
The Effect of Quenching and Nickel-Chrome Electroplating with
Variations of Voltage and Time of Coating Againts Value of Hardness
on Brass
T. Endramawan
1a
, A. Sifa
1b
, D. Suwandi
1c
and B. Laksono Jaelane
2
1
Design Manufacturing, Politeknik Negeri Indramayu, Jl. Lohbener Lama no.08, Indramayu, Indonesia
2
Department of Mechanical Engineering, Politeknik Negeri Indramayu, Jl. Lohbener Lama no.08, Indramayu, Indonesia
Keywords: Brass, Heat Treatment, Electroplating, Nickel-Chrome.
Abstract: Brass is a mixture of copper and zinc. Brass is very easy to shape into various shapes, brass is mostly used
for ship applications, one of which is the propeller, the propeller is usually damaged in the form of corrosion
or hit by a hard object so that it bends or breaks at the tip of the propeller leaf. The electroplating process is
one of the metal coating methods. The coating method is influenced by several parameters including: current
strength, electrode distance, current distribution, coating time, concentration level of the electrolyte solution
and others. The process of heat treatment (Hardening) is a process carried out to increase the hardness of the
material. Hardening at a temperature of 650
o
C for 6 hours with the highest hardness value obtained by water
quenching media of 101.78 HV (12, 67%) with a base material hardness of 90.34 HV. The results of the
Vickers hardness test after the nickel-chrome electroplating process with a voltage variation of 1.5, 3, and 4.5
volts and a time of 5, 10, and 15 minutes, with a loading of 100 gf indentation time of 10 seconds with the
highest hardness value obtained at a voltage of 4 ,5 volts and 15 minutes of 254.82 HV for nickel and 261.95
HV for nickel and 261.95 HV. The results of the thickness test after the nickel-chrome electroplating process
based on the results of the Vickers test with the highest hardness value on nickel obtained a thickness of
70.90833 m and chrome at 99.5483 m. with a loading of 100 gf indentation time of 10 seconds with the highest
hardness value obtained at a voltage of 4.5 volts and a time of 15 minutes of 254.82 HV for nickel and chrome
of 261.95 HV of 261.95 HV. The results of the thickness test after the nickel-chrome electroplating process
based on the results of the Vickers test with the highest hardness value on nickel obtained a thickness of
70.90833 m and chrome at 99.5483 m. with a loading of 100 gf indentation time of 10 seconds with the highest
hardness value obtained at a voltage of 4.5 volts and a time of 15 minutes of 254.82 HV for nickel and chrome
of 261.95 HV of 261.95 HV. The results of the thickness test after the nickel-chrome electroplating process
based on the results of the Vickers test with the highest hardness value on nickel obtained a thickness of
70.90833 m and chrome at 99.5483 m.
1 INTRODUCTION
In line with the development of the industrial
revolution 4.0 and advances in science and
technology, the use of metals cannot be separated
from human life. The development of technology that
is increasingly rapidly affecting the lifestyle of
humans has resulted in almost all equipment around
us being made of metal. The Ministry of Industry is
concentrating on developing the domestic brass-
a
https://orcid.org/0000-0002-5605-680X
b
https://orcid.org/0000-0001-9565-9884
c
https://orcid.org/0000-0001-6816-1570
based industry to make it more competitive. One of
the strategic efforts is to encourage the production of
this sector through the use of recycling raw materials
of brass or copper from the remnants of household
appliances or projects that are no longer used. The
development of the brass industry will contribute to
the performance of the national metal industry. In
2017, the metal industry recorded a growth of 5, 87%
or above the economic growth which reached 5.07%.
Currently, the growth of the base metal industry is
56
Endramawan, T., Sifa, A., Suwandi, D. and Jaelane, B.
The Effect of Quenching and Nickel-Chrome Electroplating with Variations of Voltage and Time of Coating Againts Value of Hardness on Brass.
DOI: 10.5220/0011711500003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 56-61
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

still supported by the iron, steel, aluminum, nickel,
copper and tin sectors (kemenperin.go.id).
Brass is a metal that is a mixture of copper (Cu)
and zinc (Zn). Copper is the main component of brass.
