
Effect of Pressure on Mechanical Properties and Structural Evolution
of Diffusion-Bonded Al-Ni: Insight from Molecular Dynamics
Simulation
Mohamad Zaenudin
1
, Mohammed N. Abdulrazaq
2
, Salah Al-Zubaidi
3
and Adhes Gamayel
1
1
Jakarta Global University, Indonesia
2
Gulf University, Bahrain
3
University of Baghdad, Iraq
Keywords: Al-Ni Alloy, Diffusion Bonding, Mechanical Properties, Structural Evolution.
Abstract: Molecular dynamics simulation is a method to investigate the behavior of material at atomic scale. The
application of molecular dynamics in investigation of the phenomena in joining processes at atomic scale has
significantly increased. One of the advanced joining methods that nowadays used in joining dissimilar
materials is diffusion bonding. This paper discusses the effect of pressure on the mechanical properties and
structural evolution of diffusion-bonded Al-Ni. The results showed that both concentration distribution
profiles and ultimate tensile strength showed excellent joints with low applied pressure (10 MPa), while higher
welding pressure does not come with more benefits but decreasing the material performance. Defects and
deformation that occurs during diffusion welding process has affected to the lower ultimate tensile strength
which caused by the remarkably high applied welding pressure (100 MPa and 150 MPa) while at low applied
pressure (10 MPa) defects and deformation is relatively low and thus affect to an excellent result in ultimate
tensile strength value.
1 INTRODUCTION
The joining of metallic materials in industrial
processes is particularly important due to the need to
create desired shapes which involve two dissimilar
materials. However, it has several difficulties for the
dissimilar materials mean different properties, which
lead to the other different applied parameters in
joining processes, like temperature, pressure, and
holding time. Diffusion bonding is introduced to
solve this kind of problem when the two dissimilar
joining of materials is necessary (Akca & Gu Gürsel,
2015, Cooper & Allwood, 2014). To achieve
optimum conditions using this method, several
experimental investigations are necessary. It is well
known that experiments on advanced material science
take huge amount of cost and time, thus a few
alternatives of numerical methods are introduced.
These methods have shown promising advantages to
enhance the needs of joining dissimilar materials with
optimum condition.
Apart from the other numerical methods such as
finite element method (FEM), continuum modeling,
and Monte Carlo simulation, another numerical
method like Molecular Dynamics (MD) simulation
has shown an increasing interest by researchers
extensively, especially in joining processes
(Zaenudin et al., 2018, Zaenudin et al., 2022,
Zaenudin et al., 2020). The core idea of this method
is the solution of the Newtonian equation of motion
numerically, which runs under a particular ensemble
of atoms like microcanonical and canonical
ensemble. These equations are then numerically
integrated for a tiny range of time (about 2−3),
and through the observation of elapsed time the
equilibrium of the statistical averages is computed as
interim averages. This method offers a new way to
observe and analyze the behavior of material at
atomic scales, which nowadays become more
important due to the requirement of precision and
accuracy of the estimation properties and applied
parameters for application like joining processes.
Specific on joining process using diffusion bonding
technique, MD simulations was able to deliver
several phenomena and mechanisms that have
significance influence to the final result of the as
Zaenudin, M., Abdulrazaq, M., Al-Zubaidi, S. and Gamayel, A.
Effect of Pressure on Mechanical Properties and Structural Evolution of Diffusion-Bonded Al-Ni: Insight from Molecular Dynamics Simulation.
DOI: 10.5220/0011770300003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 337-341
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
337
welded materials, whether it is based on the structural
evolution during joining processes, diffusion
mechanism based on the self-diffusion of material, or
even the phenomena that contribute to the mechanical
properties and structural evolution of the as received
material compared to the as received material. Chen
et al. has conducted research on diffusion bonding of
Cu-Ag (Chen et al., 2005) and Cu-Al (Chen, 2007)
and has successfully investigated the dominant
mechanism during diffusion bonding. Hu et al. (2013)
have demonstrated the temperature-dependence of
the mechanical properties of diffusion-bonded Ni-Al.
The significance effect of temperature also has been
demonstrated by Zhang and Jiang (Zhang and Jiang,
2013) on the stainless-steel and pure Ni materials by
simply extracting the displacement of atoms into
calculation of mean square displacement (MSD).
