
The Effect of Adding Shrimp Shell Catalyst on the Quality of
Biodiesel from Used Cooking Oil
Ayu Nindyapuspa, Vivin Setiani and Elsa Rafelia
Hartanti
Department of Waste Treatment Engineering, Politeknik Perkapalan Negeri Surabaya,
Jalan Teknik Kimia Kampus ITS Sukolilo, Surabaya, Indonesia
Keywords: Biodiesel, CaO Catalyst, Microwave, Shrimp Shell Waste Cooking Oil.
Abstract: Shrimp shell contains 40-50% calcium carbonate so that it can be used as a CaO catalyst in the manufacture
of biodiesel using waste cooking oil. High levels of free fatty acid in waste cooking oil can be reduced by
the esterification process using an acid catalyst. Biodiesel is produced by transesterification process using a
heterogeneous catalyst of calcium oxide (CaO) from shrimp shells calcined at 1000°C for 3 hours. The
esterification and transesterification processes are carried out using microwaves. The transesterification
process was carried out with a variable weight percent catalyst (0%, 1%, 3%) and microwave power (150 W,
300 W). The characteristics of the biodiesel produced indicate that the parameters of density, flash point,
and sentane number have met the standard, while the parameters of viscosity and water content have not
met the standard. The yield of biodiesel produced increased along with the increase in the percentage of
CaO catalyst concentration of shrimp shell waste and the microwave power used.
1 INTRODUCTION
Vaname shrimp is the product of pond cultivation in
Indonesia. Based on the Central Statistics Agency in
2019, Java Island produced 144,873 tons of vaname
shrimp. The large production of vaname shrimp has
resulted in the generation of shrimp shell waste.
Shrimp shell contains 45%-50% calcium carbonate,
25%-40% protein, and 15%-20% chitin (Sari et al,
2011). The content of the shrimp shells can be used
as a CaO catalyst in the manufacture of biodiesel.
One of the main ingredients for making biodiesel is
used cooking oil.
Used cooking oil is a waste that is often found in
households because of the large use of cooking oil.
The used cooking oil produced can cause problems
to the environment, especially pollution of water
bodies (Glisic and Orlović, 2014). Used cooking oil
contains fatty acids. Fatty acids are reacted with
alcohol to produce esters which are the main
compounds for making biodiesel (Darmawan and
Susila, 2013). Therefore, this study was conducted
to analyze the quality of biodiesel from used
cooking oil using shrimp shell CaO catalyst.
2 METHODS
In this research there were several variables written
in Table 1.
Table 1.
CaO Catalyst
Of Shrimp
shells (%)
Microwave Power
in the
Transesterification
Process (Watts)
150 300
1P1 P2
2P3 P4
3P5 P6
2.1 Production of Leather CaO
Catalyst
Shrimp shells were cleaned using clean water and
heated at 120°C for 1 hour then placed in a
desiccator for 10 minutes (Yasar, 2019). Shrimp
shells were mashed and sieved through a 100 mesh
sieve (Petrus et al., 2015). Shrimp shell powder was
calcined at 1000°C for 3 hours (Khodijah, 2017).
Shrimp shell powder was tested by XRF to
determine the CaO content.
532
Nindyapuspa, A., Setiani, V. and Har tanti, E.
The Effect of Adding Shrimp Shell Catalyst on the Quality of Biodiesel from Used Cooking Oil.
DOI: 10.5220/0011818600003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 532-536
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

2.2 Sample Preparation Process
Waste cooking oil was filtered with filter paper to
remove impurities. Then the sample was heated to
remove the water content at a temperature of 105°C
for 1 hour (Sartika et al., 2015).
2.3 Esterification Process
Waste cooking oil was mixed with methanol (mole
oil-methanol ratio 1:5) and 0.5% (w/w) H
2
SO
4
catalyst (Murni et al., 2016) then irradiated by
microwave (Figure 1.) in a microwave for 20
minutes with a power of 450 W and stirred with a
magnetic stirrer. (Ansori and Wibowo, 2018).
Furthermore, the esterification results are left in a
separatory funnel which aims to separate the
methanol, oil, and H2SO4 in the top layer and the
bottom layer was a mixture of oil and methyl ester
(crude biodiesel). Then the crude biodiesel was
washed with warm distilled water (60°C) with a
ratio of product weight and distilled water used 1:1
(Pahlevi et al., 2015) and the top layer in the form of
crude biodiesel was separated from the bottom layer
in the form of washing water containing catalyst and
residues. residual methanol using a separating
funnel. (Pahlevi et al., 2015). Furthermore, the
sample was heated in an oven (110°C) to reduce the
water content in crude biodiesel (Suryanto et al.,
2018). Finally, the samples were measured for
density and % FFA.