Brass is usually classified as an alloy of copper and
zinc. The color of brass varies from dark reddish
brown to light silvery yellow depending on the
amount of zinc (Zn) content. Zinc affects the color of
the brass more. Brass is stronger and harder than
copper, but not as strong or as hard as steel. Brass is
very easy to form into a variety of shapes, is a good
conductor of heat, and is generally resistant to
corrosion from salt water. Because of these
properties, brass is mostly used to make pipes, tubes,
screws, radiators, musical instruments, marine
applications, and cartridge casings for firearms. (Tata
Surdia, 1996).
Most ship propellers are also made of brass and
aluminum alloy, both of which have their own
advantages and disadvantages. The propellers on
fishing boats are rarely damaged in the form of
fractures caused by the work of the propellers.
Damage experienced by fishing boat propellers is
generally due to corrosion or bending of the leaf tips
on the fishing boat propellers.(Mad & Ellyawan,
2006).
Corrosion process is an event of damage or
decrease in the quality of a metal material caused by
a reaction to environmental conditions and at the
place where the metal is used (AR Hakim, 2012).
What is meant by environmental influences and
places of use can be in the form of air or sunlight,
dew, fresh water, sea water, river water, ground
water, lime water, and rocky sandy soil. Corrosion of
metals can also be interpreted as the reverse reaction
of metal refining. This corrosion itself can lead to a
decrease in the quality of the metal, resulting in the
metal becoming weak and damaged quickly.
Corrosion or rust is a form of metal degradation due
to electrochemical reactions with the environment
that are directly related to open air or often referred to
as corrosion. Almost all corrosion products are
caused by the atmospheric environment. This is
because in general, metals are always in contact with
open air where humidity and pollutant content can
affect metal corrosion. Atmospheric corrosion is
strongly influenced by topographic and climatic
conditions of the environment, factors such as
temperature, humidity and chemical content in the air
or water greatly determine the corrosion rate. One of
the prevention and protection against corrosion is by
electroplating nickel-chrome plating. factors such as
temperature, humidity and chemical content in the air
or water greatly determine the corrosion rate. One of
the prevention and protection against corrosion is by
electroplating nickel-chrome plating. factors such as
temperature, humidity and chemical content in the air
or water greatly determine the corrosion rate. One of
the prevention and protection against corrosion is by
electroplating nickel-chrome plating.
The electroplating process is one method of metal
plating. The electroplating process is often called
electrodeposition, which is a process of deposition of
protective metal on top of another metal by means of
electrolysis. The metal electroplating process is
basically carried out with the aim of preventing the
corrosion process that attacks the steel surface.
Currently, the electroplating method is very popular
because of its brilliant appearance, uniform
distribution of coating material throughout, not easy
to corrode and durable. The metals used as coatings
are copper, nickel, chromium, zinc, gold, silver, brass
and others (Kaban et al, 2010). In metalworking
technology, the electroplating process is a metal
finishing process. Simply, Electroplating can be
interpreted as a metal plating process using the help
of an electric current and certain chemical compounds
to transfer the coating metal particles to the material
to be coated. In chrome plating, currently there are
two kinds of chrome plating that can be done, namely
decorative chrome plating.
The electroplating coating method is influenced
by several influential parameters and needs to be
considered in order to obtain good coating results
including: current strength, electrode distance,
current distribution, plating time, agitation,
concentration level of the electrolyte solution and
others (Adnyani and Triadi, 2009: 77).
