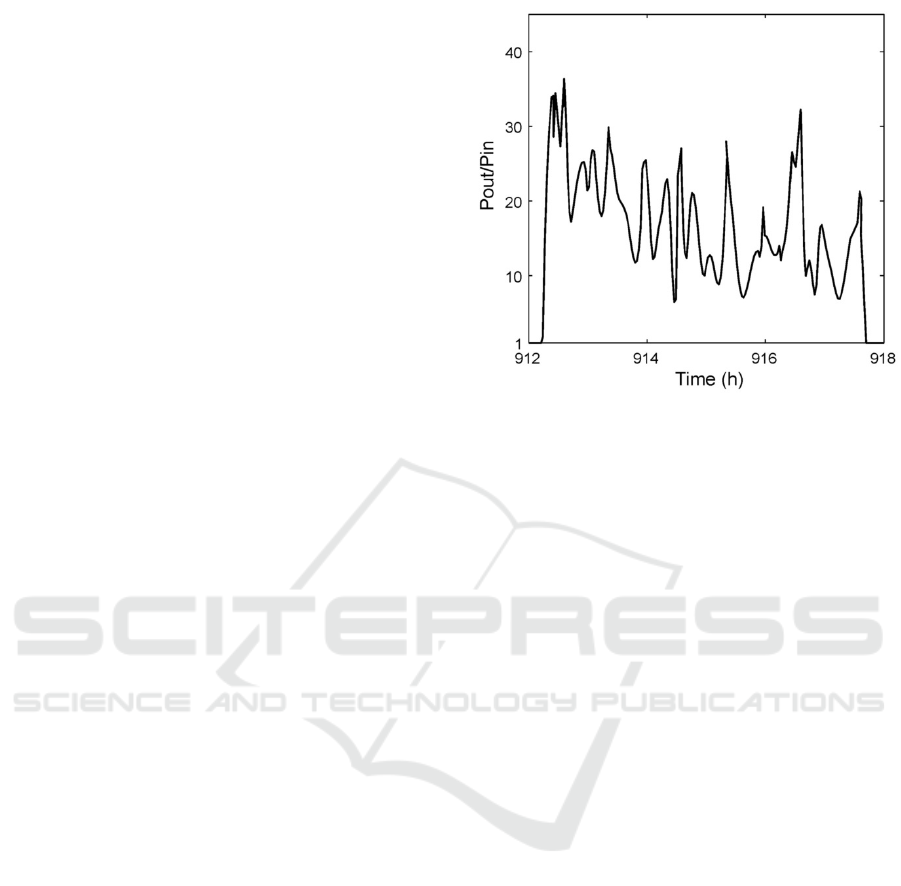
Thus, the Fleischmann-Pons (type А) effect is the
totality of the bursts occurred due to the thermal
runaway processes (3,4) in different spots of the
cathode and at different times. This is how the change
in the excess power generated by the cell looks like
(as bursts) during the Fleischmann-Pons (type А)
effect development Fig.1 (see also Fig. 3 in
(Galushkin et al., 2020), Fig. 9A,B and Fig. 8A in
(Fleischmann et al., 1990)). Many researchers of this
process, including Fleischmann and Pons
(Fleischmann et al., 1989), have noticed that the
Fleischmann-Pons (type A) effect is a series of energy
bursts. Besides, those energy bursts on cathodes were
photographed clearly in the papers (Szpak et al.,
1994; Mosier-Boss et al., 2011; etc.).
Figure 1: Change of the ratio of output power (Pout)
to input power (Pin) of the cell during the F-P (type
A) effect.
In (Galushkin et al., 2020), it was shown that
based on the mechanism of the thermal runaway (3-
6), it is possible to quantify precisely the excess
energy releasing by a cell during the Fleischmann-
Pons (type А) effect. In order to do this, it is necessary
to measure the amount of the deuterium released
during the development of the Fleischmann-Pons
(type А). effect This amount must be multiplied by
the heat release of 442.4 kJ/mol(D
2
) (i.e. by the heat
release taking place at the recombination of the
released atomic deuterium). It was shown (Galushkin
et al., 2020), that this calculated value of the excess
energy coincides with the measured experimentally
(by calorimetric method) excess energy with the
accuracy up to the experimental error.
This is the direct proof of the correctness of the
Fleischmann-Pons (type А) effect mechanism
proposed in the paper (Galushkin et al., 2020). Thus,
for the first time, the thermal runaway mechanism
(the reactions (3-6)) enabled to quantify accurately
the F-P (type A) effect.
