
Designing RNA Sequences by Self-play
Stephen Obonyo
1 a
, Nicolas Jouandeau
1 b
and Dickson Owuor
2 c
1
LIASD, Paris 8 University, Paris, France
2
School of Computing and Enfineering Sciences, Strathmore University, Nairobi, Kenya
Keywords:
Self-playing, RNA Inverse Folding, Reinforcement Learning, Computational Biology.
Abstract:
Self-play (SP) is a method in Reinforcement Learning (RL) where an agent learns from the environment by
playing against itself until the policy and value functions converge. The SP-based methods have recorded
state-of-the-art results in playing different computer games such as Chess, Go and Othello. In this paper, we
show how the RNA sequence design problem where a sequence is designed to match a given target structure
can be modelled through the SP while performing the state-value evaluation using a deep value network. Our
model dubbed RNASP recorded the best and very competitive results on the benchmark RNA design datasets.
This work also motivates the application of the self-play to other Computational Biology problems.
1 INTRODUCTION
Ribonucleic Acid (RNA) plays a critical role in bio-
logical processes such as protein synthesis and gene
expression. The design of RNA sequences which
folds to match a given target structure is often referred
to as RNA Inverse Folding. The RNA Inverse Fold-
ing is key to both in vitro and in vivo research. It
also has the potential to positively impact disciplines
such as synthetic biology, design of functional RNA
molecules, biotechnology, drug design and medicine
in general.
An RNA molecule is composed of four unique
bases; Guanine (G), Cytosine (C), Adenine (A) and
Uracil (U). The RNA primary structure is constituted
by a sequence of these bases. The molecular forces
exhibited by bases enforce the base pairing between
the A-U and G-C (the Watson–Crick base pairs).
While the latter base pairings are the most common,
G-U also tends to form base pairs in certain RNA lo-
cations such as junctions and pseudoknots.
The RNA sequence design is contingent on a pre-
defined target structure e.g. the designed sequence
must fold according to the given structural target. Fig-
ure 1 (a) shows an example of the target structure. It
is denoted using the Dot-bracket notation where an
opening and closing parenthesis represents the base
a
https://orcid.org/0000-0002-6878-7802
b
https://orcid.org/0000-0001-6902-4324
c
https://orcid.org/0000-0002-0968-5742
pairing locations which are assigned A-U, G-C, G-U
or their reverse while the dots represent single base
locations which are assigned A, U, C or G.
Following the assignment step, the designed se-
quence (Figure 1 (b)) is then folded to obtain the pre-
dicted structure which is then compared with the tar-
get structure for a match. See Figure 1 for the distinc-
tion between target structure, designed sequence and
predicted structure.
The quality of the designed RNA sequence can
be determined through different methods; (i) Ham-
ming loss (ii) Minimum Free Energy (MFE) and (iii)
the GC content. The Hamming loss is calculated be-
tween the predicted structure and target structure. The
Hamming loss between predicted structure and tar-
get sequence can be calculated using the formulae
1 − accuracy
loss. In the example shown in Figure
1, the Hamming loss is 0 since the target structure -
(((((••••••))))) - is the same as the predicted struc-
ture - (((((• •• • ••))))).
The Minimum Free Energy (MFE) determines the
stability of the sequence which affects its functional
effectiveness. Molecules with lower MFEs tend to be
more stable (Trotta, 2014). The designed sequence
must have the right proportion of G’s and C’s as
this also affects the functional effectiveness of the
molecule (Risso et al., 2011). The GC content is
calculated by Equation 1 as a proportion of G and
C values to that of the total bases contained in the
sequence. A value within the range of 50 and 60
is generally preferred. The designed sequence (CU-
Obonyo, S., Jouandeau, N. and Owuor, D.
Designing RNA Sequences by Self-play.