Based on the broad range application of Ni-Al
materials like turbine blades (Darolia et al., 2012),
batteries (Mukherjee, 1998, Young, 2013, Young,
2013), multilayers for joining of different Ti-alloys
(
Simões et al., 2010, Simões et al., 2018), coating
with a tungsten layer (Ramos et al., 2017), and mask
absorber of extreme ultraviolet (EUV) radiation [17],
this paper presents an investigation on the effect of
applied pressure on the structural evolution and
mechanical properties of diffusion bonding between
Al and Ni. The structural analysis is obtained by
employing a vector displacement analysis tool
(Stukowski, 2010) based on its slip vector properties
(Zimmerman et al., 2001) and the other properties,
such as mechanical and physical properties, are
presented in curves and tables.
2 SIMULATION PROCEDURE
This study has been performed by using LAMMPS
software package (Plimpton, 1994). Every movement
and energy of atoms in the molecular dynamics
simulation system is defined by an interatomic
potential model, thus the importance of interatomic
potential is crucial. Embedded atom method is one of
the well-established interatomic potentials. In EAM,
the total energy of a binary system A-B is defined as:
1
2
Ф
(1)
Here Ф
is the interaction of pair potential as
a function of distance
between atoms and that
have chemical sorts
and
and
is the embedding energy of an atom of chemical
sort
as a function of the host electron density
induced at site by all other atoms in the system. The
well-established EAM developed by Mishin (Mishin,
2002) is adopted in this simulation.
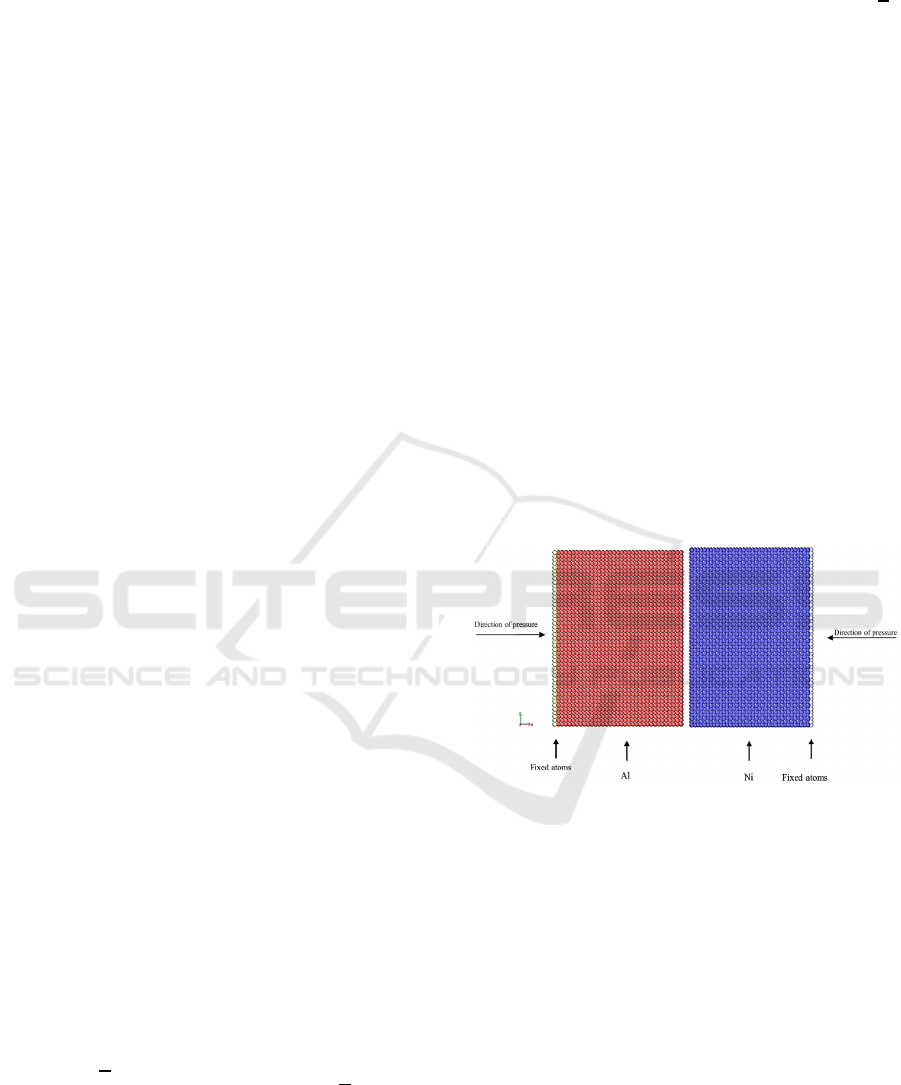
In this simulation, two slabs of Al and Ni is
deployed in this simulation with approximate
dimension of 7.29.29.2 for both Al
and Ni slabs and followed by fixed atoms in the edge
of each slab as shown in Figure 1. Lattice constant of
both monocrystalline Al and Ni slabs are 4.05 and
3.52, respectively, as reported by refs. (Mishin et al.,
2022, Mishin et al., 2004). Boundary condition is set
to periodic in all three directions , , and . This
configuration allows atoms pass through the sides of
the cell and entering on the opposite side with the
exactly with the same condition and properties such
as velocity and internal energy, and the system can
also maintain the number of atoms. In addition, each
atom in the simulation cell interacts with the closest
image of the remaining atoms. This would then avoid
the boundary effects on the simulation. Isothermal-
isobaric ensemble with Number, Pressure, and
Temperature (NPT) controlled is employed in this
simulation.