It should be noted that any other of the currently
proposed possible mechanisms of the F-P (type A)
effect do not let quantify accurately the excess energy
released. This fact is mentioned in many papers. In
(Fleischmann et al., 1994), Fleischmann and Pons
observed, "however, it remains true to say that the
generation of excess enthalpy is the major signature
and that, so far, there are no quantitative correlations
between the excess enthalpy and the expected (or
unexpected!) "nuclear ashes"."
However, the proposed in the paper (Galushkin et
al., 2020) mechanism (3,4) of the Fleischmann-Pons
ISWEE 2022 - International Symposium on Water, Ecology and Environment
42

(type A) effect solves in full the problems outlined in
the papers (Storms, 2007; Fleischmann et al., 1994).
Firstly, the strict quantitative correlation has been
proved between the products of the reactions (3,4)
(i.e. the released deuterium) and the excess enthalpy.
Secondly, based on this mechanism (3,4) of the
Fleischmann-Pons (type A) effect, the
recommendations were given that enable obtainment
of this effect reliably, whenever it could be needed
(see Section 2.2).
Thus, the Fleischmann-Pons (type A) effect does
not result in any energy production as many authors
believe (Fleischmann et al., 1989; Storms, 2007; etc.).
Since first at the long-lasted electrolysis of electrolyte
(longer that three months), in the cells electrodes, the
energy is stored in the form of the metal-deuterides.
This energy accumulates very slowly due to external
power source. Then the Fleischmann-Pons (type A)
effect occurs and all the energy stored in the
electrodes is quickly released (within a few hours);
upon this, the excess power effect is created.
The mechanism of the Fleischmann-Pons (type A)
effect (Galushkin et al., 2020) is quite similar to that
of the thermal runaway in the alkaline batteries; the
latter is studied in detail in the papers (Galushkin et
al., 2015; Galushkin et al., 2016; etc.).
Summarizing our analysis of the occurrence
mechanism of the Fleischmann-Pons (type А) effect,
we make two remarks.
Firstly, when the electrolysis decomposes the
electrolyte to the deuterium and the oxygen, only the
deuterium is accumulated in the cell electrodes. The
reason for this phenomenon is that the diffusion
permeability of atomic deuterium in nickel is 10
10
times higher than the diffusion permeability of atomic
oxygen at 20°C (Voelkl et al., 1978). That is why
during the electrolyte electrolysis, the oxygen leaves
the cell, while the deuterium partially is accumulated
in the electrodes and partially leaves the cell.
Secondly, by experiments, many researchers of
the F-P (type А) effect (Fleischmann et al., 1994;
Storms, 2007; etc.) proved the existence of the
positive feedback between the temperature increase
and the rate of generation of the excess enthalpy.
However they failed to explain this correlation based
on the “cold fusion” mechanism.
But according to the mechanism of the
Fleischmann-Pons (type A) effect proposed in the
paper (Galushkin et al., 2020) (the reactions (3-6)),
the positive feedback presence is obvious. Indeed, an
increase in the cathode temperature leads to an
increase in the decomposition rate of the deuterides
(the reaction (6)). In its turn, the deuterides
decomposition reaction (6) is the rate-limiting step for
the exothermic reactions of the thermal runaway
(3,4). Hence, in proportion to the rate of the
deuterides decomposition (6), increased will be the
intensity of the exothermic reactions of thermal
runaway (3,4) (i.e. the rate of generation of the excess
enthalpy will be increased). The increase in the
intensity of the exothermic reactions of the thermal
runaway (3,4) will result in an even higher cathode
temperature, and so on.
Thus, the positive feedback between the
temperature increase and the rate of generation of the
excess enthalpy is the basis of the F-P (type А) effect
mechanism based on thermal runaway (Galushkin et
al., 2020).
2.2 Reliable Reproduction of the
Fleischmann-Pons Effect (Type A)
According to the classical theory of deuterides
(Hagelstein, 2015) the deuterium occupies O-sites in
bulk PdD
x
near room temperature, and there is only a
single O-site per Pd atom. This leads to an upper limit
D/Pd near unity for bulk PdD
x
. However, in
(Nishimiya, 2001), it was proved that when palladium
nanoparticles or palladium nanoparticles grown in
zeolite are used, D/Pd = 2.
In our previous paper (Galushkin et al., 2020), it
was experimentally proved that in the electrodes,
where there were no microdefects of the dislocation
type, the value of x=D/Pd couldn’t be more than
unity. But in the electrodes, having a lot of
microdefects such as dislocations the deuterium
accumulation increases about 10 times. However, the
microdefects should be in the form of diverse
dislocations and other very small microdefects in
which deuterium accumulates in the atomic form (in
the form of the deuterides). In order to do this, it is
better to use the metal-ceramic electrodes.
