
Innovative Technologies for Detecting Methane in the Atmosphere
R. F. Mustafin
1,2
, M. R. Bogdanov
3
and R. R. Khisamov
1
1
Bashkir State Agrarian University, Ufa, Russia
2
Ufa State Petroleum Technical University, Ufa, Russia
3
Bashkir State Pedagogical University Named After M. Akmulla, Ufa, Russia
Keywords: Extreme environmental conditions, habitat, sustainable architecture, physical and climatic parameters.
Abstract: The article is devoted to the actual problem of the formation of sustainable architectural objects for extreme
living conditions, caused by physical and climatic parameters. It has been established that globalization, the
acceleration of the pace of scientific and technological progress, the growth of the global population and the
increasing pressure on the environment caused by these factors lead architects, engineers and researchers to
the need to quickly respond to changing conditions and form a favorable artificial environment in extreme
environments, which are determined by physico-climatic, anthropogenic physical and socio-economic param-
eters. The purpose of the study is to identify the principles of the formation of architectural objects in extreme
conditions in the context of international architectural and engineering trends. The methodological approach
to the study of this issue is based on system analysis and is based on the materials of implemented and de-
signed buildings and structures, as well as the study of open scientific research. The materials of the article
can be used for the theory and practice of the formation of an artificial environment for extreme living condi-
tions.
1 INTRODUCTION
It is impossible to imagine the life of modern society
without achievements in the field of analytical
chemistry. Accurate and timely analysis of various
compounds is required in the fuel industry,
agriculture, pharmaceuticals, food industry and many
other industries. Chemical analysis of gaseous
substances acquires a special role and importance. In
the gas industry enterprises, air cleanliness and gas
leaks in pipelines are constantly monitored, engineers
talk about “hydrogen energy”, experts in the field of
transport are developing environmentally friendly
fuel for rockets, airplanes and cars based on methane.
2 SCIENTIFIC AND LITERARY
REVIEW
The problem of determining the composition of the
gas mixture becomes particularly relevant in the light
of testing hypotheses about climate change and
natural gas leaks during production and
transportation.
Climate change research
Questions about global warming have been the
subject of scientific discussions for a number of years.
According to some estimates (Lal, 2008; Schrader,
1995; Degler, 2015; Elger, 2019; Kohl, 1989; Stuart,
2005; Mizaikoff, 2013; Wilk, 2012; Glöckler, 2020),
global surface temperatures have increased by about
0.88°C since the end of the 19th century.
Terrestrial ecosystems have been a source of
atmospheric carbon dioxide since the dawn of
agriculture, and methane since the domestication of
cattle and the cultivation of rice fields. Increasing the
concentration of carbon dioxide in the atmosphere
enhances the effect of fertilizer. The restoration of
degraded ecosystems and the combination of carbon
cycles with nitrogen and phosphorus increases the
carbon stock in terrestrial ecosystems.
Based on the above, it can be concluded that the
validity of climate forecasts depends on the accuracy
of measuring the concentration of carbon dioxide and
methane.
Natural gas leaks
Methane is the main component of natural gas.
Methane emissions can be the result of equipment
malfunctions such as pipe cracking or leaky pipe
Mustafin, R., Bogdanov, M. and Khisamov, R.
Innovative Technologies for Detecting Methane in the Atmosphere.
DOI: 10.5220/0011569000003524
In Proceedings of the 1st International Conference on Methods, Models, Technologies for Sustainable Development (MMTGE 2022) - Agroclimatic Projects and Carbon Neutrality, pages
227-233
ISBN: 978-989-758-608-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
227

connections, emission methods such as flaring, or
accidental emissions during normal transportation,
storage and distribution operations (Glöckler, 2020).
In addition, methane emissions from wells can be
caused by loss of integrity of natural gas due to
defective sealed casing pipes, cement wear in
boreholes or transverse migration along neighboring
geological formations (Fortes, 2014). Methane
released as a result of leakage through wells and
equipment accounts for 8.5% of greenhouse gas
emissions in Canada (Fortes, 2014; Dosi, 2019).