Figure 1: Planar view of simulation model of Al (red) and
Ni (blue) slabs paired with fixed atoms in each edge of the
slab.
The initialization process is started with low
temperature and pressure and maintained for 10
picoseconds. This initialization process is a key to the
achieve an equilibrated system before the production
process. Equilibrium state is indicated by the minimal
amount of potential and kinetic energy. For
production process, the temperature is designed at
500, while the pressure is set to three different
levels, they are 10, 50, 100, and
150 in direction for 200 . Finally, the
structure is cooled down from temperature of 500
to an ambient temperature to perform a tensile test
with a strain rate of 2.649/. The timestep of all
simulation process is set to 1.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
338
3 RESULT AND DISCUSSION
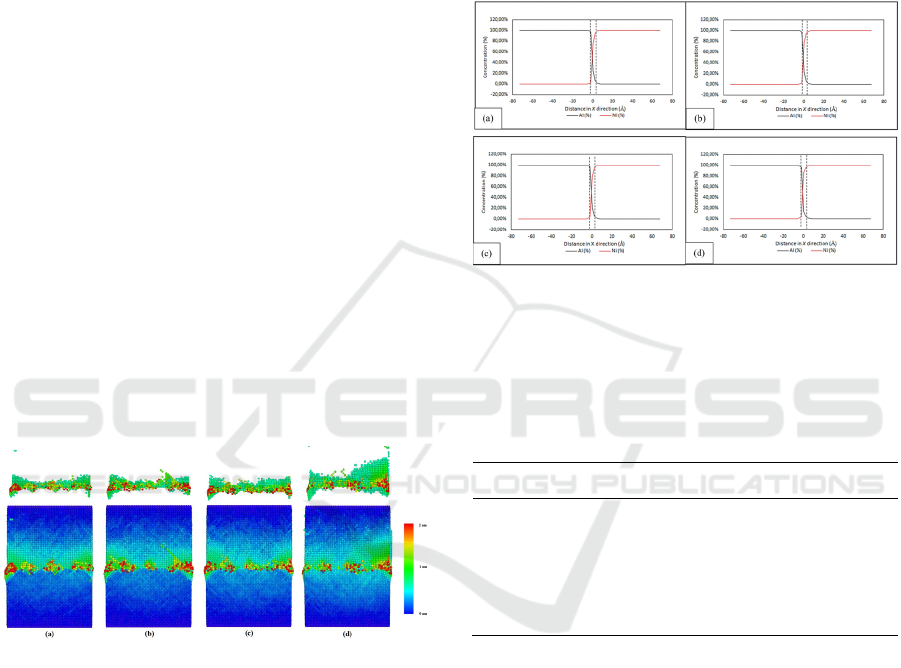
Figure 2 shows the structural evolution of diffusion-
bonded Al-Ni of four different conditions namely S1,
S2, S3, and S4. On the planar view (bottom) of the as
welded Al-Ni, it can be observed that there is no
major difference between all four different
conditions. However, at the respective diffusion
interface (above) it can be observed that the applied
pressure is shown to deform the Al slab (top) as the
pressure is increased. Even though higher pressure
influences the diffusion interface by deforming it, the
atomic exchange which is mostly shown by red-
colored atoms has no significant difference. It can be
concluded that the applied pressure (50 MPa to 150
MPa, S2-S4), instead of promoting into a better
bonding structure, mostly only deformed the Al slab
due to the lower strength of Al compared to Ni. In
addition, most of both Al-Ni slabs maintain their FCC
structure which is shown by the blue-colored atoms.