From the explanation above, the urgency of this
research is important to carry out, the aim is to
increase the hardness, physical and mechanical
resistance of the material to corrosion and to coat the
material so that it can be oxidized so as to increase the
life of the brass alloy. The method used is to treat the
brass material, this treatment includes a direct heat
treatment (hardening) process and coating the
material with the nickel-chrome electroplating
method. This research was carried out on brass
material for experimentation and testing, with the title
taken in making scientific papers, namely "The Effect
of Quenching Media and Nickel-Chrome
Electroplating with Variations in Voltage and Plating
Time on Hardness Values in Brass"
The Effect of Quenching and Nickel-Chrome Electroplating with Variations of Voltage and Time of Coating Againts Value of Hardness on
Brass
57
2 RESEARCH METHODS
2.1 Materials
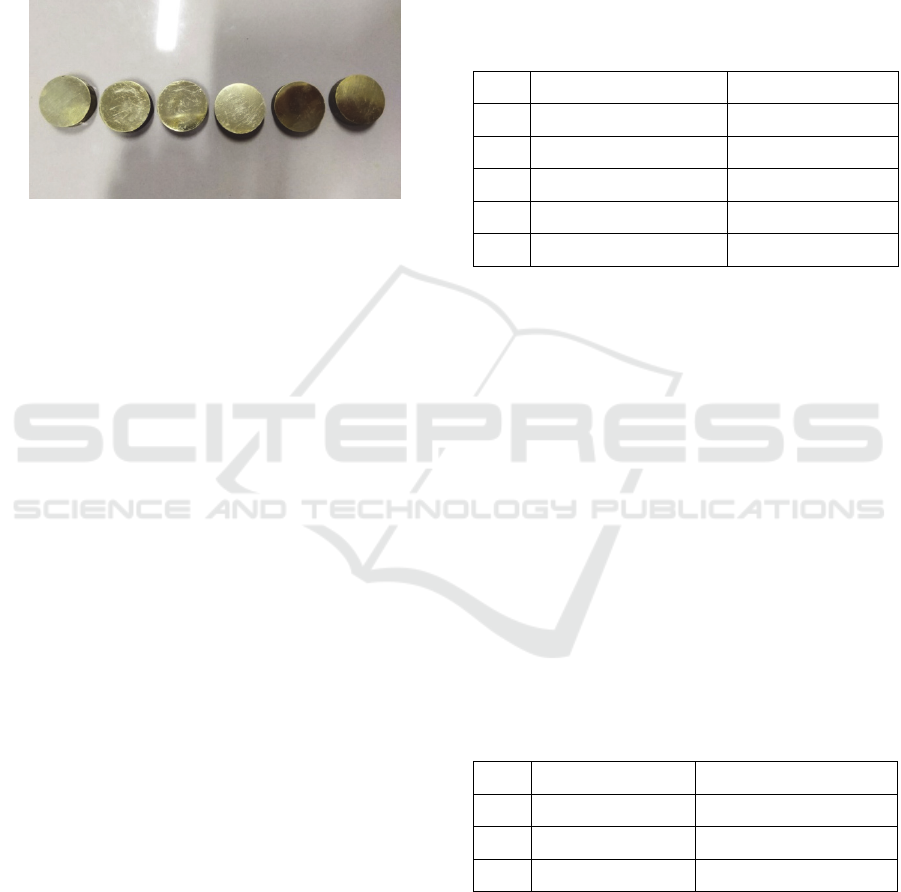
The specimen material used in this study was brass
were brass that had been cut beforehand with a cutting
saw that shown at Figure 1.
Figure 1: Brass specimen.
2.2 Heat Treatment
The heat treatment carried out in this study is uses
Bench Furnace Type BF02, where in general the heat
treatment consists of heat treatment and quenching
with water and oil. In the heat treatment process, the
test object is heated from a temperature of 650°C for
6 hours (ASM Metal Handbook V.4 – Heat Treating),
then the test object is quenched with water and oil
media after.
2.3 Pre-Treatment Process
Before doing the electroplating proses the spesimen
will be mechanical and chemical cleaning, first proses
is the mechanical cleaning process is carried out by
sanding which aims to remove dirt attached to the
surface of the brass specimen. This sanding process
uses 240 CW to 2000 CW sandpaper. After that we
continiue to the chemical cleaning process, this
cleaning is carried out by washing the specimens
using sodium carbonate (NaOH) and H2SO4. The
concentration used in this process (50 gr/liter), 5%
H2SO4 and distilled water for rinsing.
2.4 Electroplating Nickel Proses
Furthermore, in this process the specimen is
immersed in a plastic bath containing a nickel
solution that has been mixed with distilled water with
varying concentrations of the solution as shown in
Table 2 of nickel chemicals solution (Nickel Plating
Handbook, Nickel Institute 2014). In this
electroplating process, the brass specimen is in the
conductor as the cathode (-) and the coating is at the
anode (+). Furthermore, the anode and cathode
distances are set for 5 cm and the power supply
electric current is regulated before the specimen is
immersed with a current of 5 amperes, voltage
variations of 1.5 volts, 3 volts, and 4.5 volts and
immersion time of 5 minutes, 10 minutes, 15 minutes
and the temperature of the solution. ± 40 °C. After the
nickel electroplating process is complete, the
specimen will be rinsed first to neutralize it.