DOI: 10.5220/0011550300003332
In Proceedings of the 14th International Joint Conference on Computational Intelligence (IJCCI 2022), pages 305-312
ISBN: 978-989-758-611-8; ISSN: 2184-2825
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
305
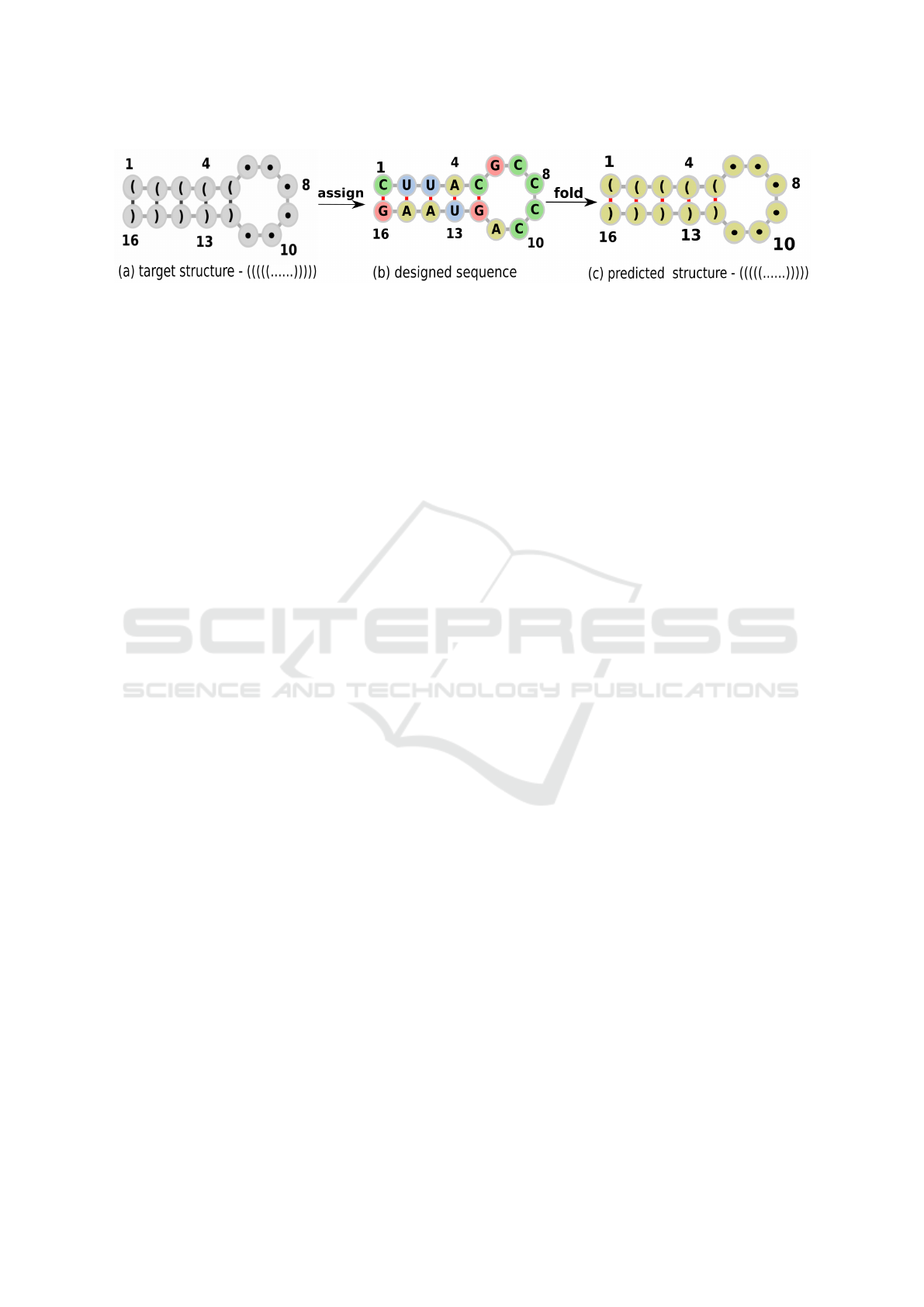
Figure 1: Target structure (a). The locations are marked by numbers 1-16 for convenience. Locations (1, 16), (2, 15), (3,
14),(4, 13) and (5, 12) are paired - opening and closing parentheses - thus can be simultaneously assigned any value from
{GC, CG, AU, UA, GU, UG}. Similarly, locations 6 - 11 are single sites - dots - and can be assigned any value from {A, U,
C, G}. In the designed sequence (b) location (1, 16) is paired hence assigned C and G simultaneously, similarly, location (3,
14) is assigned U and A respectively. The same concept applies to positions (2, 15), (3, 14), (4, 13) and (5, 12). In contrast,
locations 6 - 11 are not paired and thus have been assigned value from {A, U, C, G}. The designed sequence is folded by
Zuker and Stiegler (1981) resulting in a predicted structure (c). The Hamming loss between the predicted structure and target
structure, in this case, is 0 thus the designed sequence fold to match the target structure.