It indicates the applied pressure and temperature for
200 ps has an excellent bonding structure, except for
the highest applied pressure which can be observed
had significant defects and deformations located on
the interface. At this step, it is suggested to apply low
pressure if the temperature is high enough to allow
diffusion between two dissimilar materials to be
joined.
Figure 2: Planar view of displacement vector analysis of
diffusion-bonded between Al and Ni at temperature of
500 and hold for 200 with pressure at (a) 10,
(b) 50 , (c) 100 , and (d) 150 (upper
image) and its respective diffusion interface (lower image).
The concentration distribution curve is depicted in
Figure 3 and Table 1 shows its corresponding
interfacial region thickness. Interfacial region is
defined as the area within the diffusion zone in which
the concentration of a material is more than 5%. Table
1 is obtained from the concentration distribution data
with proper estimation. Even though the highest value
applied pressure shown has the widest interfacial
region area as listed in Table 1, the difference
between the other values of interfacial region
thickness is small. For the lowest value of applied
pressure (i.e., S1 with 10 MPa) to the highest value of
applied pressure (i.e., S4 with 150 MPa), the
difference in interfacial region thickness is only about
0.1 Angstrom. While S1 also has the highest value
among the other two conditions namely S2 and S3 (50
and 100 MPa). The applied pressure at this level of
temperature (500 K) has no significant effect on the
result of diffusion bonded Al-Ni, thus the lowest
applied pressure and vast amount of heat is suggested.
Figure 3: Curve of concentration distribution of four
different welding conditions at temperatures of 500 with
holding time of 200 and variation on pressure (a)
10, (b) 50, (c) 100, and (d) 150
referred as sample 1, 2, 3, and 4, respectively.
Table 1: Approximated interfacial region thickness of four
different conditions namely S1, S2, S3, and S4.
Condition (s) Interfacial region thickness (Å)
S1 4,485838854
S2 4,383252102
S3 4,474520861
S4 4,585924319
After the final diffusion-bonded result is acquired,
the system is then subjected to a tensile test. The
structural evolution of diffusion-bonded Al-Ni
depicted in Figure 4. The image is captured when the
as-welded Al-Ni subjected to tensile test at the time
of 25 ps, 50 ps, 75 ps, and 100 ps. At time of 25 ps,
condition S4 showed the worst behavior owing to the
huge number of defects and deformations during the
welding process and thus influencing the sample
during tensile test. While the other sample namely S1-
S3 still maintain their structure, the sample S4
deformed at the interface. This behavior proves that
during diffusion welding, the applied welding
pressure of 150 MPa is too high and is not suggested.
As the tensile test goes on to time of 50 ps to 100 ps,
sample with the lowest applied welding pressure
Effect of Pressure on Mechanical Properties and Structural Evolution of Diffusion-Bonded Al-Ni: Insight from Molecular Dynamics
Simulation
339
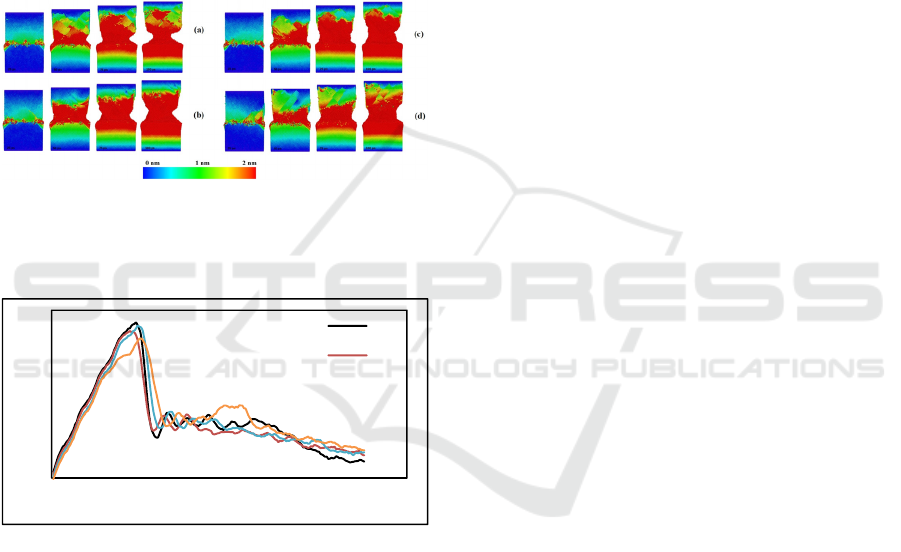
which is 10 MPa (S1) has the best behavior, as shown
it mostly maintain the structure and the deformation
during tensile test shown relatively small compared to
the other sample. For the tensile test result, the stress-
strain curve is presented in Figure 5. The ultimate
tensile strength is obtained from the lowest applied
pressure. The route with the lowest applied pressure
has the best results and behaviors, while the highest
one shows the worst. However, an inverted behavior
is obtained for pressures of 50 MPa and 100 MPa
which indicate the sensitivity of the system as the
pressure is increased. The behavior of the system
becomes harder to estimate as the pressure is
increased.