Table 1: Nickel Chemical Solution.
No. Material Name Concentration
1 Nickel Sulfate 250 gr/l
2 Nickel Chloride 70 gr/l
3 Boric acid 45 gr/l
4 Brightener 1 cc/l
5 Carrier 2 cc/l
2.5 Electroplating Chrome Proses
Furthermore, in this chrome electroplating process,
the specimen is immersed in a plastic bath containing
a chrome solution that has been mixed with distilled
water, with variations in the concentration of the
solution as shown in table 3.4 of the chemical chrome
solution. In this electroplating process, the brass
specimen is in the conductor as the cathode (-) and the
coating is at the anode (+). Furthermore, the distance
of the anode and cathode is adjusted as long as 5 cm
and the electric current of the power supply is
regulated before the specimen is immersed with a
current of 5 amperes, a voltage variation of 3 volts
and an immersion time of 1 minute and a solution
temperature of ± 40 °C. After the nickel electroplating
process is complete, the specimen will be rinsed first
to neutralize it.
Table 2: Chrome Chemical Solution.
No. Material Name Concentration
1 Chrome Acid 200 gr/l
2 Sulfuric acid 0.85 cc/l
3 Catalyst 4 gr/l
2.6 Testing
The method used to collect the test data is the vickers
hardness testing is carried out at the Mechanical
Engineering Laboratory of the Indramayu State
Polytechnic using the Innovatest Verzus 700AS type.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
58
The macro Vickers hardness testing process is carried
out through 3 data proses, the testing in this hardness
test uses an indenter load of 5 kg with a holding time
of 15 seconds, aiming to determine the hardness value
of the KTM 25 base, after hardening and aging
material.
Vickers microhardness testing aims to test the
hardness of the anodizing layer, this test was carried
out at the Mechanical Engineering Laboratory of the
Indramayu State Polytechnic using a microhardness
tester type FM-810. This test uses an indenter load of
100 gf with a holding time of 10 seconds.
Microstructure Photo Testing this test is carried out to
see the thickness of the coating resulting from the
anodizing process. The tool used in this test is the
Olympus BX3M with 20x optical magnification.
3 RESULTS AND DISCUSSION
3.1 Hardness Test Results
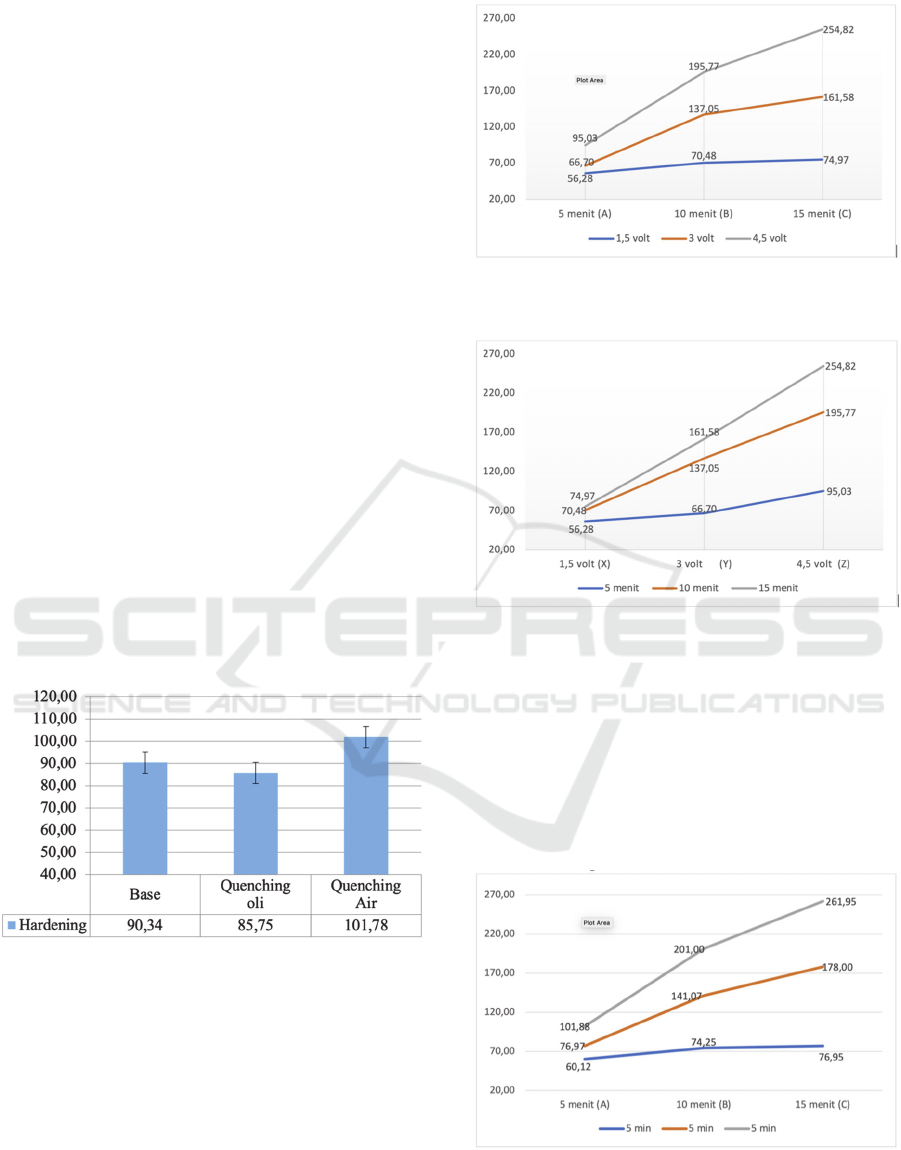
From the results of the heat treatment research, in the
hardening process with water and oil quenching
media directly with a hardening temperature of 650
o
C
with a holding time of 6 hours, then the quenching
process with water and oil, obtained hardness results
