
Ergosterol Content and Antioxidant Activity of Lion’s Mane
Mushroom (Hericium erinaceus) and Its Induction to Vitamin D
2
by UVC-Irradiation
Pinida Joradon
1
, Vilai Rungsardthong
1,*
, Uracha Ruktanonchai
2
, Khomson Suttisintong
2
,
Tawin Iempridee
2
, Benjawan Thumthanaruk
1
, Savitri Vatanyoopaisarn
1
, Nutsuda Sumonsiri
1
and Dutsadee Uttapap
3
1
Department of Agro-Industrial, King Mongkut’s University of Technology North Bangkok, Bangkok, Thailand
2
National Nanotechnology Center, Thailand Science Park, Pathumthani, Thailand
3
Division of Biochemical Technology, King Mongkut’s University of Technology Thonburi, Bangkok, Thailand
Keywords: Lion’s Mane Mushroom (Hericium erinaceus), Ergosterol, Vitamin D
2
(Ergocalciferol), UVC, Antioxidant
Properties.
Abstract: Lion’s Mane mushroom (Hericium erinaceus), LM, is a medicinal mushroom which has high protein content
and contains many bioactive compounds. However, a large amount of the irregular-shape LM (Ir-LM),
considered as by-products, are generated during the cultivation. The objectives of this research were to
determine the ergosterol content in the LM and investigate the effect of ultraviolet (UV) irradiation on the
conversion of ergosterol in the Ir-LM extract to vitamin D
2
. Ir-LM extracts were investigated for its
antioxidant properties before dissolved in methanol and irradiated with UVC for 120 min at 5 cm distance
from the lamp. The results showed that the Ir-LM contained significantly higher (p < 0.05) ergosterol content
(2.52 ± 0.13 mg/g dried LM) than that of regular-shape LM (Reg-LM), 2.15 ± 0.08 mg/g dried LM. Ergosterol
at 1.74 ± 0.09 mg/g dried LM without vitamin D
2
was detected in the non-irradiated extract, while
interestingly, the irradiated sample showed a decrease of ergosterol at 13.5% with a detection of ergocalciferol
at 30.01 ± 7.09 µg/g dried LM. These obtained results exhibited a new area of post-extraction procedure
aiming to enhance vitamin D
2
enriched extracts from mushroom by-products which can be value-added as a
nutritional supplement in foods.
1 INTRODUCTION
Lion’s Mane mushroom (Hericium erinaceus, LM) is
an edible fungus which has been used in traditional
Chinese medicine for long time (Khan et al., 2013).
LM contains a significant content of bioactive
compounds, ergosterol, hericenone C and hericene A
(Joradon et al., 2022), that would might be
responsible for several health-promoting properties
(Friedman, 2015).
In general, high molecular weight substances
including polysaccharides and low molecular weight
substances including terpenoids can be used to
categorize bioactive metabolites in the LM (Thongbai
et al., 2015). The compounds with bioactivity exist in
different part of the mushroom (Shen et al., 2010), for
instance, low-molecular weight metabolites,
erinacines were found in mycelia while hericenones
were detected in fruiting bodies of LM (Thongbai et
al., 2015).
There is an increasing interest in bioactive
compounds from natural sources such as gamma
oryzanol from rice bran oil (Rodsuwan et al., 2020),
puerarin from Pueraria (Rungsardthong et al., 2021),
ergosterol from LM (Joradon et al., 2022;
Tachabenjarong et al., 2022), antioxidants from
Sacha Inchi oil (Suwannasang et al., 2021; 2022a;
2022b), and bioactive compounds from bamboo
mushroom (Binheam et al., 2022).
Ergosterol can exhibit anti-inflammatory, anti-
tyrosinase and anti-cancer properties (Kang et al.,
2015). According to Corrêa et al. (2017), ergosterol
has the potential to lessen the negative effects from
high cholesterol. Ergosterol is the most prevalent
sterol presents in the membranes of fungi, and it is
also a precursor of vitamin D
2
. The compound is
Joradon, P., Rungsardthong, V., Ruktanonchai, U., Suttisintong, K., Iempridee, T., Thumthanaruk, B., Vatanyoopaisarn, S., Sumonsiri, N. and Uttapap, D.
Ergosterol Content and Antioxidant Activity of Lion’s Mane Mushroom (Hericium erinaceus) and Its Induction to Vitamin D2 by UVC-Irradiation.
DOI: 10.5220/0011594600003430
In Proceedings of the 8th International Conference on Agricultural and Biological Sciences (ABS 2022), pages 19-28
ISBN: 978-989-758-607-1; ISSN: 2795-5893
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
19

important for maintaining fluidity and permeability,
involves with endocytosis and cytoskeletal
organization inside the fungal cells (Abe & Hiraki,
2009). Mushroom ergosterol can be converted into
vitamin D
2
(Jasinghe et al., 2007). With the exposure
to UV irradiation, ergosterol encounters
photochemical cleavage within their structure,
causing the formation of the intermediate of vitamin
D
2
. After being heated, this intermediate then goes
through thermal isomerization to create vitamin D
2
.
(Jasinghe et al., 2007).
One of the pro-hormones that is crucial for
maintaining human health is vitamin D. Vitamin D is
well known for supporting bone health and calcium
homeostasis, as well as having a variety of non-
skeletal effects on immunological function and cell
physiology (Durrant et al., 2022). Vitamin D
2
and D
3
are found as a major forms of vitamin D (Dawson-
Hughes et al., 2010). Typically, vitamin D
2
can be
found in the fruiting bodies of mushrooms. Applying
ultraviolet (UV) irradiation to the mushroom will lead
to the conversion of ergosterol to vitamin D
2
(Jäpelt
and Jakobsen, 2013). Morales et al. (2017) revealed
that UVC irradiation to the ethanol extract of Shiitake
mushroom (Lentinula edodes) at 25°C, for 1 h (4 cm
away from the light source) could generate a high
content of vitamin D
2
than direct irradiation of the
fruiting body.
During the cultivation of LM, irregular-shape LM
(Ir-LM) were found in the mushroom farm. These
mushrooms are considered as by-products and sold at
low prices in the mushroom market because of their
inferior morphology and quality. Therefore,
alternative solutions are required to increase the value
of these mushroom by-products which still contain a
high content of health-benefit compounds. They
might be used as high nutritional food or extracts
enriched with medicinal compounds for food or
medicinal uses. Consequently, the objectives of this
research were to determine the major bioactive
compounds, ergosterol, hericenone C and hericene A
contents and antioxidant properties in the extracts
prepared from regular LM (Reg-LM) and Ir-LM.
Further investigation on the effect of ultraviolet C
(UVC) irradiation on the conversion of ergosterol in
the Ir-LM extract to vitamin D
2
was carried out.