Methane emissions from the global transportation of
liquefied natural gas account for more than 5% of the
total 932 million tons of CO
2
equivalent. Average
methane emissions from oil and gas wells in
Pennsylvania reach 55,600 tons per year. In
northeastern British Columbia, where shale gas
basins are located, 75,000 metric tons of methane
were released per year.
3 MATERIALS AND METHODS
Methane (CH
4
), a combustible gas that has neither
smell nor color, is the main component of natural gas
(Aldhafeeri, 2020). It is used as a fuel worldwide as a
source for electricity generation and heating and plays
a significant role in climate change. Methane is a
powerful greenhouse gas (GHG) with a global
warming potential 28 times higher than that of carbon
dioxide (CO
2
) over a 100-year period (Aldhafeeri,
2020). Since the beginning of the industrial
revolution, the concentration of methane in the
atmosphere has increased dramatically from about
800 parts per billion (ppb) in the early 1900s to more
than 1800 parts per billion in 2016. This increase can
be explained, first of all, by the following
anthropogenic sources of emissions: landfills,
livestock waste, coal mining, petrochemical
production, as well as oil and gas distribution and
production facilities (Aldhafeeri, 2020). In addition,
methane ignites and can be explosive if its
concentration reaches 5-15% indoors (Aldhafeeri,
2020). Despite its negative impact on the
environment, natural gas is valued for its abundance
and clean combustion process, and therefore it will be
widely used in the future (Aldhafeeri, 2020). It
replaces coal due to lower CO
2
emissions in the
process of combustion and lower production cost
(Stuart, 2005). Moreover, it is predicted that in the
future natural gas will become the second most used
source of energy (Aldhafeeri, 2020).
Since the use of natural gas in the future is
inevitable, it is necessary to develop solutions to
reduce methane emissions.
To detect leaks in the natural gas infrastructure, it
is necessary to develop reliable and cost-effective
methane detector sensors. This will allow pollutants
and law enforcement agencies to detect and eliminate
leaks in a timely manner. Therefore, many methane
sensors have been developed, each of which has its
own technology. Sensors based on different physical
principles are used to detect methane, for example:
optical sensors, capacitive sensors, calorimetric
sensors, resonance sensors, acoustic sensors,
pyroelectric sensors, semiconductor sensors based on
metal oxide (MOx), electrochemical sensors.
The purpose of the proposed work is to describe
some new approaches in the field of methane
detection. The advantages and disadvantages of these
approaches, as well as various scenarios for their
application, will be considered.
4 OPTICAL SENSORS
Optical gas sensors detect changes in electromagnetic
waves resulting from the interaction of the analyte
with the receptor (Aldhafeeri, 2020). The most
common instrument for determining methane is
infrared (IR) absorption spectroscopy, based on the
fact that methane gas has two strong absorption lines
- 2.3 and 3.26 micrometers (mid-IR range) and two
weak absorption lines – 1.33 and 1.6 micrometers
(near-IR range).
There are several approaches aimed at improving
optical methods for detecting methane. Yang et al.
(Aldhafeeri, 2020) increased the sensitivity of an
optical methane sensor with a long-period fiber
grating (LPFG) using a polycarbonate/cryptophane
overlay with a high refractive index. The change in
the thickness of the overlay led to a shift in the
resonant wavelength, and at the optimal thickness, a
significant increase in the sensitivity of the sensor
was observed. Dong et al. improved the sensor based
on the Fabry-Perot cavity (FPC) resonator to improve
the accuracy of gas measurement. The technique
consisted in recording the transmission maxima of the
resonator modes by scanning the resonator
wavelengths at each laser frequency. It was found that
the FPC sensor with integrated new technology was
able to achieve a methane detection sensitivity of 0.7–
2.9 parts per million by mass (ppm-m). Zhang et al.
developed a fiber-optic methane sensor based on
graphene-doped tin oxide. The sensor was
manufactured by coating optical fibers with lateral
MMTGE 2022 - I International Conference "Methods, models, technologies for sustainable development: agroclimatic projects and carbon
neutrality", Kadyrov Chechen State University Chechen Republic, Grozny, st. Sher
228

polishing with thin films of tin oxide doped with
graphene, while the light source operating in the
visible region was tuned to a wavelength of 1550 nm.