In (Dardik, 2004), when using the Transmission
Electron Microscopy and the Scanning Electron
Microscopy, it was proved experimentally the
following fact. In the electrodes, where the
Fleischmann-Pons (type A) effect was observed, the
microdefects & dislocations density was many times
greater than in the electrodes, where this effect had
never appeared.
As was proved in (Galushkin et al., 2020) (and
Section 2.1), for the occurrence of the Fleischmann-
Pons (type A) effect, it is necessary to accumulate a
large amount of deuterium in the electrodes and
accumulate deposits on the cathode.
Therefore, firstly, according to the studies
described above, in order for the electrodes of the F-
P cell to accumulate a very large amount of
The Process of Thermal Runaway Is the Reason of Fleischmann-Pons Effect
43

deuterium, it is necessary that they contain a very
large number of dislocations (metal-ceramic
electrodes can be used, they are guaranteed to contain
a very large number of dislocations).
Secondly, the density of deposits on the cathode
surface (Galushkin et al., 2020) (and Section 2.1) is
of great importance for the occurrence of the
Fleischmann-Pons effect. Deposits are the activation
centers for exothermic reactions of thermal runaway
(3,4). The occurrence of thermal runaway reactions
(3,4) is the F-P effect. The deposit density can be
increased by adding palladium (or nickel) salts to the
electrolyte, as was done in (Dominguez et al., 2014).
Thirdly, to start thermal runaway reactions (3,4),
it is necessary to heat the active points formed by
deposits located on the cathode (Galushkin et al.,
2020). Under natural conditions, this occurs when a
very large amount of atomic deuterium is
accumulated in highly destroyed deposits on the
cathode. Since both of these factors reduce the bond
between atomic deuterium and the metal, then it leads
to spontaneous desorption of atomic deuterium from
the metal and its recombination. The exothermic
reaction of the recombination of atomic deuterium
heats up the place where the desorption of atomic
hydrogen occurred, which leads to even more intense
desorption of deuterium (6), etc. This is the F-P (type
A) effect.
Heating of active points on the cathode can also
be obtained artificially by passing a powerful current
pulse through the cell, sufficient to decompose the
deuterides (6) stored in the electrodes. The magnitude
of the current pulse depends on the gap between the
electrodes, the density of the deposit, etc. We usually
used, to initiate the Fleischmann-Pons (type A) effect,
a current pulse that provided an electric voltage on
cell terminals of more than 50 V for 0.25–0.5s.
The Fleischmann-Pons (type A) effect can be
obtained reliably if the above recommendations are
used.
2.3 Mechanism of Fleischmann-Pons
Effect (Type B)
In their paper (Fleischmann et al., 1990) (in Appendix
6), Fleischmann and Pons indicate that in order to
obtain the effect of excess power (type B), conditions
should be sought under which, along with the reaction
(2) at the anode, an electrochemical reaction occurs
D
2
+ 2OD
−
→ 2D
2
O + 2e
−
(anode). (8)
To achieve reaction (8), Fleischmann and Pons
advise to reduce the interelectrode gap in the cells
(Fleischmann et al., 1990).
The occurrence of reaction (8) indicates that the
hydrogen released at the cathode enters the anode and
is oxidized on it. Consequently, the oxygen released
at the anode can also get to the cathode and be
reduced on it.
1/2O
2
+ D
2
O+ 2e
−
→2OD
−
(cathode). (9)
For reactions (8.9), the overall reaction is as
follows:
1/2O
2
+D
2
→D
2
O (10)
Already the reaction of Fleischmann and Pons (8)
is an exothermic reaction with heat emission in
amount of 295 kJ/mol(D
2
).
However, when calculating the energy balance of
the Fleischmann-Pons (type B) effect, the heat release
of reaction (8) is not taken into account (Fleischmann
et al., 1990). This is the reason for the appearance of
fictitious or imaginary excess power in the
Fleischmann and Pons calculations.
The F-P (type B) effect was studied in more detail
in our previous paper (Galushkin et al., 2020).
3 CONCLUSIONS
Based on the analysis performed and our previous
experimental studies (Galushkin et al., 2020;
Galushkin et al., 2015; etc), it follows that during the
long-term electrolysis of heavy water, a lot of energy
is accumulated inside the electrodes in the form of
deuterides. The release of atomic hydrogen from
deuterites and its recombination is a powerful
exoteric reaction. The occurrence of this reaction is
the F-P (type A) effect.
The reason for the F-P (type B) effect is that the
cell energy balance in (Fleischmann et al., 1990) did
not account for the exothermic reaction of
Fleischmann and Pons (8).