as shown in Figure 2.
Figure 2: Graph of Hardness Value After Heat Treatment.
The results of this study are similar to those
carried out (Cahyono, 2018) where the highest
hardness in his research was obtained in water
quenching rather than using oil and open air
quenching, and in his research showed that quenching
using oil can reduce the hardness of brass materials.
From the results of the nickel electroplating
process with a voltage variation of 1.5, 3 and 4.5 volts
with a time variation of 5, 10 and 15 minutes, the
results of the surface hardness of the coating can be
seen in Figure 3.
Figure 3: Graph Coating Hardness Value Chart Nickel
Against Time.
Figure 4: Graph Coating Hardness Value Chart Nickel
Against Voltage.
Based on the graph above, it can be seen that the
value of hardness is increasing which shows that each
voltage and the coating time, the value of hardness
will increase. This is similar to research (Sumpena, &
Wardoyo, 2020) and (Tahu, Maliwemu, & Limbong,
2015) where the highest layer hardness value is
obtained at each increase that occurs as the coating
time and voltage increases.
Figure 5: Graph Coating Hardness Value Chart Chrome
Against Voltage.
The Effect of Quenching and Nickel-Chrome Electroplating with Variations of Voltage and Time of Coating Againts Value of Hardness on
Brass
59
From the results of the chrome electroplating (3
volt, 5 ampere) againts nickel process with a voltage
variation of 1.5, 3 and 4.5 volts with a time variation
of 5, 10 and 15 minutes, the results of the surface
hardness of the coating can be seen in Figure 5.
Based on the graph above, it can be seen that the
value of hardness is increasing which shows that each
voltage and the coating time of nickel variations, the
value of hardness will increase. This is similar to
research (Sumpena & Wardoyo, 2020) and(Tahu,
Maliwemu, & Limbong, 2015) where the highest
layer hardness value is obtained at each increase that
occurs as the coating time and voltage increases.
3.2 Electroplating Nickel-Chrome
Coating Thickness Test Results
From the results of the hardness test that has been
carried out, the highest layer hardness value of the
electroplating process is obtained at 4.5 volt and 15
minutes of coating time. Then the specimen is tested
for thickness, where the thickness test results are
obtained as follows:
Table 3: Electroplating Nickel Layer Thickness Values.
. Layer Thickness Average
1 76.82
70.90833
2 69,80
3 70.23
4 73.26
5 62.38
6 72.96
Table 4: Electroplating Chrome Layer Thickness Values.