Morphology of fruiting bodies and their proximate
compositions were also performed. The results of this
study would propose an alternative way to increase
the value of the by-product by converting ergosterol
in the mushroom extracts to vitamin D
2
.
2 MATERIALS AND METHODS
2.1 Biological Materials and Chemicals
Fruiting bodies of Reg-LM and Ir-LM were
purchased from Fresh and Friendly Farm Co., Ltd. at
Thanyaburi district in Pathum Thani province,
Thailand. Morphology of the LM cultivated in the
farm for 4 lots were monitored and their production
yield and related economic data were calculated.
Ergosterol (95%) was obtained from Sigma-Aldrich
Química (Madrid, Spain). Ergocalciferol (98%)
(vitamin D
2
) was purchased from TCI, Japan. Folin-
Ciocalteu reagent and 2,2-diphenyl-1-
picreylhydrazyl (DPPH) were purchased from Sisco
Research Laboratories Pvt. Ltd., India while gallic
acid (98%) was the product from Sigma-Aldrich,
USA. Trolox reagent was obtained from M Tedia,
USA. Sodium carbonate (99.5% purity) was
purchased from Merck, India. Absolute ethanol
(analytical grade) was bought from PanReac
(Barcelona, Spain), while water and methanol (HPLC
grade) were purchased from LAB-SCAN (Gliwice,
Poland).
2.2 Cultivation of LM in the
Mushroom Farm
Temperature in the cultivation room was controlled at
16 ± 1 °C with the photo period at 12 hours of light
and 12 hours of darkness. The measurement of CO
2
in the room was measured by a sensor, and its average
intensity during the cultivation was around 900 mg/L.
Initial moisture content of the substrates for the
mycelium growth was 70-80%. Figure 1 shows the
substrate bags with LM mycelium growths in the
incubation room at Fresh and Friendly Farm.
2.3 Extraction and Determination of
Ergosterol
Ten grams of the freeze-dried samples from both
Reg-LM and Ir-LM were soaked in 200 mL of
absolute ethanol for 3 days at room temperature (25 ±
3 °C) in the dark. After filtering the suspensions, the
clean supernatant was collected and all solvents were
eliminated using a rotary evaporator (R114, Buchi,
Switzerland), at 50 °C. The dried extract was kept at
-20 °C in the dark until use. High-performance liquid
chromatography (HPLC) used to determine
ergosterol and vitamin D
2
in the dried extracts.
ABS 2022 - The International Conference on Agricultural and Biological Sciences
20

2.4 UVC-Irradiation of Ir-LM
The extract from Ir-LM was dissolved with absolute
methanol in cylindrical vessels and exposed to the
UVC at 254 nm with the intensity of 145 µw/cm
2
(determined by UVC meter, Solarmeter® version 8.0,
Solar Light Company Inc.) at room temperature for
120 min, at 5 cm away from the lamp. HPLC was
used to determine the levels of ergosterol and vitamin
D
2
.
2.5 Antioxidant Properties
2.5.1 Total Phenolic Content (TPC)
Total phenolic contents of the extracts from both Reg-
LM and Ir-LM were evaluated using Folin-Ciocalteu
reagent with the absorbance at 750 nm, measured by
a 96-well microplate reader (Bio-Rad, iMark, USA).
All experiments were performed in triplicates. The
TPC were determined as gallic acid equivalents
(GAE)/g dried LM using gallic acid as the reference
(Rosa et al., 2017).
2.5.2 DPPH Scavenging Activity
The DPPH radical scavenging experiment was
modified slightly from Ahmed et al. (2012) in order
to assess the antioxidant ability of LM extracts. DPPH
was dissolved in absolute methanol at a concentration
of 0.5 mM to create DPPH radical solution. Fifty µL
of DPPH solution were added to 50 µL of the extract
in each well. The plate was incubated for 30 minutes
in the dark at room temperature. Trolox was used to
create a calibration curve, with methanol serving as
the blank. Absorbance of the solution was measured
by a microplate reader (Bio-Rad, iMark, USA) at 540
nm. All experiments were carried out in triplicates
and scavenging ability was calculated as mg Trolox
equivalent (TE)/g dried LM followed Eq. 1:
% Inhibition = (
)×100
(1)
where A
control
is the absorbance of the control,
which has all reagents present minus the samples.
A
sample
is the absorbance of the mushroom extracts
with reagents added.
2.5.3 ABTS Scavenging Activity
The ABTS radical scavenging assay was performed
followed Ahmed et al. (2012) with minor
modification. The ABTS radical was determined by
reacting 200 mL of 140 mM K
2
S
2
O
8
solution with 7
mM of ABTS solution, and allowed the mixture to
react for 16 hours at room temperature in the dark.
Absorbance of the working ABTS solution was
measured on a microplate reader (Bio-Rad, iMark) at
750 nm. ABTS solution used for the measurement
was diluted with absolute methanol to gain the
absorbance approximately 1.1 to 1.2 at 750 nm. In 96-
well microtiter plates, 50 L of extract solution was
combined with 100 L of ABTS solution. Methanol
was used as a blank. The absorbance was read within
30 minutes at room temperature. All measurements
were performed in triplicate. The antioxidant activity
was determined as mg TE/g dried LM as detailed in
Eq. 1.
2.6 Analysis of Bioactive Compounds
by HPLC
Ergosterol and other compounds in the Reg-LM and
Ir-LM extracts were analyzed using HPLC (Agilent
Technology 1,200 series, Germany). Eclipse Zorbax
XDB-C 18 (Agilent, 250 4.6 mm, 5 m) analytical
column and Zorbax XDB-C 18 (Agilent, 12.5 4.6
mm, 5 m) guard column were used with the HPLC
system to measure the compounds at 282 nm using
the UVVIS LC detector. Methanol and water were
mixed in the mobile phase at a 98:2 ratio. Ergosterol
was quantified from the calibration curve of
ergosterol standard. The content of hericenone C and
hericene A were calculated from their peak area
compared with the area of ergosterol.
The content of each bioactive compound after
extraction was presented in the unit of mg/g extract,
mg/g dried LM, and mg/g fresh LM. The unit mg/g
extract was calculated as mg bioactive compound per
gram of dried extract while mg/g dried LM and mg/g
fresh LM were calculated as the mg bioactive
compound per gram of dried LM powder and fresh
LM fruiting body, respectively.
2.7 Statistical Data Analysis
All experiments were performed with three
replications. Data were analyzed using IBM SPSS 26
for Windows (SPSS Inc.) with independent t-test to
compare the means of each treatment. To compare the
difference between each sample, the significance
level at p < 0.05 was employed.