Figure 1: Microwave for Esterification.
2.4 Transesterification Process
Crude biodiesel was mixed with a mixture of shrimp
shell CaO catalyst according to the specified
variable with methanol with an oil ratio of 1:9 into a
one neck flask (Ansori and Wibowo, 2018). Then,
the sample was irradiated by microwaves using a
microwave with microwave power according to the
specified variable while stirring with a magnetic
stirrer for 10 minutes. After that, the sample was put
into a separatory funnel and allowed to stand at
room temperature to form two layers (biodiesel and
glycerol) (Sartika et al., 2015). Crude biodiesel was
washed with warm water (60°C) with a ratio of
product weight and distilled water used 1:1 (Pahlevi
et al., 2015) and separation of two layers (crude
biodiesel and biodiesel (FAME) (Pahlevi et al.,
2015). The sample was heated in an oven (110°C) to
reduce the water content in crude biodiesel
(Suryanto et al., 2018) and lastly, the calculation of
biodiesel yield on all variables of this study.
2.5 Analysis of Biodiesel
Characteristics and CaO Catalysts
The characteristics of biodiesel tested in this study
were samples that had the highest yield (3% catalyst
at 300 Watt microwave power). The characteristics
of biodiesel consist of density, viscosity, Free Fatty
Acid (FFA), biodiesel yield, water content, Flash
point, sentane number, X-Ray Fluorence (XRF) and
Gas Chromatography Mass Spectroscopy (GC-MS).
Density testing in this study used a pycnometer.
According to Dewi (2015) and Cahyati and
Pujaningtyas (2017), the working principle of this
test was that a clean and dry pycnometer was
weighed to determine the mass of an empty
pycnometer. First, Crude biodiesel to be tested was
heated at a temperature of 40°C and then put into the
pycnometer until it was full. Close the pycnometer
and make sure there are no bubbles. The sample was
cleaned and the pycnometer containing the sample is
weighed. Then, record the mass of the pycnometer
and the sample.
(1)
Viscosity testing in this study using the Ostwald
viscometer. According to Sinta et al. (2016), the
working principle of this test was the Ostwald
viscometer in a container filled with water at a
temperature of 40°C (artificial water bath) in a
vertical position. A certain amount of sample was
pipetted into reservoir A. The liquid was brought to
reservoir B and its surface crosses the line m, so
reservoir A was still half filled. The viscometer and
its contents were left in a container of water for 10
minutes to reach the desired temperature. Liquid B
was sucked or blown to slightly above the m line.
The liquid was allowed to flow freely. Record the
time it takes for the liquid to flow from M to N, this
step was repeated several times.
The Effect of Adding Shrimp Shell Catalyst on the Quality of Biodiesel from Used Cooking Oil
533
Kinematic viscosit
y
(
μ
k) = C×
t
(2)
Where:
C= Ostwald viscosity constant (0.4994 cSt/second)
t = time required for sample from point A to point B
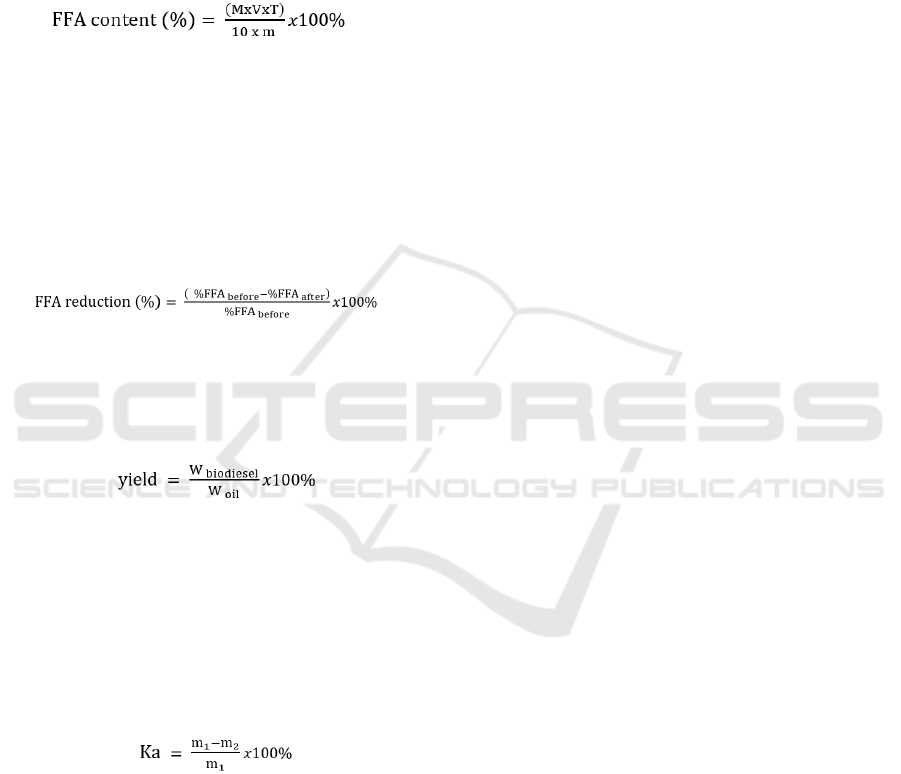
FFA testing was carried out on waste cooking oil
before and after the esterification process.