An interesting implementation of the optical
method of methane detection is the combination of IR
spectroscopy and remote chemical sensing. Yutaka
and his collaborators (Matsumi, 2016) have
developed a method for measuring methane by the
open optical path method by detecting the second
harmonic, using a near-IR diode laser for IR
absorption spectroscopy. During field measurements
in rice fields in India, the laser beam is returned by a
reflector located tens of meters from the device and
detected by a photodetector in the device (Figure 1).
The measurement error at a distance of 50 m was 2%.
Figure 1: A field system for measuring methane
concentration (CH
4
) using a LaserMethane miniG detector
(LMm) and a solar power source (Matsumi, 2016).
5 CALORIMETRIC SENSORS
Calorimetric sensors are used to detect methane and
other combustible gases in coal mines, drilling rigs,
oil refineries, and landfills (Aldhafeeri, 2020). There
are three types of calorimetric sensors: a catalytic gas
sensor, an adsorbent-based gas sensor, and a gas
thermal conductivity sensor. A calorimetric gas
sensor often consists of a temperature meter, a
catalytic combustion chamber and a heating element.
The principle of operation of a calorimetric sensor is
based on the fact that a chemical reaction or a physical
adsorption process absorbs or releases heat. The most
important part of the calorimetric sensor is the
material that interacts with the gas. The surface layer
is often used as a combustion reaction catalyst to
reduce the combustion temperature. Platinum (Pt),
palladium (Pd) and rhodium (Rh) are the most
commonly used catalysts in calorimetric gas sensors.
Conventional calorimetric sensors use a catalytic coil
made of platinum or palladium, also known as a
pellistor. For methane, gas oxidation in contact with a
catalyst is an exothermic reaction with the release of
heat. This leads to a change in the temperature of the
catalytic surface due to a chemical reaction, which is
used by calorimetric sensors to obtain a useful signal.
Calorimetric gas sensors are simple, cheap and
convenient to use. These sensors are affected by
temperature, pressure and humidity, however, they
are sensitive to methane and other hydrocarbons.
Purely calorimetric methane sensors have a
measurement error of 5%. Calibration using gas with
a known concentration is proposed to increase
accuracy.
6 PYROELECTRIC SENSORS
Pyroelectric sensors register electromagnetic
radiation at a certain wavelength range. They convert
electromagnetic or thermal energy into electricity.
They are non-contact thermometers operating at room
temperature. In the sensor, the dielectric is located
between two electrodes. The advantages of the
pyroelectric methane sensor include its ability to
work without oxygen, good sensitivity and a wide
operating range. These sensors can operate at room
temperature. In addition, the pyroelectric effect is a
thermal process in which no chemical reactions are
involved, so the risk of degradation of the sensor is
reduced. However, pyroelectric sensors are expensive
and require a high-energy power source and a
constant source of heat or infrared radiation, which
makes them unsuitable for many applications. In
addition, they are difficult to manufacture, since a thin
pyroelectric element must be fixed on a supporting
base.
Dong et al. have developed a multi-gas sensor
system that uses one broadband light source and
several pyroelectric sensors for carbon monoxide,
carbon dioxide and methane using time-division
multiplexing (TDM) technology. A rotating system
based on a stepper motor and a spherical optical
Innovative Technologies for Detecting Methane in the Atmosphere
229

mirror with a single reflection have been developed
and integrated to improve the detection of multiple
gases. Experimentally, it was determined that the
detection limit of methane is 2.84 ppm.