Ergosterol Content and Antioxidant Activity of Lion’s Mane Mushroom (Hericium erinaceus) and Its Induction to Vitamin D2 by
UVC-Irradiation
21

3 RESULTS AND DISCUSSIONS
3.1 Cultivation of LM in the
Mushroom Farm
Production yield, percentage of Reg-LM and Ir-LM
as well as the calculated economic losses were
presented in Table 1. Irregular-shape LM (Ir-LM)
occurred during the cultivation were sold as low
price, consequently leads to economic loss for the
mushroom farm. Ir-LM is considered as by-products,
equals to around 20% of total LM cultivated in the
farm. Total production of LM in the farm was about
3,270 Bahts/batch or 156,967 Bahts/year since
approximately 48 batches were cultivated per year
(Table 1). Similar information was reported by
Aguayo et al. (2017) that high amounts of irregular-
shape of Button mushroom (Agaricus Bisporus)
fruiting bodies (around 20% of total production) were
generated during the mushroom cultivation. They are
considered as by-products since their misshaped caps
or stalks did not meet the product specifications set
by retailers.
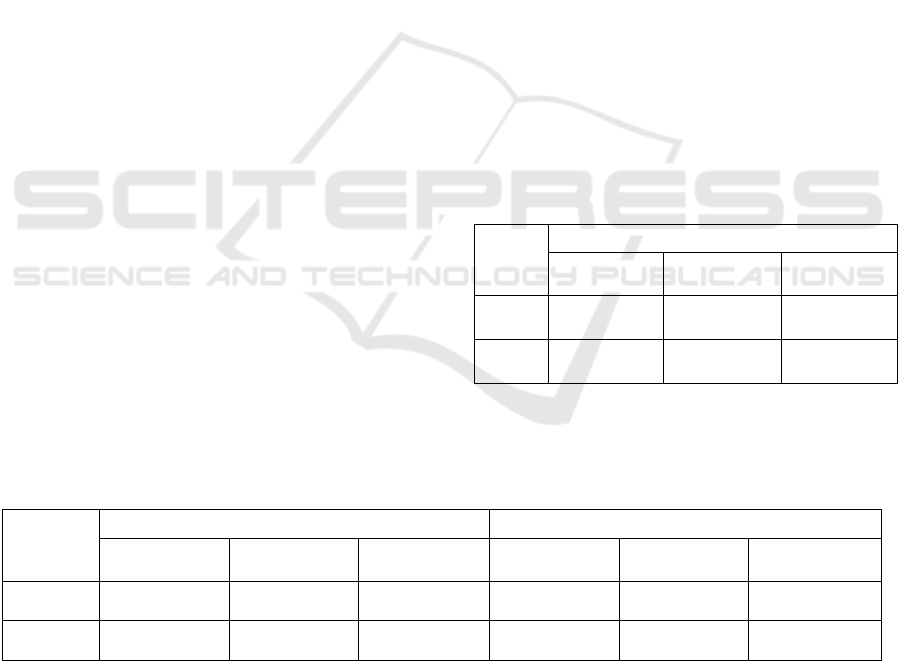
Table 1: Statistical data for the production of Lion’s Mane
mushroom at Fresh & Friendly Farm mushroom farm
*
.
Re
g
-LM I
r
-LM
Production yield (Kg/batch)
168
(80%)
25 (20%)
Sale amount (Bahts/batch) 109,473 13,081
Economic loss (Bahts/batch) - 3,270
Economic loss (Bahts/year) - 156,967
Notes:
*
Data were collected and averaged from 4 batches of
the cultivation during February, 2022.
Irregular-shape LM was found with the covered
lids, randomly in the incubation room for mycelium
and primordium induction. Most of the Ir-LM was
found at the top of the shelf in the incubation room
(Figure 1).
Figure 1: Incubation for the mycelium growths of Lion’s
Mane mushroom in the substrate bags at Fresh and Friendly
Farm. The temperature in the incubation room was
controlled at 16 ± 1 °C.
Morphological study indicated that the fruiting
bodies of Reg-LM exhibited long spore-bearing
spines and intricately branches with primary (PB),
secondary (SB) and tertiary (TB) branches (Figure 2,
A-C), while the fruiting bodies of Ir-LM aggregated
thickly in branches which presented only primary
(PB) and secondary (SB) branches with short spore-
bearing spines (Figure 2, D-F).
Figure 2: Macroscopic images on the fruiting bodies of
Lion’s Mane mushroom. (A-C): regular-shape (Reg-LM),
and (D-F): irregular-shape (Ir-LM) mushroom. SP: spore-
bearing spine, PB: primary branch, SB: second branch, TB:
tertiary branch.
There are various environmental factors affecting
the formation of primordia and fruiting bodies. The
occurrence of Ir-LM might be due to both intrinsic
and extrinsic factors. The spawning rate and the
synthesis of volatile organic molecules like ethylene
and 1-octen-3-ol may be examples of intrinsic factors.
Yang et al. (2013) reported that an increase of
spawning rate could reduce the time for mycelial
colonization development of the fruiting bodies.
According to Zhang et al. (2016), reducing the
generation of ethylene and its precursor 1-
aminocyclopropane-1-carboxylic acid (ACC)
resulted in twice as many primordia that developed
more quickly than those in the wild type strain of the
button mushroom. Eastwood et al. (2013), reported
that temperature and the reduced content of octenol
(1-octen-3-ol) were the switches that controlled the
plenary morphogenesis process, led to the production
of fruiting bodies from the vegetative mycelium.
Before directing the formation of undifferentiated
hyphae into mature mushrooms, the volatile 1-octen-
3-ol would first affect the differentiation of mycelial
condenses into hyphal knots. Undifferentiated
primordia would subsequently become differentiating
primordia, depending on the temperature (Baars et al.,
2020).
The possible extrinsic factors such as air
composition and luminosity in the room might
involve with the occurrence of Ir-LM since large
amounts of Ir-LM were found at the top of the shelf
ABS 2022 - The International Conference on Agricultural and Biological Sciences
22
in the incubation room. According to Bellettini et al.
(2019), mushroom fruiting body in a cultivation room
might be generated by reducing the amounts of
carbon dioxide and the rate of air circulation. The
spawn bottles on the top shelf might expose to higher
light intensity and air ventilation than the other shelf
at lower levels (Figure 1). The higher light intensity
in the environment caused the reduction of coloration,
deformations and elongated stipe of mushroom
fruiting bodies (Urben, 2004). In order to promote pin
head production in button mushrooms, Visscher et al.
(1979) hypothesized that an optimal concentration of
ethylene and carbon dioxide are required.