UACGCCCCAGUAAG) in Figure 1 for example has
a GC content of 56.23%.
GC = SUM(G,C)/SUM(G,C,A,U) (1)
2 RELATED WORK
Several RNA sequence design methods have been
proposed by different researchers. These models are
heavily influenced by methods spanning different dis-
ciplines such as computer game playing, brute force,
genetic and evolutionary search algorithms, dynamic
programming, constraint satisfaction modelling, opti-
mization, tree search, general AI search and thermo-
dynamics.
Yang et al. (2017) proposed a Monte Carlo Tree
Search (MCTS) which optimized both the GC content
and the Hamming loss. The model also allowed the
user to specify the target GC content value and using
unique substitution rules, it designed RNA sequences
which fold according to the target structure and the
specified GC content value.
Cazenave and Fournier (2020) also proposed an
MCTS-based model which optimized the Hamming
loss and the GC content with a Generalized Nested
Policy Adaptation (GNPA) algorithm.
Apart from the Tree search-based models,
other researchers have also proposed dynamic
programming-based models including the INFO-
RNA (Busch and Backofen, 2006) which was rein-
forced by the local search to improve the candidate
RNA sequences to optimize the Hamming loss be-
tween the target and predicted structure.
The method adopted by INFO-RNA is an exten-
sion of RNAinverse (Hofacker et al., 1994). This is
one of the earliest RNA Inverse Folding models which
has inspired several RNA Inverse Folding models.
RNA sequence design model proposed by (An-
dronescu et al., 2004) dubbed RNA-SSD also focused
on minimizing the Hamming loss while reinforcing
the candidate RNA solutions by a recursive stochastic
local search. The method employed by the RNA-SSD
has stark similarities with Levin et al. (2012) where
the candidate RNA solutions were reinforced through
the global search.
Genetic and Evolutionary algorithms have also
been applied to the RNA sequence design problem.
The AntaRNA model proposed by Kleinkauf et al.
(2015b) is precisely inspired by the Evolutionary al-
gorithm named Ant Colony. In this work, both the
Hamming loss and the GC content were jointly min-
imized as well. The design of the AntaRNA was ex-
tended by Kleinkauf et al. (2015a) on a more compli-
cated RNA design problem; the pseudoknotted struc-
tures.
The evolutionary-based model by Esmaili-Taheri
and Ganjtabesh (2015) varies from the previous ones
as it allowed the user to specify the energy constraints
(the MFE) of the resulting sequence from the RNA
Inverse Folding.
The Constraint satisfaction model, RNAiFOLD,
by Garcia-Martin et al. (2013) employed a unique
candidate solution method allowing for the speci-
fication of the GC content and certain RNA inter-
molecular properties. The ability to specify user-
defined constraints is a property which has been ex-
plored by Modena (Taneda, 2012); a genetic and
multi-objective model which also jointly optimized
both the Hamming loss and the GC content.
Runge et al. (2018) introduced a Proximal Policy
Model (PPO) based model to design RNA sequences
by directly modelling the best single bases or paired
bases to select at any given time. In contrast to learn-
ing the policy or value functions which then inform
the base assignment, a PPO (Schulman et al., 2017)
model can directly learn the best action to select at
NCTA 2022 - 14th International Conference on Neural Computation Theory and Applications
306

any given time. The model was further augmented
by meta-learning and local search methods recording
competitive results on the benchmark datasets.
3 CONTRIBUTION
We propose a self-play RNA sequence design agent
(RNASP) which learns the state-value function
through SP to improve its policy over time. The state-
value evaluation function is represented by a Deep
Neural Network which allows for wider state gener-
alization as the agent explores the environment. Ac-
cording to the best of our knowledge and extensive
related work review, this is the first research which
investigates how the SP concept can be leveraged in
RNA sequence design. The application of the SP mo-
tivates the application of the concept to other Compu-
tational Biology problems which are majorly charac-
terized by the scarce labelled datasets.
4 METHODS
4.1 Self-play
Self-play (SP) can be defined as an ”algorithm that
learns by playing against itself without requiring any
direct supervision (Bai and Jin, 2020). SP has been
widely applied in Reinforcement Learning (RL) for
game playing and general game intelligence to im-
prove agent’s state-action or state-value evaluation
functions.
General self-play logic in computer games is as
illustrated by Algorithm 1. Given a state, the next
best move is selected and then applied to the environ-
ment. When the terminal state is reached, the value of
the terminal state is then assigned to all the observed
states during the episode.