7 SENSORS BASED ON METAL
OXIDES MOX
Sensors based on metal oxides are attracting more and
more attention of analytical specialists (Glöckler,
2020). The principle of operation of such devices is
based on the reactions of transformation of target
molecules on their semiconductor surface. Johannes
and his collaborators (Glöckler, 2020) combined two
optical sensing methods - luminescence quenching
for molecular oxygen and infrared spectroscopy for
carbon dioxide and methane to study the behavior of
a sample of a semiconductor MOx sensor of methane
integrated into a small volume gas cell. As a result of
the experiments, it became possible to quantitatively
control oxygen consumption, as well as the formation
of carbon dioxide as a result of the methane
conversion reaction during the operation of the MOx
sensor. The latter was analyzed using a gas analyzer
in the mid-infrared range, based on the technology of
a hollow waveguide integrated into the substrate
(substrate-integrated hollow waveguide, iHWG), in
combination with a portable infrared spectrometer
with Fourier transform, which can not only determine
the amount of carbon dioxide released, but also the
consumption of methane during the operation of a
MOx sensor. This approach made it possible to
quantify organic compounds (CH
4
) in real time in
traces. The use of chemical-resistant gas sensors
based on semiconductor metal oxides makes it
possible to detect explosive gases such as propane
and toxic gases such as carbon monoxide or nitrogen
dioxide, to detect gas leaks in atmospheric conditions
and identify volatile organic compounds. This
approach can be used in agriculture, automotive
industry, indoor air quality control and monitoring of
gases in the environment. The disadvantages of MOx
sensors include limited selectivity, the advantages are
small area, fast response and cost-effectiveness
compared to traditional analytical methods, such as
gas chromatography combined with mass
spectrometry (GC-MS) and infrared Fourier
transform spectroscopy (FTIR) using bulky multi-
pass gas cells.
MOx sensors consist of a substrate equipped with
electrodes (for example, ceramic Al
2
O
3
), which is
covered with a sensitive layer. The electrodes allow
analyzing changes in the conductivity of the sensitive
layer. In addition, resistive heaters are integrated into
the sensors, which are electrically separated by an
insulating shield from the sensor, which later allows
heating the measuring electrode in the range of 200-
400 °C. Heating of the sensor layer increases the
sensitivity of MOx sensors due to the higher
conductivity of the semiconductor and faster
adsorption/desorption of target particles on/off the
surface.
Studies of SnO
2
surfaces have shown that
methane oxidation occurs during several intermediate
stages compared to acetate before complete oxidation
to water and carbon dioxide. Acetaldehyde could be
detected both on the surface and in the gas phase.
However, these reactions do not proceed in the same
way on all SnO
2
surfaces.
The surface structure and alloying impurities
along with the layer thickness play an important role
in surface processes. Temperature probably has a big
influence on the type of absorption and other surface
reactions that occur. The initial oxygen adsorption is
largely determined by the surface temperature of the
MOx sensor. In addition, humidity plays a significant
role and worsens the characteristics of the device. For
example, deoxidization of SnO
2
in a humid
environment directly correlates with the formation of
surface hydroxyl groups. The target molecules also
react with the oxygen of the crystal lattice, which
makes it difficult to study the change in resistance
depending on the oxygen concentration.
The registered MOx sensor signal indicates a
change in the resistance of the sensitive layer, which
is caused by the oxidation of methane by previous
adsorbed oxygen forms on the surface by a rather
complex mechanism, eventually with the formation
of carbon dioxide (CO
2
) and water (H
2
O). The
resulting carbon dioxide, together with the remaining
methane, can be detected using infrared spectroscopy.
As a detector, the TGS2611-C00 sensor is often
used - a semiconductor thick-film sensor consisting
of tin oxide SnO
2
, designed to detect flammable
gases in the air. According to the manufacturer's
passport, the TGS2611 sensor has a high sensitivity
to methane, propane and butane with a similar
sensitivity. These properties are determined by the
characteristics of the SnO
2
surface and the presence
of impurities acting as catalysts. The thermistor was
made of RuO
2
, the lead wires were made of Pt - W
alloy, and the connections to the sensor substrate were
Ni - Fe contacts (50%). SnO
2
is an n-type wide-band
semiconductor. The advantages of SnO
2
include high
sensitivity and resistance to a reducing atmosphere,
MMTGE 2022 - I International Conference "Methods, models, technologies for sustainable development: agroclimatic projects and carbon
neutrality", Kadyrov Chechen State University Chechen Republic, Grozny, st. Sher
230
and its disadvantages are low selectivity and
dependence on humidity.
Figure 2 shows a sensor circuit based on SnO
2
.
The MOx sensor allows simultaneous real-time
measurements of methane, carbon dioxide and
oxygen using the principles of orthogonal direct
optical sensing - infrared radiation and luminescence.
The detection limit of methane is 41 ppm (parts per
million).