3.2 Extraction and Determination of
Ergosterol, Hericenone C and
Hericene A
The ergosterol content shown in Table 2 reveals that
Ir-LM contained significantly higher (p < 0.05)
ergosterol content (2.52 ± 0.13 mg/g dried LM) than
that of Reg-LM (2.15 ± 0.08 mg/g dried LM).
Gąsecka et al. (2020) reported that the sample
preparation techniques had a substantial impact on the
ergosterol content of the LM mushroom.
Specifically,
the authors noted that ergosterol level was higher in
the fresh mushroom samples (4.5 mg ergosterol/kg
LM), and it declined when drying temperature was
increased from 20 to 70 °C. Heleno et al. (2016)
presented the use of ethanol as the extraction solvent
in a soxhlet extraction of button mushrooms for 4
hours, yielding maximum concentration of ergosterol
at 676 ± 3 mg/100 g. Mushroom by-products can be
used for the recovery of bioactive compound from
fruiting bodies or mycelium of mushrooms. Wang et
al. (2015) found antichronic atrophic gastritis activity
from the mycelium of LM by-products extracted with
hot water (70 °C for 12 h) followed by precipitation
with ethanol (80%). The antioxidant and antifungal
activities in the ultrasound-assisted extract of LM
mycelium with ethyl acetate were reported by Lu et
al. (2014). Ergosterol was also extracted from the by-
product of button mushroom fruiting bodies using
ethanol and microwave-assisted at 132.8 °C and 1.6
g/L CO
2
flow rate for 19.4 min (Heleno et al., 2016).
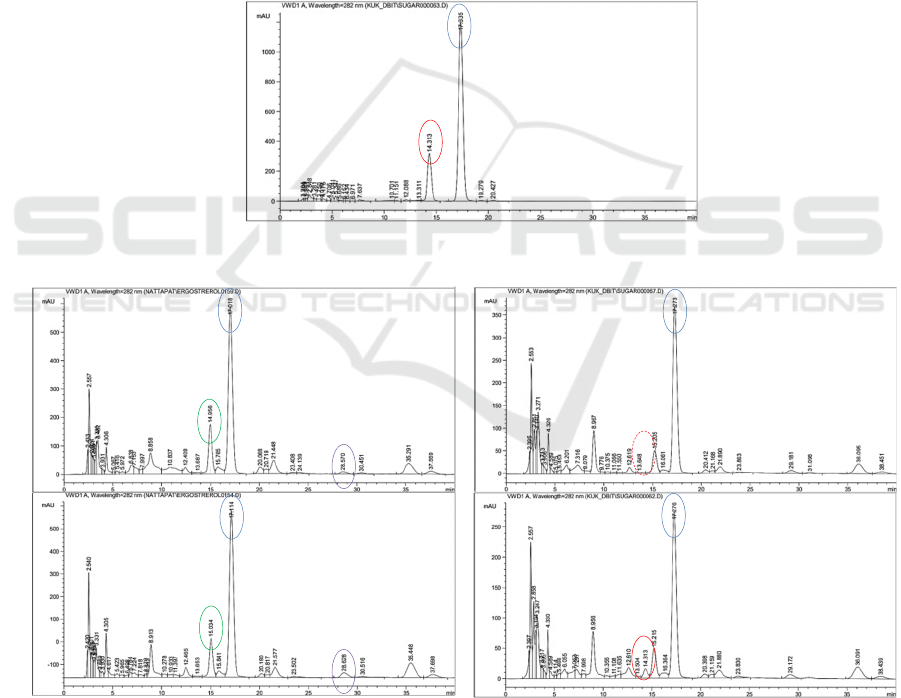
Several chromatogram peaks were detected in
both LM extracts, Reg-LM and Ir-LM and the
contents of hericenone C and hericene A were
compared (Figure 3, B-C). Ir-LM extract showed
significantly higher (p < 0.05) in hericene A (1.25 ±
0.08 mg/g extract) than Reg-LM extracts (0.42 ± 0.02
mg/g extract) (Table 3). One of the bioactive
substances in LM was hericene A, which had an IC
50
value of 6.7 M and significantly reduced glucosidase
activity (Lee at al., 2020). However, the content of
hericenone C in both Reg-LM and Ir-LM were not
significantly different. Ergosterol, hericenone C, and
hericene A in the fresh Ir-LM fruiting bodies were
found at 0.49 ± 0.03 mg/g fresh LM, 0.11 ± 0.01 mg/g
fresh LM, and 0.02 ± 0.01 mg/g fresh LM,
respectively.
Table 2: Ergosterol content in the extracts prepared from
regular-shape (Reg-LM) and irregular-shape (Ir-LM)
Lion’s Mane mushroom.
Extract
Ergosterol
mg/g extract
mg/g dried
LM
mg/g fresh
LM
Reg-
LM
19.42
±0.66
2.15
±0.08
0.41
±0.02
Ir-LM
26.77
±1.20
2.52
±0.13
0.49
±0.02
Notes: Different superscripts in the same column mean
significant difference at p < 0.05.
Table 3: Concentration of the hericenone C and hericene A in regular-shape (Reg-LM) and irregular-shape (Ir-LM) Lion’s
Mane mushroom.
Extract
Hericenone C
*
Hericene A
*
mg/g extract
mg/g dried
LM
mg/g fresh
LM
mg/g extract
mg/g dried
LM
mg/g fresh LM
Reg-LM
5.40 ± 0.18
0.60 ± 0.02
0.12 ± 0.01
0.42 ± 0.02
0.05 ± 0.01
0.01 ± 0.01
Ir-LM
5.79 ± 0.36
1.25 ± 0.08
0.11 ± 0.01
0.55 ± 0.04
0.12 ± 0.01
0.02 ± 0.01
Notes: Different superscripts in the same column mean significant difference at p < 0.05.
*
The content of hericenone C and
hericene A were calculated from their peak area compared with that area of ergosterol.
3.3 Antioxidant Properties
Reg-LM and Ir-LM extracts were tested for their
capacity to inhibit the DPPH radical, one of the few
stable organic nitrogen radicals that presents purple
color. This test relies on the determination of DPPH
•
loss upon sample response. Additionally, the
antioxidant activity in the ABTS experiment was
Ergosterol Content and Antioxidant Activity of Lion’s Mane Mushroom (Hericium erinaceus) and Its Induction to Vitamin D2 by
UVC-Irradiation
23
calculated as the capacity of the extract to reduce
color when they have direct contact with the radical
ABTS
•+
(Rivero-Cruz et al., 2020). DPPH and ABTS
scavenging ability for Ir-LM extract were 0.27 ± 0.01
mg TE/g dried LM and 0.52 ± 0.04 mg TE/g dried
LM, respectively as presented in Table 4. These
antioxidant activities of Ir-LM were significantly
higher (p < 0.05) than Reg-LM extract which
presented 0.20 ± 0.01 mg TE/g dried LM and 0.46 ±
0.01 mg TE/g dried LM of DPPH and ABTS
scavenging ability, respectively. The antioxidant
concentration required needed to reduce the initial
radical concentration by 50% is known as IC
50
, which
is a factor commonly used to evaluate antioxidant
activity (Rivero-Cruz et al., 2020). The IC
50
determined by DPPH (85.28 mg/ml), and ABTS
scavenging ability (164.84 mg/ml) of Reg-LM
extracts were higher than those of Ir-LM extract,
67.03 mg/ml and 151.27 mg/ml, respectively. In
conclusion, Ir-LM exhibited higher antioxidant
activities in terms of DPPH and ABTS than Reg-LM.