N
o. Layer Thickness Average
1 95.69
99.5483
2 100.79
3 91.88
4 90.04
5 117.37
6 101.52
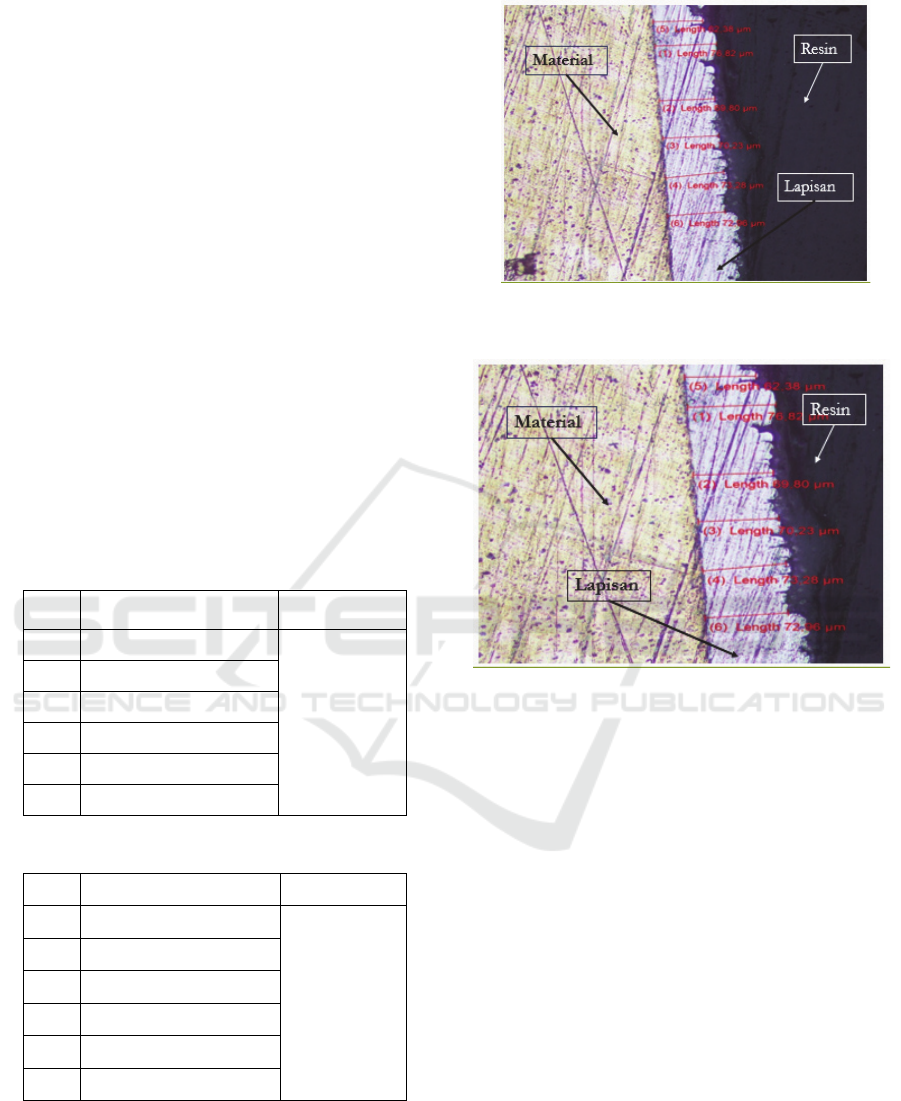
For more details where the measurement is located
taken from the process of testing the thickness of the
electroplating layer with a time of 15 minutes and 4.5
volt can be seen in the following figure:
Figure 6: Electroplating Nickel Layer Microstructure
(Measurement Position).
Figure 7: Electroplating Chrome Layer Microstructure
(Measurement Position).
Based on the table data above, the lowest thickness
value is at point 5, which is62.38 µm, and the highest
thickness is obtained at point 1 which is 76.82 µm.
While the value of the average thickness obtained
from the results of the electroplating process with a
voltage of 4.5 volts and a time of 15 minutes
is70.90833 µm in nickel coating.
Based on the table data above, the lowest thickness
value is at point 4, which is90.04 µm, and the highest
thickness is obtained at point 5 which is117.37 µm.