Figure 4: Planar view of displacement vector analysis of
structural evolution of diffusion-bonded Al-Ni with four
different welding conditions (a) S1, (b) S2, (c) S3, and (d)
S4 subjected to tensile test.
Figure 5: Stress-strain curve of as-welded Al-Ni with four
different welding conditions (S1-S4) subjected to tensile
test.
During diffusion welding, there are at least three
parameters that contribute to the result of the as
welded materials, they are temperature, holding time,
and pressure. The effect of pressure in this study
indicates significant to the performance of the as
welded material. For example, when the applied
welding pressure is too high, even before being
subjected to tensile test defects and deformations has
occurred and it has affected the lower ultimate tensile
strength during tensile test. Thus, diffusion welding
with relatively high pressure is not suggested.
Another aspect to be considered when the applied
welding pressure too high is the ability of the welded
materials to recover their structure is not utilized,
which means if defects are occur during diffusion
bonding, the structure will keep the defects or even
make it bigger and thus affecting the ultimate tensile
strength of the as welded material. Instead of applying
high welding pressure which has no significant
difference in term of benefits, applying low welding
pressure, such as 10 MPa, is suggested because it has
indicates resulting the highest ultimate tensile
strength and excellent bonding structure while
interfacial region thickness shown indication the
ability to maintain its bonding that can avoid any
dissolving phenomena. This low-pressure diffusion-
bonding could be used for nano-scale welding (Lu et
al., 2010) to macroscale. Even though the applied
welding pressure is low, the other parameters like
temperature and pressure still need to be estimated
properly. Here, at a temperature of 500 K, good joints
are achieved with a relatively wide enough interfacial
region and relatively high ultimate tensile strength.
As reported in refs. (Chen et al., 2007, Hu et al.,
2013), the influence of temperature will decrease the
ultimate strength, thus the applied heating
temperature has to be considered properly. If the
temperature is high enough to promote diffusion
between the two dissimilar materials, thus avoiding
making the temperature higher is reasonable.
4 CONCLUSIONS
This study has successfully examined the effect of
pressure on diffusion bonding between Al and Ni.
The conclusions are drawn as listed below:
1. From the concentration distribution profiles, the
welding at low pressure with high enough holding
time (200 ps) and temperature (500 K) shows
good joints and it is enough to avoid any
dissolving phenomena indicated by the high value
of thickness of the interfacial region. Instead of
performing at high pressure that shows no
significant impact, the welding of Al-Ni is better
selecting high enough temperature and long
enough holding time.
2. If the pressure is high enough to perform diffusion
bonding of Al-Ni and has shown satisfactory
results on concentration profile, it is a good
decision to consider the lowest value of pressure
to avoid deformation/defect and efficiency of
resources. Another reason to consider the lowest
possible applied pressure is that with the lowest
value, as discussed in this section, the highest
value of ultimate tensile strength is achieved.
0
0,5
1
1,5
2
2,5
3
3,5
4
4,5
0 0,05 0,1 0,15 0,2 0,25 0,3
Stress (GPa)
Strain (%)
10
Mpa
50
Mpa
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
340

ACKNOWLEDGEMENTS
The authors would like to acknowledge the support
from Jakarta Global University and Management &
Science University throughout this project.
REFERENCES
Akca, E., & Gürsel, A. (2015). The importance of
interlayers in diffusion welding-A review. Periodicals
of Engineering and Natural Sciences (PEN), 3(2).
Liu, Z., Nouraei, H., Papini, M., & Spelt, J. K. (2014).
Abrasive enhanced electrochemical slurry jet micro-
machining: Comparative experiments and synergistic
effects. Journal of Materials Processing Technology,
214(9), 1886-1894.
Zaenudin, M., Mohammed, M. N., & Al-Zubaidi, S. (2018).