The reasons that Ir-LM expressed significantly higher
antioxidant capacity might be because it contained
higher phenolic content (0.11 ± 0.02 mg GAE/g dried
LM) than that of Reg-LM (0.06 ± 0.01mg GAE/g
dried LM). Phenolic acids are important compounds
contributing to antioxidant activity due to OH groups
that can scavenge free radicals are present in their
structures (Heleno et al., 2012).
(1) Ergosterol and vitamin D
2
standard
(2) Bioactive compounds extracted from LM by Maceration (3) Ergosterol and vitamin D
2
before and after UVC irradiation
Figure 3: HPLC chromatograms of ergosterol and vitamin D
2
standard (A), crude ethanolic extract of regular-shape Lion’s
Mane mushroom (B), irregular-shape Lion’s Mane mushroom (C), non-irradiated Ir-LM extract (D), and Ir-LM extract
irradiated with UV-C (E).
Moreover, the higher ergosterol content in Ir-
LM (Table 2) might involve with its antioxidant
capacity. Dupont et al. (2021) described that the B-
ring of ergosterol has two double bonds, which may
A
B
C
D
E
Ergosterol
Vitamin D
Hericenone C
Hericene A
Ergosterol
Ergosterol
Vitamin D
Hericenone C
Hericene A
Ergosterol
Ergosterol
Standard
Reg-LM
extract
Ir-LM
extract
Non-irradiated
Ir-LM extract
Irradiated
Ir-LM
extract
No peak of
Vitamin D
ABS 2022 - The International Conference on Agricultural and Biological Sciences
24
have antioxidant effects. Shao et al. (2010) also
observed that, ergosterol was primarily responsible
for the antioxidant activity in the lipophilic fraction
of button mushroom. Therefore, Ir-LM was selected
to be used for ultraviolet C (UVC) irradiation to
convert ergosterol to vitamin D
2
in the extract.
3.4 Induction of Vitamin D
2
by UVC
Irradiation
The most abundant sterol in cell membranes of fungi
is ergosterol. It is critical for preserving permeability,
trafficking, fluidity, and cytoskeletal structure (Abe
and Hiraki, 2009). The transformation of ergosterol to
vitamin D
2
can be obtained by the use of UV
radiation, either artificially or naturally (Jäpelt and
Jakobsen, 2013). Ergosterol undergoes
photochemical cleavage at the B ring upon exposure
to UV radiation, resulting in the synthesis of pre-
vitamin D
2
, an intermediate of vitamin D
2
. After
being heated, this intermediate then goes through
thermal isomerization to produce vitamin D
2
. The
equilibrium between thermal and photochemical
processes is crucial for the production of vitamin D
2
(Jasinghe et al., 2007).
The UV radiation for food processing and
preservation is effective and favorable for the
environment (Singh et al., 2021). Food irradiation is
a technology that is secure and effective. The flavor
of the product, taste, and odor, is unaffected by the
radiation, and neither are the residues or poisons
generated in the process (Bisht et al., 2021).
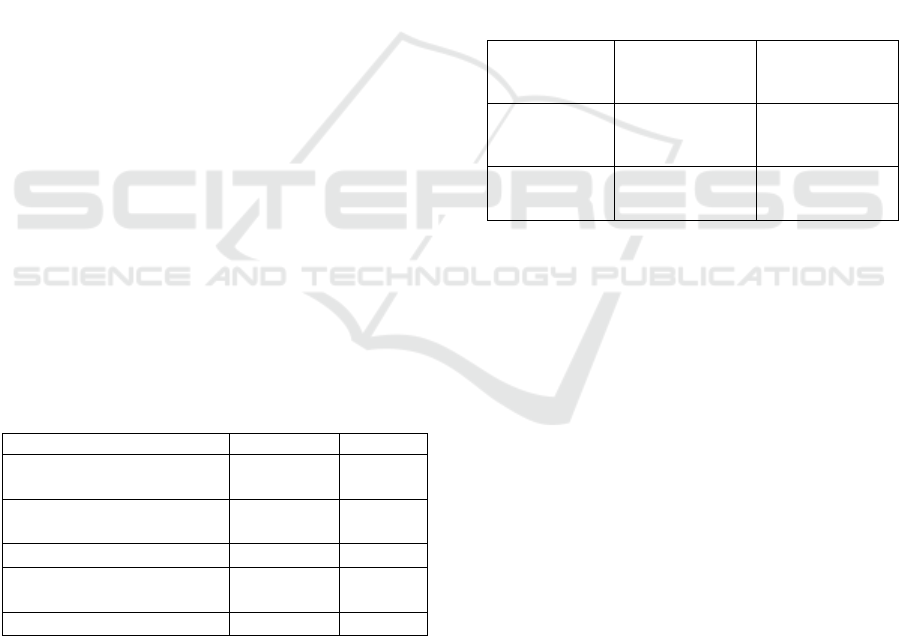
Table 4: Radical scavenging ability and total phenolic
content in the extracts from the fruiting bodies of regular-
shape (Reg-LM) and irregular-shape (Ir-LM) Lion’s Mane
mushroom.
Re
g
-LM I
r
-LM
Total phenolic content
(mg GAE/g dried LM)
0.06
±0.01
0.11
±0.02
DPPH radical scavenging
ability (mg TE /g dried LM)
0.20
±0.01
0.27
±0.01
IC
(mg/ml) by DPPH
85.28 67.03
ABTS radical scavenging
ability (mg TE /g dried LM)
0.46
±0.01
0.52
±0.04
IC
(mg/ml) by ABTS
164.84 151.27
Notes: Different superscripts in the same row mean
significant difference at p < 0.05. IC
50
is the concentration
of antioxidants required to decrease the initial radical
concentration by 50%; DPPH: 2,2-diphenyl-1-
picrylhydrazyl, ABTS: 3-ethylbenzthiazoline-6-sulphonic
acid.