8 ELECTROCHEMICAL
METHANE SENSORS BASED
ON LASER-INDUCED
GRAPHENE WITH A SOLID
POLYMER ELECTROLYTE
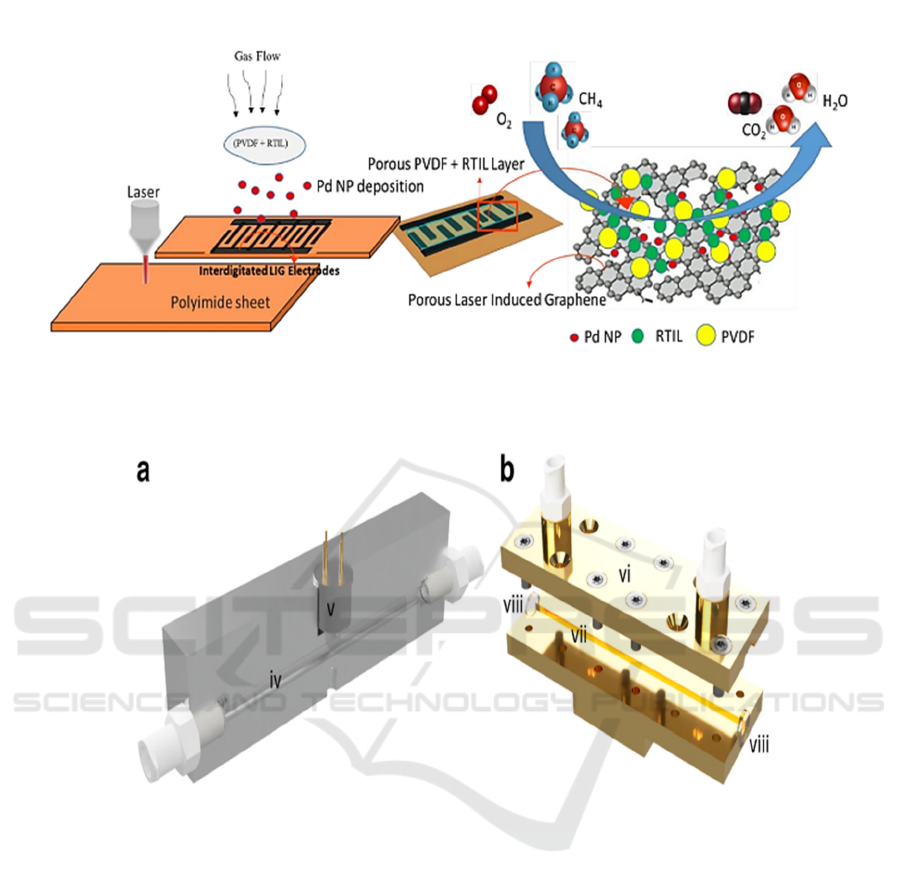
Manan Dosi and his collaborators (Dosi, 2019) have
developed an electrochemical gas sensor that allows
detecting methane at room temperature for 40
seconds in concentrations of less than one part per
million. The sensor was manufactured as follows.
Porous electrodes made of laser-induced graphene
(LIG) form a pattern in polymer films and are
impregnated with a dispersion of palladium
Figure 2: MOx sensor gas cell. a - an aluminum block with a gas channel (iv) and a MOx sensor (v), b - an open
iHWG with an upper substrate (vi), a base substrate (vii), windows from BaF
2
(viii) (Glöckler, 2020).
Fi
g
ure 3. Dia
g
ram of the manufacturin
g
p
rocess of an electrochemical methane sensor.
Innovative Technologies for Detecting Methane in the Atmosphere
231

nanoparticles to distribute the electrocatalyst inside a
carrier with a large surface area. An ionic liquid in a
pseudo-solid state / polyvinylidene fluoride
electrolyte is applied to a flexible element to form a
porous electrolyte inside a porous electrode made of
laser-induced graphene, which simultaneously
promotes rapid gas transfer and electrooxidation of
methane at room temperature (Figure 3). The gas
analyzer thus obtained is an amperometric sensor. Its
ability to detect methane is tested in the presence of
moisture and interfering gases.