Molecular dynamics simulation of welding and joining
processes: an overview. Int. J. Eng. Technol, 7(4),
3816-3825.
Zaenudin, M., Abdulrazaq, M. N., & Al-Zubaidi, S. S.
(2022). A Review on Molecular Dynamics Simulation
of Joining Carbon-Nanotubes and Nanowires: Joining
and Properties. International Journal of Integrated
Engineering, 14(4), 137-159.
Zaenudin, M., Abdulrazaq, M. N., Al-Zubaidi, S., &
Sunardi, A. (2020). Atomistic Investigation on the Role
of Temperature and Pressure in Diffusion Welding of
Al-Ni. Journal of Engineering & Technological
Sciences, 52(2).
Chen, S. D., Soh, A. K., & Ke, F. J. (2005). Molecular
dynamics modeling of diffusion bonding. Scripta
Materialia, 52(11), 1135-1140.
Chen, S., Ke, F., Zhou, M., & Bai, Y. (2007). Atomistic
investigation of the effects of temperature and surface
roughness on diffusion bonding between Cu and Al.
Acta Materialia, 55(9), 3169-3175.
Hu, Z., Zhang, J., Yan, Y., Yan, J., & Sun, T. (2013).
Molecular dynamics simulation of tensile behavior of
diffusion bonded Ni/Al nanowires. Journal of
Mechanical Science and Technology, 27(1), 43-46.
Zhang, Y., & Jiang, S. (2018). Atomistic investigation on
diffusion welding between stainless steel and pure Ni
based on molecular dynamics simulation. Materials,
11(10), 1957.
Darolia, R., Walston, W. S., & Nathal, M. V. (1996). NiAl
alloys for turbine airfoils. Superalloys, 1996, 561-570.
Mukherjee, A. B. (1998). Nickel: a review of occurrence,
uses, emissions, and concentration in the environment
in Finland. Environmental Reviews, 6(3-4), 173-187.
Young, K. H. (2016). Research in nickel/metal hydride
batteries 2016. Batteries, 2(4), 31.
Simões, S., Viana, F., Ventzke, V., Koçak, M., Sofia
Ramos, A., Teresa Vieira, M., & Vieira, M. F. (2010).
Diffusion bonding of TiAl using Ni/Al multilayers.
Journal of Materials Science, 45(16), 4351-4357.
Simões, S., Viana, F., Ramos, A. S., Vieira, M. T., & Vieira,
M. F. (2018). Microstructural characterization of
dissimilar titanium alloys joints using Ni/Al nanolayers.
Metals, 8(9), 715.
Ramos, A. S., Maj, L., Morgiel, J., & Vieira, M. T. (2017).
Coating of Tungsten Wire with Ni/Al Multilayers for
Self-Healing Applications. Metals, 7(12), 574.
Luong, V., Philipsen, V., Hendrickx, E., Opsomer, K.,
Detavernier, C., Laubis, C., ... & Heyns, M. (2018). Ni-
Al alloys as alternative EUV mask absorber. Applied
Sciences, 8(4), 521.
Stukowski, A. (2009). Visualization and analysis of
atomistic simulation data with OVITO–the Open
Visualization Tool. Modelling and simulation in
materials science and engineering, 18(1), 015012.
Zimmerman, J. A., Kelchner, C. L., Klein, P. A., Hamilton,
J. C., & Foiles, S. M. (2001). Surface step effects on
nanoindentation. Physical Review Letters, 87(16),
165507.
Prieve, D. C., & Russel, W. B. (1988). Simplified
predictions of Hamaker constants from Lifshitz theory.
Journal of Colloid and Interface Science, 125(1), 1-13.
Mishin, Y., Mehl, M. J., & Papaconstantopoulos, D. A.
(2002). Embedded-atom potential for B 2− NiAl.
Physical review B, 65(22), 224114.
Mishin, Y. (2004). Atomistic modeling of the γ and γ′-
phases of the Ni–Al system. Acta Materialia, 52(6),
1451-1467.
Lu, Y., Huang, J. Y., Wang, C., Sun, S., & Lou, J. (2010).
Cold welding of ultrathin gold nanowires. Nature
nanotechnology, 5(3), 218-224.
Plimpton, S., & Root, J. (1994). Materials and strategies
that work in low literacy health communication. Public
Health Reports, 109(1), 86.
Effect of Pressure on Mechanical Properties and Structural Evolution of Diffusion-Bonded Al-Ni: Insight from Molecular Dynamics
Simulation
341