Vitamin D (D
2
, or D
3
, or both) ingested in the
human body is incorporated into chylomicrons, which
are absorbed by the lymphatic system and penetrated
into venous blood. Vitamin D
2
cannot be
biosynthesized by human body. Most oil-rich fish
including the oil fish from salmon contains high
content of vitamin D
3
. Vitamin D sufficiency can
enhance the absorption of calcium and phosphorus by
30-40% and 80%, respectively (Nair & Maseeh,
2012). According to the World Health Organization,
low vitamin D intake, both in the form of D
2
and D
3
,
causes bone problems and raises the risk of other
chronic diseases (Holick & Chen, 2008). Ergosterol
was detected in the non-irradiated extract at 1.74 ±
0.09 mg/g dried LM, but no vitamin D
2
was found.
Interestingly, a decrease of ergosterol at 13.5% with
a detection of vitamin D
2
at 30.01 ± 7.09 µg/g dried
LM were found in the irradiated extract (Table 5).
Table 5: Ergosterol and vitamin D
2
content in the extracts
of non-irradiated and irradiated Lion’s Mane mushroom
with UV-C at 5 cm distance from UV-C lamp, for 2 h.
Samples
Ergosterol
ns
(mg/g dried
LM)
Vitamin D
(µg/g dried LM)
Non-irradiated
extract
(Control)
1.74 ± 0.09
nd
Irradiated
extract
1.51 ± 0.15
30.01 ±7.09
Notes: ns: no significant difference at p < 0.05.
Different superscripts in the same column mean significant
difference at p < 0.05. nd: not detected
In addition, a decrease of ergosterol content was
observed in the irradiated extract. Ergosterol might be
transformed into other derivatives of vitamin D such
as lumisterol or tachysterol (Morales et al., 2017).
There are very few studies on the process of directly
exposing mushroom extract to UVC to convert
ergosterol to vitamin D
2
. Morales et al. (2017)
revealed that UVC irradiation to Shiitake mushroom
extracts in ethanol at 25°C, for 1 h, at 4 cm distance
from the lamp could generate vitamin D
2
enriched
extracts higher than the irradiation of direct fruiting
body. Generally, vitamin D
2
was induced by direct
UV-light irradiation to fresh fruiting bodies or dried
mushroom powder. Xu et al. (2020) reported that
after being exposed to a high level of UVC (4 kJ/m
2
)
for 40 minutes, vitamin D
2
content of ground shiitake
and Jew's ear powder increased from 1.38 g/g to 20.11
g/g and 4.13 g/g to 39.93 g/g, respectively.
With a UVB lamp at 25 °C, for 2 hours, and 19
cm away from the lamp, Huang et al. (2015) were able
to irradiate oyster mushrooms and obtain 69 g/g
vitamin D. In addition, Wittig et al. (2013) exposed
Ergosterol Content and Antioxidant Activity of Lion’s Mane Mushroom (Hericium erinaceus) and Its Induction to Vitamin D2 by
UVC-Irradiation
25

the same mushrooms to UVB radiation at 20 and 30
°C with a 10 cm distance from the light and found that
after only 10 minutes of exposure, a higher vitamin D
content (80 g/g) was obtained. These studies present
that the mushrooms placed more closely to the UV
lamp could generate a higher amount of vitamin D.
Currently, several industrial mushroom farms in
the United States, Ireland, the Netherlands, and
Australia have exposed their fresh mushrooms to UV
light, producing at least 10 µg of vitamin D per 100 g
of fresh weight. Therefore, a 100 g serving of the
mushroom can satisfy 50 to 100% of a person's daily
requirement for vitamin D. Additionally, UV-light-
exposed dry mushrooms can also create sufficient
content of vitamin D
2
for nutritional purposes
(Cardwell et al., 2018).
4 CONCLUSIONS
Irregular-shape Lion’s Mane (Ir-LM), mushroom
considered as by-products, was used in the
experiment to investigate the effect of UVC
irradiation on the conversion of ergosterol in the
mushroom extract to vitamin D
2
. Ergosterol,
hericenone C, hericene A, total phenolic content and
antioxidant activities of Ir-LM were found
significantly higher (p < 0.05) than those of Reg-LM.
Irradiation with a low dose of UVC (145 µW/cm
2
) for
120 minutes at 5 cm distance from the lamp caused
the detection of vitamin D
2
in the irradiated extracts,
but no detection in the non-irradiated sample. This
research provides possible methods for a conversion
to vitamin D
2
enriched extracts from mushroom by-
products and use as nutritional supplement in
medicinal foods. It is necessary to conduct additional
research on the negative effects of UVC irradiation on
the antioxidant activity, physical characteristics, and
other significant nutritional parameters of irradiated
mushroom extracts. The study on the effect of
individual environmental factors on the induction of
Ir-LM fruiting bodies are required.
ACKNOWLEDGEMENTS
This research was funded by King Mongkut’s
University of Technology North Bangkok and
National Science and Technology Development
Agency, Thailand (Contract no. 024/2563). This
research was also partially supported by NRCT
Senior Research Scholar Program (Contract No.814-
2020). Special thanks go to Mr. Wuttipong
Ruksavong, Managing Director, Fresh and Friendly
Farm Co., Ltd., for his kind supports during the
cultivation of the Lion’s Mane mushrooms.
REFERENCES
Abe, F., and Hiraki, T. (2009). Mechanistic role of
ergosterol in membrane rigidity and cycloheximide
resistance in Saccharomyces cerevisiae. Biochimica et
Biophysica Acta (BBA)-Biomembranes, 1788:743-752.
Aguayo, I. A., Walton, J., Vinas, I. and Tiwarii, B. K.,
(2017). Ultrasound assisted extraction of
polysaccharides from mushroom by-products. LWT -
Food Science and Technology, 77:92e99.
Ahmed, A. S., Elgorashi, E. E., Moodley, N., McGaw, L.
J., Naidoo, V. and Eloff, J. N. (2012). The
antimicrobial, antioxidative, anti-inflammatory activity
and cytotoxicity of different fractions of four South
African Bauhinia species used traditionally to treat
diarrhoea, Journal of Ethnopharmacology, 143:826-
839.
Baars, J. J. P., Scholtmeijer, K., Sonnenberg, A. S. M. and
Peer, A. V. (2020). Critical factors involved in
primordia building in agaricus bisporus: a review.
Molecules, 25:2984.
Bellettini, M. B., Assump¸ F., Fiorda, A., Maieves, H. A.,
Teixeira, G. L., A´vila, S., Hornung, P. S., Ju´nior, A.
M. and Ribani, R. H. (2019). Factors affecting
mushroom Pleurotus spp. Saudi Journal of Biological
Sciences, 26:633-646.