A CO
2
laser is used to transform a polyamide
sheet into a patterned graphene structure. Pd
nanoparticles are absorbed into the structure of laser-
induced graphene (LIG) with a large surface area,
after which a porous layer of polyvinylidene fluoride
(PVDF) / ionic liquids operating at room temperature
(room temperature ionic liquids, RTIL) is applied to
the electrodes. This provides a large number of three-
phase contacts between gas, Pd and RTIL, which
ensures high sensitivity and fast response (Dosi,
2019).
The described device is a flexible flat device of a
small area (~ 2 cm
2
), the voltage consumed is 0.6 V,
the current consumed is 1.1 µA, the methane
detection limit is ~ 9 ppm.
For a long time, most analytical instruments were
cumbersome, expensive and time-consuming to
maintain. However, recent advances in materials
science, microelectronics, communications and data
analysis make it possible to implement several very
interesting scenarios. Let's look at some of them.
Wireless electrochemical platform based on RFID
technology. Radislav Poteryailo from General
Electric has proposed an interesting solution designed
for the analysis of multicomponent mixtures, having
a small size, low price, high selectivity, sensitivity at
the level of ppm units and does not require energy
(Potyrailo, 2012). The device works as follows. As
you know, passive tags with radio frequency
identification consist of an antenna, a microprocessor,
a transmitter and a memory on which service
information is recorded. Separately, I would like to
note the fact that such devices do not need power
supply. For the first time, this concept was
implemented by Lev Semenovich Termen, who
developed radio microphones for the NKVD in the
30s. The energy is taken from an external source of
electromagnetic radiation, which induces induction
EMF on the antenna. At the same time, the capacitor
is charged. Its charge is enough to operate the
transmitter. Radislav Poteryailo opened the passive
RFID tag (Figure 4) and placed the nafion material on
the antenna surface.
Figure 4: The multi-gas sensor is located on an American
one-penny coin.
https://www.ge.com/research/newsroom/ge-researchers-
demonstrate-grain-size-gas-sensor-bloodhound-sensing-
capabilities-ideal
9 RESULTS AND DISCUSSION
The development of materials science,
communications, nanotechnology, and data analysis
makes it possible to implement very interesting
scenarios in the development and application of
chemical sensors for detecting methane in the
atmosphere. The wireless electrochemical platform
has already been mentioned above. To control the
ecological environment, a wireless sensor network
can be deployed. A number of companies are
developing chemical sensors that can be embedded in
cell phones, flexible and stretchable sensors are being
developed, work is underway in the field of "sensitive
skin" for cyborgs, advances in
micro/nanoelectromechanics are designed to create
an "electronic nose".
10 CONCLUSIONS
Methane plays a very important role in the life of
society. First of all, this concerns the fuel and energy
complex. Thermal power plants running on natural
gas are much more environmentally friendly than
thermal power plants running on fuel oil or coal. In
Russia, the transition of trucks and commercial
vehicles to methane is a reasonable alternative to
electric vehicles. Methane is the most important
greenhouse gas, which makes it relevant to research
in the field of anthropogenic impact of methane.
Timely and accurate detection of methane
emissions into the atmosphere is an urgent problem.
MMTGE 2022 - I International Conference "Methods, models, technologies for sustainable development: agroclimatic projects and carbon
neutrality", Kadyrov Chechen State University Chechen Republic, Grozny, st. Sher
232

This is important both for fire safety and for reducing
losses in the fuel and oil and gas industry, protecting
the environment and preserving the climate balance.
There are classical methods for detecting
methane, such as gas chromatography or mass
spectrometry. Recently, optical, colorimetric, and
pyroelectric methods for detecting methane have also
become widespread. Sensors based on metal oxides
and electrochemical methane sensors based on laser-
induced graphene with a solid polymer electrolyte are
being developed (Blair, 1991). Such methods will
make it possible in the future to create fairly compact,
inexpensive and sensitive methane sensors. There are
new scenarios for detecting methane, for example,
remotely. The team of authors, together with
colleagues from related industries, is ready to conduct
scientific research with the above-mentioned high-
tech equipment and devices.
REFERENCES
Lal, R., 2008. Carbon sequestration. Phil. Trans. R. Soc. B.