The SP has been successfully applied in computer
games e.g. Alpha Go (Silver et al., 2016) and Al-
pha Zero (Silver et al., 2017). The former and latter
recorded state-of-the-art results in playing computer
games such as Go and Chess. Alpha Go and Alpha
Zero agents both learned through SP, however, the Al-
pha Go learning was reinforced by the expert knowl-
edge moves which guided the move selection process.
Alpha Zero on the other hand learned entirely
through SP without any expert knowledge reinforce-
ment. Its design incorporated a value network for
evaluating the game state constrained within the range
of {1, −1} and a policy network which returned the
optimal move at a given game state. Furthermore, in
Alpha Zero, Monte Carlo Tree Search (MCTS) was
Algorithm 1: Self-play Logic in games. If it is win at the
end of the game then a target of +1 is assigned to all the
observed states otherwise -1. Given the observed states and
targets, the agent can improve its policy or value function
through supervised learning.
1 function selfPlay(state) :
2 X ←
/
0 ;
3 y ←
/
0 ;
4 while not state.terminal( ) do
5 action ← state.select move( ) ;
6 state ← state.make move(action) ;
7 X ← X ∪ state.state value ;
8 for each x ∈ X do
9 if state.win( ) then score ← 1 ;
10 else score ← −1 ;
11 y ← y ∪ score ;
12 return X , y ;
used to improve the policy and bias the exploration
weight value.
Following the success of Alpha Go and Alpha
Zero, the SP concept has since been extended to
general game playing and game intelligence (Berner
et al., 2019; Cazenave et al., 2020).
4.2 RNASP Design
Similar to Alpha Go, the RNASP does not require
any expert knowledge regarding the RNA sequence
design except the pairing rules, however, while Alpha
Zero learned both the policy and state evaluation func-
tions, RNASP only learns the state-value evaluation
function using a Deep Neural Network (also referred
to as the value network).
We model the SPRNA as an RL problem. Gener-
ally any RL problem can be formally described using
five parameters {S,A,R , P ,R, γ}; where S is a finite
set of states, A set of possible actions, P the state
transition model, R the reward and γ the discount fac-
tor (Sutton and Barto, 2018).
If P and R are known then solving the MDP is
tractable through iterative or dynamic programming
methods. Modelling P and R, however, for most RL
problems can be resource-intensive and intractable
leading to the sampling of the environment through
methods such as Monte Carlo (MC) and Temporal
Difference (TD) Learning.
When the number of states is large e.g number
of states corresponding to the number of pixels in an
image or video stream, a Deep Learning model can
be used to approximate P and R. This concept has
been illustrated by Mnih et al. (2013) and Schaul et al.
Designing RNA Sequences by Self-play
307

(2015).The RNASP RL constrains are defined as fol-
lows;
• Action Space (A): Set of valid actions is defined
by Equation 2. If the current move location is
paired the valid actions are also paired or unpaired
otherwise.
A =
(
{GC,CG, AU,UA,GU,U G} paired(move)
{A,U,C,G} otherwise
(2)
• State Space (S ): Every symbol in state value is
coded using the binary code sequences shown in
Table 1 and the resulting values are concatenated.
The state space of sequence L can therefore be de-
fine as
S
|L|
i=1
C
i
where C
i
any binary code of a given
state value symbol and i is the the base/nucleotide
assignment location and |L| the length of the RNA
sequence to be designed.
• Reward (R ): The immediate reward in every
non-terminal state is 0. In the terminal state (all
paired and unpaired locations assigned) the re-
ward is 1 if the Hamming loss between the pre-
dicted and target structure is 0 and −1 otherwise.
This is defined by Equation 3.
R =
(
+1 hamming(target, predicted) = 0
−1 otherwise
(3)
• Transition Function (P ): The value of each valid
action at time step t is predicted by the value net-
work ( f
θ
) shown in Figure 2. It is stochastic (de-
cayed ε-greedy; to encourage exploration) during
training time and deterministic during evaluation
time.
The state value is a sequence of the same length as
the target structure. Initially, the state values are as-
signed characters similar to the target structure. At
any given time step, a one-step look ahead is per-
formed by the value network over all the possible
actions in the set {GC, CG, AU, UA, GU, UG} for
paired locations and {A, U, C, G} otherwise.
