
Paroxysmal Atrial Fibrillation Detection by Combined Recurrent
Neural Network and Feature Extraction on ECG Signals
Xinqi Bao
1a
, Fenghe Hu
1
, Yujia Xu
1
, Mohamed Trabelsi
2
and Ernest Kamavuako
1b
1
Department of Engineering, King’s College London, London, U.K.
2
Department of Electronic and Communications Engineering, Kuwait College of Science and Technology, Kuwait
Keywords: Electrocardiogram (ECG), Paroxysmal Atrial Fibrillation (Afib), Recurrent Neural Network (RNN).
Abstract: Paroxysmal atrial fibrillation (AFib) or intermittent atrial fibrillation is one type of atrial fibrillation which
occurs rapidly and stops spontaneously within days. Its episodes can last several seconds, hours, or even days
before returning to normal sinus rhythm. A lack of intervention may lead the paroxysmal into persistent atrial
fibrillation, causing severe risk to human health. However, due to its intermittent characteristics, it is generally
neglected by patients. Therefore, real-time monitoring and accurate automatic algorithms are highly needed
for early screening. This study proposes a two-stage algorithm, including a BiLSTM network to classify
healthy and atrial fibrillation, followed by a feature-extraction-based neural network (NN) to identify the
persistent, paroxysmal atrial fibrillation onsets. The extracted features include the entropy and standard
deviation of the RR intervals. The two steps can achieve 90.14% and 92.56% accuracy in the validation sets
on small segments. This overall algorithm also has the advantage of the low computing load, which shows a
high potential for a portable embedded device.
1 INTRODUCTION
Atrial fibrillation (AFib) is an irregular heartbeat
(arrhythmia) caused by the ectopic impulses in the
atrium. It may lead to blood clots, stroke, and heart
failure, which are severe hidden dangers to human
lives. Furthermore, the AFib is a common issue for
approximately 2% of people younger than 65 and 9%
older than 65 (Kornej et al., 2020). The American
Heart Association guideline (January et al., 2014)
classified Afib into four types: paroxysmal AFib,
persistent AFib, long-standing persistent AFib, and
permanent AFib based on the duration and
recoverability. While in clinics, physicians usually
sort them into paroxysmal and persistent types only.
Paroxysmal AFib episodes can last several seconds,
hours, or even days before returning to normal sinus
rhythm. Lack of intervention may lead the
paroxysmal into persistent AFib, which is
irreversible. Due to the intermittent characteristics of
the paroxysmal AFib, it is generally neglected by
patients before deteriorating into a persistent type. As
a result, the all-cause mortality rate is approximately
a
https://orcid.org/0000-0002-7117-1267
b
https://orcid.org/0000-0001-6846-2090
6.3% on AFib patients (Lee et al, 2018). Therefore, it
is vital to have an algorithm that can work
automatically in the early screening to prevent the
paroxysmal AFib from worsening to persistent AFib
or more severe health issues.
Electrocardiogram (ECG) is the most commonly
used approach in cardiac diagnosis. It represents the
electrical activity of the heart. The whole electrical
process starts with the spontaneous impulse generated
at the Sinoatrial node (SA node), then propagates to
the atrioventricular node (AV node), causing the
squeezing of the atria as represented by the P wave.
Afterwards, the electrical signal is transmitted to the
His bundle and Purkinje fibres, causing the
contraction of the ventricles. The ventricles will be
repolarized and ready for the next heart cycle. The
QRS complex indicates the depolarization, and the T
wave shows the repolarization of the ventricles,
respectively. However, AFib is caused by irregular
fast squeezing of the atria leading the heart walls
quiver, or fibrillate. This phenomenon it is reflected
by disorganized electrical activity (ectopic impulses
instead of SA impulse) in the atrium, so its ECG
Bao, X., Hu, F., Xu, Y., Trabelsi, M. and Kamavuako, E.
Paroxysmal Atrial Fibrillation Detection by Combined Recurrent Neural Network and Feature Extraction on ECG Signals.