363. pp. 815-830.
Schrader, B., Bougeard, D., 1995. Infrared and Raman
spectroscopy: methods and applications. VCH.
Degler, D., Wicker, S., Weimar, U., Barsan, N., 2015.
Identifying the active oxygen species in SnO2 based
gas sensing materials: an operando IR spectrsocopy
study. J Phys Chem C. 119. pp. 11792-11799.
Elger, A. K., Hess, C., 2019. Elucidating the mechanism of
working SnO2 gas sensors using combined operando
UV/Vis, Raman, and IR spectroscopy. Angew Chem Int
Ed. 58. pp. 15057-15061.
Kohl, D., 1989. Surface processes in the detection of
reducing gases with SnO2-based devices. Sensors
Actuators. 18. pp. 71-113.
Stuart, B. H, 2005. Infrared spectroscopy: fundamentals
and applications.
Mizaikoff, B., 2013. Waveguide-enhanced mid-infrared
chem/bio sensors. Chem Soc Rev. 42. p. 8683.
Wilk, A., Seichter, F., Kim, S. S., Tütüncü, E., Mizaikoff,
B., Vogt J. A. et al., 2012. Toward the quantification of
the 13CO2/12CO2 ratio in exhaled mouse breath with
mid-infrared hollow waveguide gas sensors. Anal
Bioanal Chem. 402. pp. 397-404.
Glöckler, J., Jaeschke, C., Tütüncü, E., Kokoric, V.,
Kocaöz, Y., Mizaikoff, B., 2020. Characterization of
metal oxide gas sensors via optical techniques.
Analytical and Bioanalytical Chemistry. 412, pp. 4575-
4584.
Fortes, P. R., da Silveira Petruci, J. F., Wilk, A., Cardoso, A.
A., Raimundo, I. M. Jr., Mizaikoff, B., 2014. Optimized
design of substrate-integrated hollow waveguides for
mid-infrared gas analyzers. J Opt. 16, pp. 1-6.
Dosi, M., Lau, I., Zhuang, Y., Simakov, D. S. A., Fowler,
M. W., Pope, M. A., 2019. Ultra-Sensitive
Electrochemical Methane Sensors based on Solid
Polymer Electrolyte-Infused Laser-Induced Graphene.
ACS Appl. Mater. Interfaces, Just Accepted Manuscript.
Aldhafeeri, T., Tran, M.-K., Vrolyk, R., Pope, M., Fowler,
M., 2020. A Review of Methane Gas Detection Sensors:
Recent Developments and Future Perspectives.
Inventions. 5. 28.
Matsumi, Y., Hidemori, T., Nakayama, T., Imasu, R.,
Dhaka, S. K., 2016. Measuring methane with a simple
open-path gas sensor A near-IR laser spectroscopic
technique enables continuous in situ measurements of
methane emissions in rural northern India.
https://spie.org/news/6283-measuring-methane-with-a-
simple-open-path-gas-sensor.
Potyrailo, R. A., Surman, C., Lai, H., Kelley-Loughnane,
N., Naik, R. R., 2012. Wireless sensors and sensor
networks for homeland security applications. Trends
Analyt Chem. 40. pp. 133-145.
Potyrailo, R. A., Bonam, R. K., Hartley, J. G., Starkey, T.
A., Vukusic, P., Vasudev, M., Bunning, T., Naik, R. R.,
Tang, Z., Palacios, M. A., Larsen, M., Le Tarte, L. A.,
Grande, J. C., Zhong S., Deng, T., Towards
outperforming conventional sensor arrays with
fabricated individual photonic vapour sensors inspired
by Morpho butterflies.
Joshi, G. P., Nam, S. Y., Kim, S. W., 2013. Cognitive Radio
Wireless Sensor Networks: Applications, Challenges
and Research Trends. Sensors, 13, pp. 11196-11228.
Blair, J. M., Crossley, D. A., Callaham, L. C., 1991. A
litterbasket technique for measurement of nutrient
dynamics in forest floors. Agriculture, Ecosystems and
Environment, 34. pp. 465-471.
Innovative Technologies for Detecting Methane in the Atmosphere
233