Determination of FFA levels refers to SNI 01-3555-
1998 concerning Oil and Fat Test Methods.
(3)
Where:
M = molecular weight of fatty acids (grams)
V = volume of NaOH required for titration (ml)
T = normality of NaOH m = weight of sample
(grams)
The calculation of the conversion of reducing
FFA levels in waste cooking oil was carried out to
determine the decrease in FFA levels using the
esterification process.
(4)
1. Biodiesel yield was the percentage of
conversion of oil into biodiesel (Efendi et al., 2018).
The amount of yield produced according to Zuhra et
al. (2015) can be calculated using the following
formula:
(5)
Where:
W
𝑏𝑖𝑜𝑑𝑖𝑒𝑠𝑒𝑙
= weight of methyl ester (biodiesel) from
washing and separation
W
oil
= weight of waste oil and fat used in the reactor
2. Moisture content in waste cooking oil refers
to SNI 01-3555-1998 on Oil and Fat Test Method
using the gravimetric method. Samples that already
have a fixed weight can be calculated for their water
content by the formula:
(6)
Where:
Ka = moisture content (%)
m
1
= sample weight (grams)
m
2
= sample weight after drying (grams)
Flash point testing was used to determine the
indication of the boiling distance. This test was
carried out with reference to SNI 7182:2015
regarding Biodiesel using the ASTM D 93 method.
The tool used for the flash point test was the Pensky-
Martens closed cup tester.
The cetane number test was used to determine
the ability of the fuel to ignite quickly after being
injected. This test was carried out with reference to
SNI 7182:2015 regarding Biodiesel.
XRF test aims to determine the composition of
the elements in the calcined shrimp shell powder.
The most important component in calcined shrimp
shell powder was CaO.
Gas Chromatoraphy Mass Spectroscopy (GC-
MS) Test
Analysis of fatty acid composition in (Waste
Cooking Oil) used GC-MS method.
3 RESULTS AND DISCUSSION
3.1 Density
Testing the density of biodiesel was carried out at a
temperature of 40°C using a pycnometer. The
biodiesel quality requirements for the density
parameter regulated in ISN 7182:2015 are 850 – 890
kg/m
3
. Biodiesel with the highest yield has a density
value of 858.69 kg/m
3
. The density value in this
study has met the standards that have been set. The
smaller density value indicates that there has been a
breakdown of glycerol from triglycerides, so that a
compound with a smaller molecular size is formed
(Petrus et al., 2015).
3.2 Viscosity
Viscosity testing using an Ostwald viscometer at a
temperature of 40°C. Viscosity in biodiesel based on
ISN 7182:2015 concerning biodiesel has a value of
2.3-6 cSt. The result of viscosity testing on biodiesel
waste cooking oil which has the highest % yield is
14.77 cSt. This value is not included in the standard
range that has been set. Triglycerides have a higher
viscosity than methyl esters, this is what causes high
biodiesel viscosity if the transesterification reaction
is not perfect (Zulhardi et al., 2018). Several
approaches have been proposed to reduce the
viscosity of biodiesel so as to improve the flow
properties of biodiesel at low temperatures which
include mixing with diesel fuel, the use of additives,
physical modification, fractionation crystallization,
and winterization (Sukarno, 2012).
3.3 Flash Point
The flash point test refers to ISN 7182:2015
regarding Biodiesel using the ASTM D 93 method.
The tool used for the flash point test is the Pensky-
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
534

Martens closed cup tester. The minimum
requirement for flash point parameters for biodiesel
is 100°C. Flash point test results show 205.83°C.