Given •(••)• as the target structure, the move lo-
cations is defined by the set {1, (2, 5), 3, 4, 6}. At the
beginning, the state value is •(••)•. Assuming that
in the next time step the move location is (2, 5) and
the value network (Figure 2) returns GC as the best
action then the state value becomes •G • •C•. Simi-
larly, if the move location of the following time step
is 6 and the value network returns A as the best action
then the state value becomes •G • •CA. The process
is repeated until all the possible move locations have
been assigned.
At each time step, the state value can be coded us-
ing the binary encodings shown in Table 1 and the re-
sulting values concatenated to form the input feature
with a single channel. In addition, the observed coded
state values in each step of the episode are saved for
training the value network.
Table 1: Coding Values.
state value character code
known
unpaired
A 0000
G 0001
U 0001
C 0011
known paired
GC 0100
CG 0111
AU 0101
UA 1000
UG 1001
GU 0110
unknowns
unknown paired [( and )] 1010
unknown unpaired [.] 1011
When an episode terminates, the designed se-
quence is folded using Zuker and Stiegler (1981)
which was built as part of the ViennaRNA package
(Lorenz et al., 2011) to obtain the predicted structure.
If the Hamming loss between the predicted structure
and the target structure is 0 then the observed coded
state values are assigned target +1 and −1 otherwise.
The value network shown in Figure 2 is trained by
minimizing the Mean Squared Error (MSE) loss be-
tween the predicted state value and the targets. This
is capture by Equation 4 which shows the computa-
tion over the batches of size N.
The network design is inspired by AlexNet
(Krizhevsky et al., 2012), however, it has important
to note that it has additional layers; BatchNorm (Ioffe
and Szegedy, 2015), Adaptive Pool, Drouput (Srivas-
tava et al., 2014) and Softmax. The value network
was trained at the end of one full pass over the target
RNA batch sequences for 30 epochs with a batch size
of 64 with Adam optimizer (α=0.0001). A summary
of all the the differences has been captures in Table 2.
After multiple iterations, SPRNA eventually starts
to learn the patterns over the state spaces which leads
to correct sequence design. The playout and the state-
evaluation algorithm are shown in Algorithm 2 and
Algorithm 3 respectively.
L =
s
1
N
N
∑
i=1
( f
θ
(s
t
) − y)
2
(4)
NCTA 2022 - 14th International Conference on Neural Computation Theory and Applications
308

s
t
CONV1 1, 64
BN1 64
ReLU1
CONV2 64,128
BN2 128
ReLU2
AD. POOL
FC1+ReLU4 1024,256
FC2+Dropout 256,64
FC3+ReLU6 64,32
FC4 32X1
v
Figure 2: SPRNA value network. It accepts the coded state value (s
t
) as the input and computes the value of that state.
The network is composed of Convolutional Layers (CONV) (LeCun et al., 1998), Rectified Linear Units (ReLU), Batch
Normalization (BN) (Ioffe and Szegedy, 2015), Drouput (with p = 0.5) (Srivastava et al., 2014), Adaptive Pooling (AD.
Pool) and Fully Connected layers (FC). The tanh activation was used in the output layer. CONV 1 is the the first Convolution
while BN2 is the second Batch Normalization. Each layer(s) input/output value(s) have also been shown e.g. in CONV 1
layer the numbers 1,64 corresponds to the input channels and output channels respectively while in layer BN2 the number
128 corresponds to the size of input volume. In FC layers, the numbers corresponds to the size of input and output neurons.
Adaptive Pool allowed playing variable sized state episodes.
Table 2: A comparison between the value network ( f
θ
) and
AlexNet. f
θ
has additional layers; Batch Normalization and
Adaptive Pool. The kernel size over the layers varies be-
tween the two architectures; 2, 4, 4 in the first, second and
third convolutional layers respectively in f
θ
and 11, 5, 3, 3,
3 in AlexNet.
Property f
θ
AlexNet
Batch Norm. 3 0
Adaptive Pool 1 0
Output layer size 1 1000
Input channel 1 3
Convolution layers 3 5
Kernel size 2,4,4 11,5,3,3,3
Fully Connected layers 4 3
Dropout layers 1 2
Learnable parameter layers 10 8
Padding 0 1-2
Output activation tanh sotfmax
4.3 Dataset
Table 3 shows the information related to the dataset
used in this research. The RNASP training environ-
ment was created by Dataset E while the evaluation
environment was created using datasets A, B, C and
D. The training and testing environments were cre-
ated using Gym (Brockman et al., 2016) to ensure the
extensibility of the RNA sequence problem to the al-
ready existing RL algorithms on the platform.