Undoubtedly, the Fleischmann-Pons effect
requires further both experimental and theoretical
studies.
REFERENCES
Dardik, I., Zilov, T., et al. (2004) Excess heat in electrolysis
experiments at Energetics Technologies. In Eleventh
International Conference on Condensed Matter
Nuclear Science. Marseille.
ISWEE 2022 - International Symposium on Water, Ecology and Environment
44

Dominguez, D. D., Kidwell, D. A., Grabowski, K. S., and
Knies, D .L. (2014). Evidence for excess energy in
fleischmann – pons-type electrochemical experiments.
Journal of Condensed Matter Nuclear Science, 14: 15–
28.
Fleischmann, M., Pons, S., and Hawkins, M. (1989).
Electrochemically induced nuclear fusion of deuterium.
Journal of Electroanalytical Chemistry, 261: 301–308.
Fleischmann, M., Pons, S., Anderson, M. W., Li, L. J., and
Hawkins, M. (1990). Calorimetry of the palladium-
deuterium-heavy water system. Journal of
Electroanalytical Chemistry, 287: 293–348.
Fleischmann, M., Pons, S., Le Roux, M., and Roulette, J.
(1994). Calorimetry of the Pd-D
2
O System: the Search
for Simplicity and Accuracy. In Proceedings: Fourth
International Conference on Cold Fusion, Volume 1.
page 1. EPRI. Palo Alto.
Galushkin, N. E., Yazvinskaya, N. N., and Galushkin, D. N.
(2020). Mechanism of thermal runaway as a cause of
Fleischmann-Pons effect. Journal of Electroanalytical
Chemistry, 870: 114237.
Galushkin, N. E., Yazvinskaya, N. N., and Galushkin, D. N.
(2015). Study of thermal runaway electrochemical
reactions in alkaline batteries. Journal of The
Electrochemical Society, 162: A2044–A2050.
Galushkin, N. E., Yazvinskaya, N. N., and Galushkin, D. N.
(2016). Thermal runaway as a new high-performance
method of desorption of hydrogen from hydrides.
International Journal of Hydrogen Energy, 41: 14813–
14819.
Greenwood, N. N., and Earnshaw, A. (1997). Chemistry of
the elements. Butterworth Heinemann. Oxford. 2
nd
edition.
Hagelstein, P. L. (2015). Empirical Models for Octahedral
and Tetrahedral Occupation in PdH and in PdD at High
Loading. Journal of Condensed Matter Nuclear
Science, 17: 35–66.
Lewis, N., Heben, M. J., et al. (1989). Searches for low-
temperature nuclear fusion of deuterium in palladium.
Nature, 340: 525–530.
Luo, Y.-R. (2007) Comprehensive handbook of chemical
bond. CRC Press. New York.
Mosier-Boss, P. A., Dea, J. Y., Gordon, F. E., Forsley, L.
P. G., and Miles, M. H. (2011). Review of twenty years
of LENR research using Pd/D co-deposition. Journal of
Condensed Matter Nuclear Science, 4: 173–187.
Nishimiya, N., Kishi, T., Mizushima, T., Matsumoto, A.,
and Tsutsumi, K. (2001). Hyperstoichiometric
hydrogen occlusion by palladium nanoparticles
included in NaY zeolite. Journal of Alloys and
Compounds, 319: 312- 321.
Sakamoto, Y., Imoto, M., Takai, K., Yanaru, T., and
Ohshima, K. (1996). Calorimetric enthalpies for
palladium - hydrogen (deuterium) systems at H(D)
contents up to about [H]([D])/[Pd] = 0.86. Journal of
Physics: Condensed Matter, 8: 3229.
Shanahan, K. L. (2010). Comments on “a new look at low-
energy nuclear reaction research ” . Journal of
Environmental Monitoring, 12: 1756–1764.
Storms, E. K. (2007). The Science of Low Energy Nuclear
Reaction. World Scientific. Singapore.
Szpak, S., Mosier-Boss, P. A., and Smith, J. J. (1994).
Deuterium uptake during Pd-D codeposition. Journal of
Electroanalytical Chemistry, 379: 121–127.
Voelkl, J., and Alefeld, G. (Eds.), (1978). Hydrogen Topics
in Applied Physics. and Metals II, page 321. Springer-
Verlag. Berlin.
Williams, D. E., Findlay, D. J. S., et al. (1989). Upper
bounds on ‘cold fusion’ in electrolytic cells. Nature,
342: 375–384.
The Process of Thermal Runaway Is the Reason of Fleischmann-Pons Effect
45