The test results indicate that the flash point
parameter has met the requirements. According to
Permana et al, (2020), the higher the flash point of
biodiesel, the safer it will be to use because it will
minimize the occurrence of explosions due to
heating.
3.4 Water Content
Testing the water content using the oven drying
method. The water content value that has been
determined in ISN 7182:2015 regarding biodiesel is
a maximum of 0.05%-volume. Based on the research
that has been done, the results obtained water
content of 0.58%. The water content contained in
biodiesel is above the maximum level. The biodiesel
heating process is carried out to evaporate the water
content after the washing process using water in
biodiesel. The results of the water content that
exceeds the standard cannot be used as fuel because
the water content in the methyl ester can form
paraffin crystals at cold temperatures which can clog
the fuel flow. The water content can also cause
corrosion of the engine (Busyairi., et al 2020). One
approach to reduce water content is to use the dry
washing method in the purification process. The dry
washing method uses magnesium silicate as a
substitute for the role of water in absorbing
contaminants in biodiesel (Ayu & Zibbeni, 2012).
3.5 Centane Number
Centane number is one of the test parameters to
determine the quality of diesel fuel combustion. The
minimum requirement for the cetane number for
biodiesel based on ISN 7182: 2015 is 51. The
biodiesel cetane number in this study based on the
test results is 0 because the cetane number value is
outside the test range (20-100). This value is a sign
that biodiesel has a cetane number that is outside the
reading range of the analysis tool, which is between
20 - 100. The raw materials used in this study
contained 52.51% saturated fatty acids and 47.49%
unsaturated fatty acids. According to Damayanti., et
al (2020), a higher content of saturated fatty acids
than unsaturated fatty acids will cause the biodiesel
cetane number in this study to be high (> 100).
Based on these data and the results of previous
studies, it can be used as a reference that the
biodiesel centane number value in this study is
above 100. The resulting centane number value
exceeds the minimum cetane number value in the
specified SNI standard. So that the value of the
centane number in this study has met the standard.
3.6 Fatty Acids Methyl Ester Level
Fatty Acids Methyl Ester analysis was carried out
using Gas Chromatography-Mass Spectrometry
(GC-MS). Based on the results of GC-MS analysis,
the dominant FAME components are Octanoic acid,
methyl ester and Decanoic acid, methyl ester with
area percentages of 46.4% and 43.07%, respectively.
The compounds contained in the GC-MS test results
indicate that the triglycerides contained in the waste
cooking oil have been converted into methyl esters.
According to ISN 7182-2015 regarding biodiesel, it
is stated that the minimum methyl ester content is
96.5%. In this study, the methyl ester content
produced was 95.31%. This value does not meet the
standard so it can be seen that there are still some
triglyceride components that have not been
converted to FAME (Oktavian., et al 2019).
4 CONCLUSIONS
Characteristics of biodiesel produced from used
cooking oil (waste cooking oil) indicates that the
parameters of density, flash point, and the acid
number has met the standard, while the viscosity and
parameters water content does not meet the
standard..
REFERENCES
Ansori, A., & Wibowo, S. A. (2018), Biodiesel Production
from Nyamplung Oil (Calophyllum inophyllum L.)
Using Microwave with Heterogeneous Base Catalyst
(Doctoral dissertation, Sepuluh Nopember Institute of
Technology).
Ayu, D., & Zibbeni, A. (2012), Pengaruh stir washing,
bubble washing, dan dry washing terhadap kadar metil
ester dalam biodiesel dari biji nyamplung
(Calophyllum inophyllum).
Busyairi, M., Za'im Muttaqin, A., Meicahyanti, I., &
Saryadi, S. (2020), "The Potential of Used Cooking
Oil as Biodiesel and the Effect of Catalysts and
Reaction Time on Biodiesel Quality Through the
Transesterification Process". Serambi Engineering
Journal, 5(2).
Cahyati, E. D. and L. Pujaningtyas. (2017), “Producing
Biodiesel from Used Cooking Oil by
Transesterification Process Using KOH Catalyst”.
Thesis. Ten November Institute of Technology.
The Effect of Adding Shrimp Shell Catalyst on the Quality of Biodiesel from Used Cooking Oil
535

Damayanti, R. N., Setiawan, A., & Mayangsari, N. E.
(2020), "Production of Biodiesel from Waste Oil and
Fat in the Dairy Processing Industry Using
Esterification-Transesterification Reaction". In
Conference Proceedings on Waste Treatment
Technology (Vol. 3, No. 1, pp. 227-231).
Darmawan, Ferry Indra dan I wayan Susila. 2013.