5 EXPERIMENTS
All the experiments we run on the NVIDIA Quadro
T2000 GPU card running for a period of three days.
Algorithm 2: RNASP playout algorithm. The algorithm ac-
cepts list of RNA target structures (D), paired bases (P =
{GC, CG, AU, UA, GU, UG}) and unpaired bases (F =
{A, U, C, G} ). The call to to the value function invokes
the value network to perform the current state evaluation.
The state values are designed following the Gym (Brock-
man et al., 2016) Interface.
1 function RNA playouts(D,P ,F ) :
2 R ←
/
0 ;
3 for seq ∈ D do
4 state ← RNA GymEnv(seq) ;
5 sites ← state.available sites( ) ;
6 sites ← state.shuffle sites( ) ;
7 for each s ∈ sites do
8 if pair(s) then
best ← value(state,P ) ;
9 else best ← value(state,F ) ;
10 state ← state.apply move(best) ;
11 R ← R ∪ {state} ;
12 return R ;
Algorithm 3: The state evaluation function. The network
accepts coded state s
t
and returns the ε-greedy best action
a ∈ P or a ∈ F .
1 function value(state,A) :
2 v ←
/
0 ;
3 for each a ∈ A do
4 state ← state.apply move(a) ;
5 input ← state.code( ) ;
6 v(state,a) ← f
θ
(input) ;
7 state ← state.undo move(a) ;
8 return εgreedy(v) ;
Designing RNA Sequences by Self-play
309

Table 3: Dataset Summary. Dataset E was used in training
the model while A-D used in validation. The Length entry
is average value calculated over all the sequences.
Dataset Size Source Length
A 63 Kleinkauf et al. (2015b) 100.86
B 83 Kleinkauf et al. (2015b) 105.63
C 29 Taneda (2012) 191.87
D 100 Runge et al. (2018) 247.87
E 65K Runge et al. (2018) 243.70
Table 4: Comparative Analysis on validation datasets.
Data Model MFE GC Correct
A
RNAInverse -27.47 48.20 40/63
incaRNAfbinv -35.05 62.50 2/63
Modena -49.35 50.52 27/63
RNAiFold -53.70 49.76 48/63
RNASP (Ours) -60.35 54.62 50/63
B
RNAInverse -29.02 48.23 47/83
incaRNAfbinv -34.47 46.67 3/83
Modena -49.90 51.00 37/83
RNAiFold -53.30 49.15 72/83
RNASP (Ours) -62.19 54.55 67/83
C
RNAInverse -26.08 48.00 20/29
incaRNAfbinv 0.00 0.00 0/29
Modena -59.64 52.09 11/29
RNAiFold -57.47 45.89 18/29
RNASP(Ours) -57.47 51.82 20/29
D
RNAInverse -28.56 51.31 79/100
incaRNAfbinv -19.70 57.50 60/100
Modena -47.48 48.40 75/100
RNAiFold -52.10 53.76 80/100
RNASP (Ours) -58.90 57.24 86/100
6 RESULTS
The results are shown in Table 4. The GC and MFE
entries are mean values while the Correct entry corre-
sponds to instances where the Hamming loss between
the predicted and target structure was 0. A compar-
ative analysis of the mean running time (in seconds)
was also carried out. The results are shown in Table
5, Table 6, Table 7 and Table 8.
In the time comparison Tables, given a target
structure, each model was assigned a maximum of 60
seconds to design the RNA sequence. If the sequence
could not be solved within this time constraint, its
running time is marked by −.
Table 5: Time on A.
Model Time
RNAInverse 0.17
incaRNAfbinv -
Modena 9.01
RNAiFold 0.06
RNASP 1.01
Table 6: Time on B.
Model Time
RNAInverse 0.16
incaRNAfbinv -
Modena 9.52
RNAiFold 0.07
RNASP 1.00
Table 7: Time on C.
Model Time
RNAInverse 2.12
incaRNAfbinv -
Modena 14.99
RNAiFold 3.23
RNASP 1.42
Table 8: Time on D.
Model Time
RNAInverse 3.5
incaRNAfbinv -
Modena 41.78
RNAiFold 3.47
RNASP 1.73
7 DISCUSSION
While the existing RNA sequence models are heav-
ily reinforced by user-defined constraints and expert
knowledge such as the distribution of the GC content
and other base pairings constraints, in this work we
have presented a self-play RL agent which designs
RNA sequences which can fold to match a given tar-
get structure.