Binheam, F., Thumthanaruk, B., Vatanyoopaisarn, S.,
Rungsardthong, V. and Poodchakarn, S. (2022).
Antioxidant capacity of the bamboo mushroom
(Dictyophora indusiata) and the effects of temperature
and relative humidity on its growth. In IAMBEST 2022,
3rd International Conference on Informatics,
Agriculture, Management, Business administration,
Engineering, Science and Technology.
Bisht, B., Bhatnagar, P., Gururani, P., Kumar, V., Tomarf,
M. S., Sinhmar, R., Rathi, N. and Kumar, S. (2021).
Food irradiation: effect of ionizing and non-ionizing
radiations on preservation of fruits and vegetables-a
review. Trends in Food Science & Technology, 114:
372-385.
Cardwell, G., Bornman, J. F. James, A.P., and Black L.J.
(2018). A review of mushrooms as a potential source of
dietary vitamin D. Nutrients, 10: 1498.
doi:10.3390/nu10101498
Corrêa, R. C. G., Peralta, R. M., Bracht, A. and Ferreira, I.
C. F. R. (2017). The emerging use of mycosterols in
food industry along with the current trend of extended
use of bioactive phytosterols. Trends in Food Science
& Technology, 67:19-35.
Dawson-Hughes, B., Mithal, A., Bonjour, J. P., Boonen, S.,
Burckhardt, P., Fuleihan, G. E. H., Josse, R. G., Lips,
P., Morales-Torres, J. L. A. and Yoshimura, N. (2010).
IOF position statement: vitamin D recommendations
ABS 2022 - The International Conference on Agricultural and Biological Sciences
26

for older adults. Osteoporosis International, 21: 1151-
1154.
Dupont, S., Fleurat-Lessard, P., Cruz, R.G, Lafarge, C.,
Grangeteau, C., Yahou, F., Gerbeau-Pissot, P., Júnior,
O.A., Gervais, P., Simon-Plas, F., Cayot, P. and Beney,
L. (2021). Antioxidant properties of ergosterol and its
role in yeast resistance to oxidation. Antioxidants, 10:
1024.
Durrant, L. R., Bucca, G., Hesketh, A., Möller-Levet, C.,
Tripkovic, L., Wu, H., Hart, K. H., Mathers, J. C.,
Elliott, R. M., Lanham-New, S. A. and Smith, C. P.
(2022). Vitamins D
2
and D
3
have overlapping but
different effects on the human immune system revealed
through analysis of the blood transcriptome. Frontiers
in Immunology, 13:790444.
Eastwood, D. C., Herman, B., Noble, R., Dobrovin-
Pennington, A., Sreenivasaprasad, S. and Burton, K. S.
(2013). Environmental regulation of reproductive phase
change in Agaricus bisporus by 1-octen-3-ol,
temperature and CO
2
. Fungal Genetics and Biology,
55:54-66.
Friedman, M. (2015). Chemistry, nutrition, and health-
promoting properties of hericium erinaceus (lion's
mane) mushroom fruiting bodies and mycelia and their
bioactive compounds. Journal of Agricultural and
Food Chemistry, 63(32):7108-7123.
Gasecka, M., Siwulski, M., Magdziak, Z., Budzyn´ska, S.,
Stuper-Szablewska, K., Niedzielski, P. and Mleczek,
M. (2020). The effect of drying temperature on
bioactive compounds and antioxidant activity of
Leccinum scabrum (Bull.) Gray and Hericium
erinaceus (Bull.) Journal of Food Science and
Technology, 57(2):513-525.
Heleno, S. A., Barros, L., Martins, A., Queiroz, M. J. R. P.,
Santos-Buelga, C. and Ferreira, I. C. F. R. (2012).
Phenolic, polysaccharidic, and lipidic fractions of
mushrooms from North eastern Portugal: chemical
compounds with antioxidant properties. Journal of
Agricultural and Food Chemistry, 60:4634-4640.
Heleno, S. A., Prieto, M. A., Barros, L., Rodrigues, A.A.,
Barreiro, M.F. and Ferreira, I.C.F.R. (2016).
Optimization of microwave-assisted extraction of
ergosterol from Agaricus bisporus L. by-products using
response surface methodology. Food and Bioproducts
Processing, 100:25-35.
Holick, M. F. and Chen, T. C. (2008). Vitamin D
deficiency: A worldwide problem with health
consequences. The American Journal of Clinical
Nutrition,87:1080-1086.
Huang, S. J., Lin, C. P. and Tsai, S. Y. (2015). Vitamin D
2
content and antioxidant properties of fruit body and
mycelia of edible mushrooms by UV-B irradiation.
Journal of Food Composition and Analysis, 42:38-45.
Jäpelt, R. B. and Jakobsen, J. (2013). Vitamin D in plants:
A review of occurrence, analysis, and biosynthesis.
Frontiers of Plant Science, 4:136-136.
Jasinghe, V. J., Perera, C. O. and Sablani, S. S. (2007).
Kinetics of the conversion of ergosterol in edible
mushrooms. Journal of Food Engineering, 79(3):864-
869.
Joradon, P., Rungsardthong, V., Ruktanonchai, U.,
Suttisintong, K., Iempridee, T., Thumthanaruk, B.,
Vatanyoopaisarn, S. and Uttapap, D. (2022). A
comparative study of conventional and supercritical
carbon dioxide extraction methods for the recovery of
bioactive compounds from Lion’s Mane mushroom
(Hericium erinaceus). In RI
2
C 2022, International
Conference on Research, Invention, and Innovation
Congress.
Kang, J. H., Jang, J. E., Mishra, S. K., Lee, H. J., Nho, C.
W., Shin, D., Jin, M., Kim, M. K., Choi, C. and Oh, S.
H. (2015). Ergosterol peroxide from Chaga mushroom
(Inonotus obliquus) exhibits anti-cancer activity by
down-regulation of the β-catenin pathway in colorectal
cancer. Journal of Ethnopharmacology, 173:303-312.
Khan, M. A., Tania, M., Liu, R. and Rahman, M. M. (2013).
Hericium erinaceus: an edible mushroom with
medicinal values. Journal of Complementary and
Integrative Medicine, 24(10).
Lee, S.K., Ryu, S.H., Turk, A., Yeon, S.W., Jo, Y.H., Han,
Y.K., Hwang, B.Y., Lee, K.Y. and Lee, M.K. (2020).
Characterization of α-glucosidase inhibitory
constituents of the fruiting body of lion’s Mane
mushroom (Hericium erinaceus), Journal of
Ethnopharmacology, 262:113197.
Lu, Q. Q., Tian, J. M., Wei, J. and Gao, J. M. (2014).
Bioactive metabolites from the mycelia of the
basidiomycete Hericium erinaceum. Natural Product
Research, 28:288-1292.