DOI: 10.5220/0010987300003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 4: BIOSIGNALS, pages 85-90
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
85
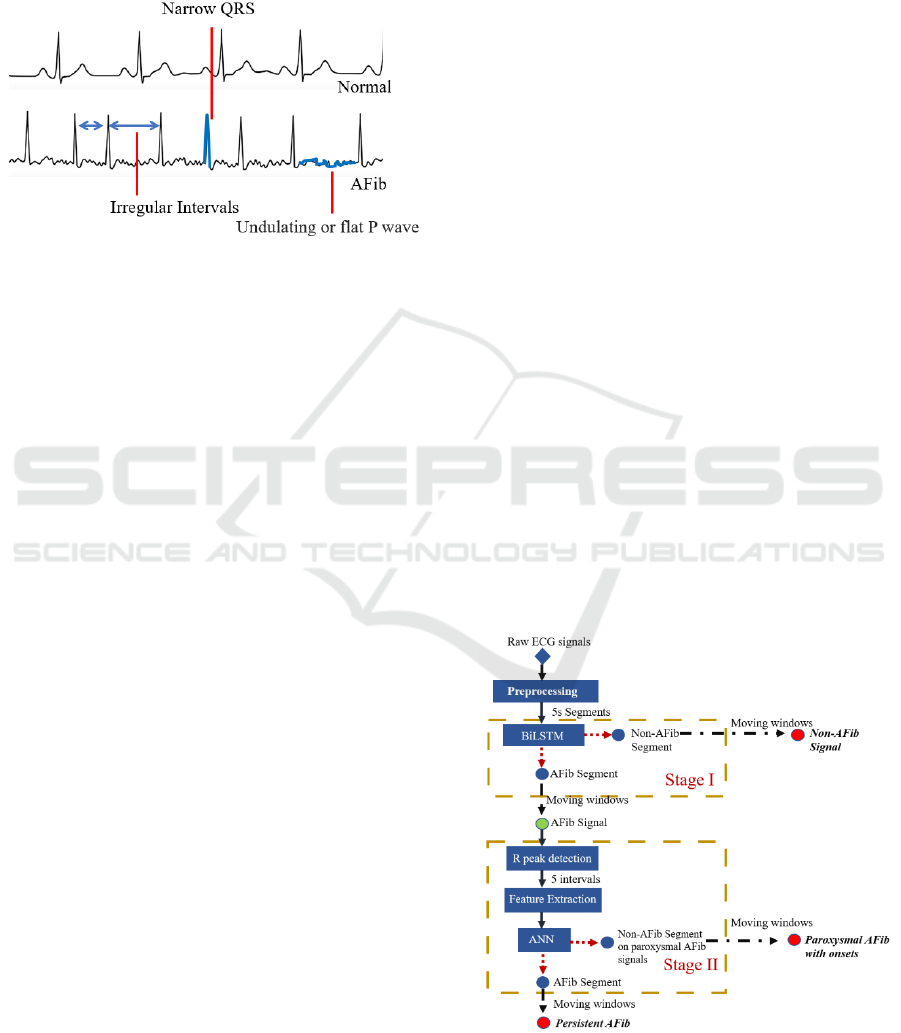
signal differs from normal, as shown in Figure 1.
Morphologically, the AFib ECG has irregular
intervals, a narrow QRS complex, and undulating P
waves. Thus, using ECG signals to identify the AFib
is a practical approach in designing automatic
classification algorithms.
Figure 1: The cardiac cycles of normal and AFib ECG.
Computer-aided algorithms for AFib detection
have been developed for decades, and the proposed
algorithms covered the conventional machine
learning (ML) methods such as support vector
machine (SVM), k-nearest neighbours algorithm
(KNN), random forest, discriminant analysis, etc
(Zhou et al., 2015; De Giovanni et al., 2017; Kalidas
& Tamil,2019; Pourbabaee et al., 2018; Annavarapu
et al., 2016; Rizwan et al., 2020). These conventional
ML approaches relied on manually extracted features
such as average, standard deviation, and entropy of
RR intervals in the time domain (Liu et al., 2018),
power spectral density in the frequency domain, and
statistical features such as kurtosis and skewness
(Rizwan et al., 2020). With the development of deep
learning (DL) in recent years, approaches such as
convolutional neural network (CNN) and recurrent
neural network (RNN) have also been tested on AFib
detection (Xiong et al., 2017; Petmezas et al., 2021;
Ping et al., 2020). They hold the advantage of
neglecting feature extraction and using raw ECG
signals as input and have also achieved promising
performance. Though there are tons of researches
focusing on AFib classification, only few pieces of
research work have focused on paroxysmal AFib
detection due to the lack of suitable databases. As a
result, paroxysmal AFib is often unrecognized
(Michaud & Stevenson, 2021). Therefore, it is pretty
meaningful to explore the capability of the neural
network (NN) in the identification of paroxysmal
AFib.
In this study, the primary aim is to propose an
algorithm that can classify the non-AFib, persistent
AFib, paroxysmal AFib, and their onsets. The
secondary task is to constrain the computing load
while achieving comparable performance, making it
available for a standard laptop or embedded system.