“Biodiesel Production Process from Used Cooking Oil
with Dry-Wash System Washing Method”. Jurusan
Teknik Mesin UNESA.
Dewi, D. C. (2015), "Production of Biodiesel from Castor
Oil (Ricinus communis) with Microwave". Thesis.
Semarang State University. Semarang.
Efendi, R., H. A. N. Faiz, and E. R. Firdaus. (2018),
“Producing Cooking Oil Biodiesel Using the
Esterification – Transesterification Method Based on
the Amount of Used Cooking Oil”. 9 th Industrial
Research Workshop and National Seminar (IRONS):
402 – 409.
Glisic, S.B., Orlović, A.M., 2014. “Review of biodiesel
synthesis from waste oil under elevated pressure and
temperature: Phase equilibrium, reaction kinetics,
process design and techno-economic study”. Renew.
Sustain. Energy Rev. 31, 708–725.
Indonesian National Standard. (1998), Oil and Fat Test
Method. Indonesian National Standard 01-3555.
Jakarta.
Indonesian National Standard. (2015), Biodiesel.
Indonesian National Standard 7182. Jakarta.
Khodijah, S. (2017), Making Crude Palm Oil-Based
Biodiesel with Cao Catalyst From Shrimp Shells
Based on Methanol Addition Ratio and
Transesterification Time (Doctoral dissertation,
Sriwijaya State Polytechnic).
Murni, S. W., G. Kusumawardani, and T. Arifin. (2016),
“Producing Biodiesel from Sunan Candlenut Oil with
a Two-Stage Process”. Proceedings of the “Struggle”
National Seminar on Chemical Engineering J11: 1 – 5.
Oktavian, R., Poerwadi, B., Supriyono, S., Septiadi, H. T.,
& Yuniardi, M. I. (2019), Performance Study of
Silica-Based Hydrophobic Membrane in Biodiesel
Purification Process. Journal of Natural Materials
Engineering and Sustainable Energy, 3(1), 20-24.
Pahlevi, M. R., Nurhayati, and S. Anita. (2015),
"Variation of Catalyst Weight and Reaction
Temperature of Crude Palm Oil Transesterification
Using 800°C Calcined Blood Shells Catalyst". JOM
FMIPA 2(1): 186 – 191.
Permana, E., Naswir, M., Sinaga, M. E. T., Alfairuz, A., &
Murti, S. S. (2020), "Quality of Biodiesel from
Cooking Oil Based on Saponification Process and
Without Saponification". JTT (Journal of Applied
Technology), 6(1), 26-31.
Petrus, B., A. P. Sembiring, and M. S. Sinaga. (2015),
“Utilization of Blood Shell Ash (Anadara Granosa) as
a Catalyst in Making Methyl Esters from Waste
Cooking Oil”. USU Journal of Chemical Engineering
4(2): 13 – 19.
Sari T.I., Said M., Summa A.W., Sari A.K. 2011.
“Heterogeneous Base Catalyst Mixed CaO and SrO in
Palm Oil Transesterification Reaction”. Proceeding of
3
rd
AVOER National Conference Seminar Nasional
AVOER, p. 482-493.
Sartika, A., Nurhayati, and Muhdarina. (2015),
“Esterification of Used Cooking Oil with H2SO4
Catalyst and Transesterification with CaO Catalyst
from Blood Clam Shells: Variations in Esterification
Conditions”. JOM FMIPA 2(1): 178 – 185.
Sinta, T., Daniel, and C. Saleh. (2016), “Optimization of
Transesterification Reaction Temperature in Rice
Straw Oil (Oryza sativa L.) into Biodiesel using CaO
Catalyst from Chicken Eggshell”. Journal of
Chemistry Mulawarman 14(1): 19 – 23.
Sukarno, S. (2012), Study of the effect of mixing additives
on biodiesel viscosity at low temperatures (Doctoral
dissertation, Undip Postgraduate Program).
Suryanto, A. (2018), "Study of Biodiesel Production
Process from Castor Oil With the Help of Sound
Waves". Journal of Chemical Process Engineering,
51(54), 2303-3401.
Yasar, F. (2019), Biodiesel Production Via Waste
Eggshell as A Low-Cost Heterogeneous Catalyst: Its
Effect On Some Critical Fuel Properties and
Comparison with CaO. Fuel 225: 1 – 6.
Zulhardi, R., Restuhadi, F., & Zalfiatri, Y. (2018),
"Addition of Methanol in Making Biodiesel from
Waste Cooking Oil with Rubbing Ash Catalyst". vols,
5, 1-10.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
536