Morales, D., Gil-Ramirez, A., Smiderle, F. R., Piris, A. J.,
Ruiz-Rodriguez, A. and Rivasa, C. (2017). Vitamin D-
enriched extracts obtained from shiitake mushrooms
(Lentinula edodes) by supercritical fluid extraction and
UV-irradiation. Innovative Food Science and Emerging
Technologies, 41:330-336.
Nair, R. and Maseeh, A. (2012). Vitamin D: The “sunshine”
vitamin. Journal of Pharmacology and
Pharmacotherapeutics, 3(2).
Rodsuwan, U., Pithanthanakul, U., Thisayakorn, K.,
Uttapap, D., Boonpisuttinant, K., Vatanyoopaisarn, S.,
Thumthanaruk, B. and Rungsardthong, B. (2020).
Preparation and characterization of gamma oryzanol
loaded zein nanoparticles and its improved stability.
Food Science and Nutrition, 9(2):616-624.
Rosa, A., Maxia, A., Putzu, D., Atzeri, A., Era, B., Fais, A.,
Sanna, C. and Piras, A. (2017). Chemical composition
of Lycium europaeum fruit oil obtained by supercritical
CO
2
extraction and evaluation of its antioxidant
activity, cytotoxicity and cell absorption. Food
Chemistry, 230:82-9.
Rivero-Cruz, J. F., Granados-Pineda, J., Pedraza-Chaverri,
J., Pérez-Rojas, J. M., Kumar-Passari, A., Diaz-Ruiz,
G. and Rivero-Cruz, B. E. (2020). Phytochemical
Constituents, antioxidant, cytotoxic, and antimicrobial
activities of the ethanolic extract of Mexican Brown
propolis. Antioxidants, 9(70).
Rungsardthong, V., Pithanthanakul, U., Puttanlek, C.,
Uttapap, D. and Boonpisuttinant, K. (2021).
Preparation of puerarin-loaded zein nanoparticles:
Ergosterol Content and Antioxidant Activity of Lion’s Mane Mushroom (Hericium erinaceus) and Its Induction to Vitamin D2 by
UVC-Irradiation
27

characterization and stability study. Journal of Current
Science and Technology, 11(1):60-70.
Shao, S., Hernandez, M., Kramer, J. K., Rinker, D. L. and
Tsao, R. (2010). Ergosterol profiles, fatty acid
composition, and antioxidant activities of button
mushrooms as affected by tissue part and
developmental stage. Journal of Agricultural and Food
Chemistry, 58:11616-11625.
Shen, J. W., Yu, H. Y., Ruan, Y., Wu, T. T. and Zhao, X.
(2010). Hericenones and erinacines: stimulators of
nerve growth factor (NGF) biosynthesis in Hericium
erinaceus. Mycology An International Journal on
Fungal Biology, 1:92-98.
Singh, H., Bhardwaj, S. K., Khatri, M., Kim, K. H. and
Bhardwaj, N. (2021). UVC radiation for food safety:
An emerging technology for the microbial disinfection
of food products. Chemical Engineering Journal, 417:
128084.
Suwannasang, S., Thumthanaruk, B., Zhong, Q., Uttapap,
D., Puttanlek, C., Vatanyoopaisarn, S. and
Rungsardthong, V. (2021). The improved properties of
zein encapsulating and stabilizing Sacha Inchi oil by
surfactant combination of lecithin and Tween 80. Food
and Bioprocess Technology, 14(11):2078-2090.
Suwannasang, S., Zhong, Q., Thumthanaruk, B.,
Vatanyoopaisarn, S., Uttapap, D., Puttanlek, C. and
Rungsardthong, V. (2022a). Physicochemical
properties of yogurt fortified with microencapsulated
Sacha Inchi oil. LWT, 61:113375.
Suwannasang, S., Zhong, Q., Thumthanaruk, B.,
Vatanyoopaisarn, S., Uttapap, D., Puttanlek, C. and
Rungsardthong, V. (2022b). Optimization of wall
material composition for production of spray-dried
Sacha Inchi oil microcapsules with desirable
physicochemical properties. Food and Bioprocess
Technology. (Major revision)
Tachabenjarong, N., Rungsardthong, V., Ruktanonchi, U.,
Suttisintong, K., lempridee, T., Thumthanaruk, B.,
Vatanyoopaisam, S., Poodchakarn, S. and Uttapap, D.
(2022). Bioactive compound and antioxidant activity of
Lion’s mane mushroom (Hericium erinaceus) from
different growth period. In RI
2
C 2022, International
Conference on Research, Invention, and Innovation
Congress.
Thongbai, B., Rapior, S., Hyde, K. D., Wittstein, K. and
Stadler, M. (2015). Hericium erinaceus, an amazing
medicinal mushroom. Mycological Progress, 14(91).
Visscher, H. R. (1979). Fructification of Agaricus bisporus
(Lge) Imb. in relation to the relevant microflora in the
casing soil. Mushroom Science, 10:631-663.
Urben, A. F. (2004). Produc¸ao de cogumelos por meio de
tecnologia chinesa modificada. Embrapa Recursos
Gene´ticose Biotecnologia, Brasılia (in Portuguese).
Wang, M., Gao, Y., Xu, D. and Gao, Q. (2015). A
polysaccharide from cultured mycelium of Hericium
erinaceus and its anti-chronic atrophic gastritis activity.
International Journal of Biological Macromolecules,
81:656-661.
Wittig, M., Krings, U. and Berger, R. G. (2013). Single-run
analysis of vitamin D photoproducts in oyster
mushroom (Pleurotus ostreatus) after UV-B treatment.
Journal of Food Composition and Analysis, 31:266-
274.
Xu, Z., Meenu, M. and Xu, B. (2020). Effects of UV-C
treatment and ultrafine-grinding on the
biotransformation of ergosterol to vitamin D
2
,
physiochemical properties, and antioxidant properties
of shiitake and Jew’s ear. Food Chemistry, 309:125738.
Yang, W., Guo, F., Wan, Z., and Saudi, J. (2013). Yield and
size of oyster mushroom grown on rice/wheat straw
basal substrate supplemented with cotton seed hull.
Journal of Biological Sciences, 20:333-338.
Zhang, C., Huang, T., Shen, C., Wang, X., Qi, Y., Shen, J.,
Song, A., Qiu, L. and Ai, Y. (2016). Downregulation of
ethylene production increases mycelial growth and
primordia formation in the button culinary-medicinal
mushroom, Agaricus bisporus (agaricomycetes).
International Journal of Medicinal Mushrooms, 18:
1131-1140.
ABS 2022 - The International Conference on Agricultural and Biological Sciences
28