A Deep Neural Network has been used to model
the state-value function. By performing a one-step
look-ahead over the value network given a valid set
of actions {GC, CG, AU, UA, GU, UG} or {A, U, C,
G} the agent can learn to design stable RNA sequence
with desirable GC content value.
As shown in Table 4, RNASP has recorded com-
petitive yet promising results. It recorded the best
Hamming score on Datasets A and D while ty-
ing the score with RNAInverse on dataset C. While
RNAiFold recorded the best result on dataset B
(72/83), RNASP recorded the second-best result of
67/83. In addition, the RNASP model recorded the
best MFE score on all the datasets (except dataset C)
and registered a mean GC content within the gener-
ally recommended range between 50 and 60.
In relation to the mean time, all the models were
assigned a maximum of 60 seconds to design a se-
quence conforming to the given target structure. The
RNAInverse and RNAiFold models recorded the best
times (in seconds) on datasets A and B. However it is
important to note that RNASP recorded the best time
on datasets C and D.
The average sequence length in datasets A and
B is 100.86 and 105.63 respectively and 191.87 and
NCTA 2022 - 14th International Conference on Neural Computation Theory and Applications
310

243.70 respectively in datasets C and D. We argue
that the RNASP has a desirable mean runtime even
when the sequence size increases. RNASP is ideal for
designing longer sequences within a shorter period.
Modena recorded the longest amount of mean
time while incaRNAfbinv could not solve any se-
quence within the 60 seconds constraint.
8 CONCLUSIONS AND FUTURE
WORK
In this research, we have shown that Self-play can be
used to model an agent which learns to design RNA
sequences which fold to match a given target struc-
ture. By performing state evaluation using a Deep
Neural Network, we have shown that RNASP can
learn to design RNA sequences with desirable energy
and GC content values.
The RNASP recorded the best score on two bench-
mark datasets and the best run time on longer se-
quences. As future research, it would be interesting
to investigate if other encoding scheme or different
value network architecture or novel learning methods
would yield better results.
In addition, extension of Self-play to other real
world problems such as drug design, genetics, protein
folding and protein-protein interaction also remains
an interesting future research endeavor.
9 SUPPLEMENTARY MATERIAL
The code and data used in the research is available at
https://github.com/kobonyo/sprna
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support
of the French Embassy in Kenya and Strathmore Uni-
versity in Nairobi. The two entities facilitated a work-
ing environment which enabled the success of this re-
search work.
REFERENCES
Andronescu, M., Fejes, A. P., Hutter, F., Hoos, H. H., and
Condon, A. (2004). A new algorithm for rna sec-
ondary structure design. Journal of molecular biology,
336(3):607–624.
Bai, Y. and Jin, C. (2020). Provable self-play algorithms
for competitive reinforcement learning. In Interna-
tional conference on machine learning, pages 551–
560. PMLR.
Berner, C., Brockman, G., Chan, B., Cheung, V., Debiak,
P., Dennison, C., Farhi, D., Fischer, Q., Hashme, S.,
Hesse, C., et al. (2019). Dota 2 with large scale deep
reinforcement learning. arXiv preprint 1912.06680.
Brockman, G., Cheung, V., Pettersson, L., Schneider, J.,
Schulman, J., Tang, J., and Zaremba, W. (2016). Ope-
nai gym.
Busch, A. and Backofen, R. (2006). Info-rna a fast approach
to inverse rna folding. Bioinformatics, 22(15):1823–
1831.
Cazenave, T., Chen, Y.-C., Chen, G.-W., Chen, S.-Y., Chiu,
X.-D., Dehos, J., Elsa, M., Gong, Q., Hu, H., Khali-
dov, V., et al. (2020). Polygames: Improved zero
learning. ICGA Journal, pages 1–13.
Cazenave, T. and Fournier, T. (2020). Monte carlo inverse
folding. arXiv preprint 2005.09961.
Esmaili-Taheri, A. and Ganjtabesh, M. (2015). Erd: a fast
and reliable tool for rna design including constraints.
BMC bioinformatics, 16(1):1–11.
Garcia-Martin, J. A., Clote, P., and Dotu, I. (2013).
Rnaifold: a constraint programming algorithm for
rna inverse folding and molecular design. Jour-
nal of bioinformatics and computational biology,
11(02):1350001.