While the value of the average thickness obtained
from the results of the electroplating process with a
voltage of 4.5 volts and a time of 15 minutes is
99.5483 µm in chrome coating.
4 CONCLUSION
Vickers hardness test results with a loading of 5 kg
indentation time 15 seconds after the heat treatment
(Hardening) process that is gradually starting from a
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
60

temperature of 350oC to 650oC for 6 hours with oil
quenching media decreasing the hardness value by
85.75 HV (5.08%) and water quenching experienced
a significant increase of 12.67% with a hardness value
of 101.78 HV compared to the hardness of the base
material 90.34 HV, it can be concluded that the
highest hardness value was obtained using water
media.
The results of the Vickers hardness test after the
nickel-chrome electroplating process with a voltage
variation of 1.5 volts, 3 volts, and 4.5 volts and a time
of 5 minutes, 10 minutes, and 15 minutes, with a
loading of 100 gf indentation time of 10 seconds
increased the hardness value. with increasing stress
and time on the coating, the variation of the increase
in at a voltage of 4.5 volts with a time of 15 minutes
with the highest hardness value for nickel of 254.82
HV and chrome of 261.95 HV. The increase in
voltage and time in the nickel-chrome electroplating
coating affects increase in the hardness of the coating.
The results of the thickness test after the nickel-
chrome electroplating process of 4.5 volts and 15
minutes based on the results of the Vickers test with
the highest hardness value on nickel obtained a
thickness of 70.90833 µm and on chrome the
thickness is 99.5483 µm.
ACKNOWLEDGEMENTS
The author would like to thank the Ministry of
Research and Technology, Indramayu State
Polytechnic and the Department of Mechanical
Engineering, Indramayu State Polytechnic.
REFERENCES
https://kemenperin.go.id/artikel/18999/Tingkatkan-
Efisiensi,-Industri-Kuningan-Manfaatkan-Bahan-
Baku-Daur-Ulang.
Surdia, Tata. 1996. Teknik Pengecoran Logam, Edisi ke-2,
Cetakan ke-7, PT. Pradnya Paramita, Jakarta.
Mad, Y., & Ellyawan, A. (2006). Studi Kasus Laju Korosi
Baling-Baling Perahu Nelayan. Jurnal Teknologi
Academia Ista, 43.
Hakim, AR. 2012. Analisa Korosi Atmosfer pada material
Baja Karbon Sedang di Kota Semarang. Semarang.
Universitas Diponegoro.
Kaban, Hadir, dkk. 2010. Menguji kekuatan bahan
6 ubtract 6 ating pelapisan nikel pada 6 ubtract besi
dengan uji impak. Jurnal Penelitian Sains Vol. 13 No
3B. September 2010.
Adnyani, I.A.S., dan Triadi A.A.A., (2009). Pengaruh Kuat
Dan Distribusi Arus Terhadap Ketebalan dan
Kekerasan Lapisan Krom Pada Stoneware Dan
Earthenware. Mataram: Jurusan Teknik Elektro
Fakultas Teknik, Universitas Mataram.
ASM International Metals Handbook Volume 4 – Heat
Treating
Nickel Plating Handbook, Nickel Institute 2014.
Cahyono, I., 2018. Analisa Hasil Pengecoran (Cu, Zn)
Dengan Variasi Media Pendinginan (Air Sumur, Oli
SAE 40 Dan Udara) Menggunakan Cetakan Pasir CO2,
Fakultas Teknik Univeritas Muhammadiyah.
Sumpena, Wardoyo, 2020. Analisa Kuat Arus Listrik dan
Waktu Electroplating Nickel-Chrome terhadap
Kekerasan dan Ketebalan Lapisan Permukaan Baja
Karbon Rendah. Jurnal Engine Vol. 4, No.2, 2020 : 96-
102.
Tahu, F., Maliwemu, E. U., 7 Limbong, i.S. (2015).
Pengaruh Tegangan Listrik dan Waktu Terhadap Mikro
Pelapisan Nikel-Krom Pada Produk Pengecoran
Alumunium Bekas (Scrap), Jurnal Teknik Mesin
UNDANA.
The Effect of Quenching and Nickel-Chrome Electroplating with Variations of Voltage and Time of Coating Againts Value of Hardness on
Brass
61