Hofacker, I. L., Fontana, W., Stadler, P. F., Bonhoeffer,
L. S., Tacker, M., and Schuster, P. (1994). Fast folding
and comparison of rna secondary structures. Monat-
shefte f
¨
ur Chemie/Chemical Monthly, 125(2):167–
188.
Ioffe, S. and Szegedy, C. (2015). Batch normalization: Ac-
celerating deep network training by reducing internal
covariate shift. In International conference on ma-
chine learning, pages 448–456. PMLR.
Kleinkauf, R., Houwaart, T., Backofen, R., and Mann, M.
(2015a). antarna–multi-objective inverse folding of
pseudoknot rna using ant-colony optimization. BMC
bioinformatics, 16(1):1–7.
Kleinkauf, R., Mann, M., and Backofen, R. (2015b). an-
tarna: ant colony-based rna sequence design. Bioin-
formatics, 31(19):3114–3121.
Krizhevsky, A., Sutskever, I., and Hinton, G. E. (2012). Im-
agenet classification with deep convolutional neural
networks. Advances in neural information processing
systems, 25:1097–1105.
LeCun, Y., Bottou, L., Bengio, Y., and Haffner, P. (1998).
Gradient-based learning applied to document recogni-
tion. IEEE, 86(11):2278–2324.
Levin, A., Lis, M., Ponty, Y., O’donnell, C. W., Devadas,
S., Berger, B., and Waldisp
¨
uhl, J. (2012). A global
sampling approach to designing and reengineering
rna secondary structures. Nucleic acids research,
40(20):10041–10052.
Lorenz, R., Bernhart, S. H., H
¨
oner zu Siederdissen, C.,
Tafer, H., Flamm, C., Stadler, P. F., and Hofacker, I. L.
(2011). Viennarna package 2.0. Algorithms for molec-
ular biology, 6(1):1–14.
Designing RNA Sequences by Self-play
311

Mnih, V., Kavukcuoglu, K., Silver, D., Graves, A.,
Antonoglou, I., Wierstra, D., and Riedmiller, M.
(2013). Playing atari with deep reinforcement learn-
ing. arXiv preprint 1312.5602.
Risso, D., Schwartz, K., Sherlock, G., and Dudoit, S.
(2011). Gc-content normalization for rna-seq data.
BMC bioinformatics, 12(1):1–17.
Runge, F., Stoll, D., Falkner, S., and Hutter, F.
(2018). Learning to design rna. arXiv preprint
arXiv:1812.11951.
Schaul, T., Quan, J., Antonoglou, I., and Silver, D.
(2015). Prioritized experience replay. arXiv preprint
1511.05952.
Schulman, J., Wolski, F., Dhariwal, P., Radford, A., and
Klimov, O. (2017). Proximal policy optimization al-
gorithms. arXiv preprint arXiv:1707.06347.
Silver, D., Huang, A., Maddison, C. J., Guez, A., Sifre, L.,
Van Den Driessche, G., Schrittwieser, J., Antonoglou,
I., Panneershelvam, V., Lanctot, M., et al. (2016).
Mastering the game of go with deep neural networks
and tree search. nature, 529(7587):484–489.
Silver, D., Schrittwieser, J., Simonyan, K., Antonoglou, I.,
Huang, A., Guez, A., Hubert, T., Baker, L., Lai, M.,
Bolton, A., et al. (2017). Mastering the game of go
without human knowledge. nature, 550(7676):354–
359.
Srivastava, N., Hinton, G., Krizhevsky, A., Sutskever, I.,
and Salakhutdinov, R. (2014). Dropout: a simple way
to prevent neural networks from overfitting. The jour-
nal of machine learning research, 15(1):1929–1958.
Sutton, R. S. and Barto, A. G. (2018). Reinforcement learn-
ing: An introduction. MIT press.
Taneda, A. (2012). Multi-objective genetic algorithm for
pseudoknotted rna sequence design. Frontiers in ge-
netics, 3:36.
Trotta, E. (2014). On the normalization of the minimum
free energy of rna’s by sequence length. PloS one,
9(11):e113380.
Yang, X., Yoshizoe, K., Taneda, A., and Tsuda, K. (2017).
Rna inverse folding using monte carlo tree search.
BMC bioinformatics, 18(1):1–12.
Zuker, M. and Stiegler, P. (1981). Optimal computer
folding of large rna sequences using thermodynam-
ics and auxiliary information. Nucleic acids research,
9(1):133–148.
NCTA 2022 - 14th International Conference on Neural Computation Theory and Applications
312