All the findings will provide knowledge on using
NNs to classify paroxysmal AFib and contribute to
designing small-scale portable ECG devices which
can do real-time monitoring of the heart conditions.
2 METHODOLOGY
2.1 Database
The database used in this research was CPSC2021
(Wang et al., 2021). It includes 1436 ECG recordings
(475 Persistent AFib, 229 Paroxysmal AFib, 732
Non-AFib) from 100 subjects (24 Persistent AFib, 23
Paroxysmal AFib, 53 Non-AFib).
2.2 Proposed Algorithm
In this study, a two-stage algorithm was designed to
conduct the detection of paroxysmal AFib and its
onsets. The flowchart of the proposed algorithm is
shown in Figure 2. In Stage I, a Bidirectional Long
short-term memory (BiLSTM) network was used to
classify the ECG segments into Non-AFib and AFib
segments. Then the ECG signals consisting of AFib
segments were transferred to Stage II and classified
into Persistent AFib or Paroxysmal AFib. A moving
window was employed to classify the whole signal
and detect AFib onsets. The processing was
conducted in Matlab® R2021a environment, using a
laptop (CPU: i7-8650U, RAM: 16G, no GPU).
Figure 2: The flowchart of the designed algorithm.
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
86
2.2.1 Pre-processing
Before the two classification stages, the ECG signals
were pre-processed. The raw ECG signals were
normalized (z-score), filtered with 0.5 – 30 Hz
bandpass filter (3
rd
order Butterworth), then
segmented into 5s segments for training (without
overlap). After segmentation, 699040 ECG segments
were generated (421022 Non-AFib, 212098
Persistent, and 65920 Paroxysmal) for training.
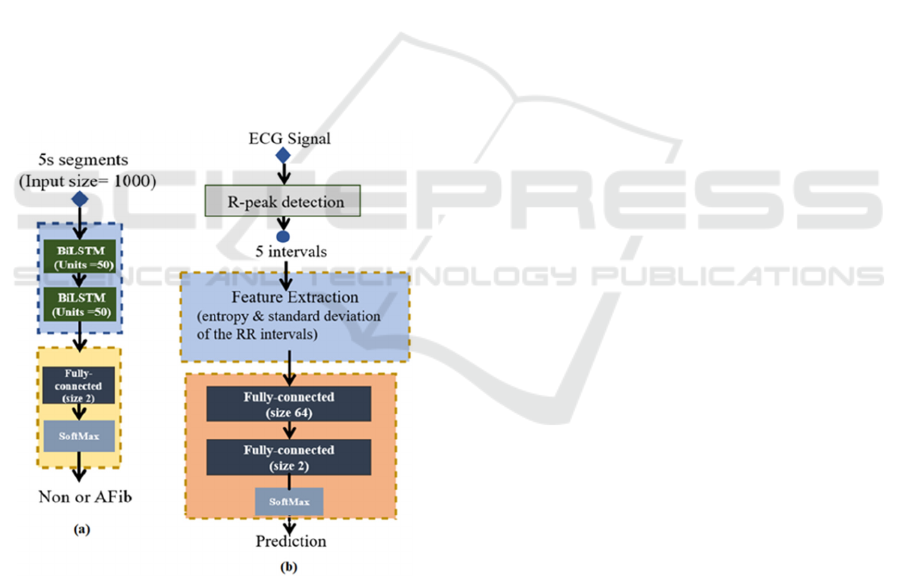
2.2.2 Stage I: BiLSTM
BiLSTM is one type of RNN algorithms that showed
outstanding performance in the sequence data, such
as speech and text recognition (Graves et al., 2005,
Liu & Guo, 2019). In the proposed algorithm, a
simplified structure with two layers of BiLSTM
(hidden units: 50) was applied. The inputs for the
BiLSTM layers were 5s segments. After BiLSTM, it
is connected with a fully connected layer to project
the results into Non-AFib (0) and AFib (1) two
classes. The overall structure of Stage I is shown in
Figure 3(a).
Figure 3: (a) The Stage I structure, (b) The Stage II
structure.
During training, the used training sets included
non-AFib segments labeled (0), persistent AFib
segments labeled (1), while paroxysmal AFib
segments were also labeled (1) to increase the
sensitivity. Training and Validation Proportion was
7:3. 20% recordings (285) were randomly left for the
whole signal testing, including 145 non-AFib, 95
persistent, and 45 paroxysmal recordings. The
optimizer selected in this study was stochastic
gradient descent with momentum (SGDM). The
initial learning rate was 0.001 with a drop factor of
0.2, the max epoch of 10, and the batch size of 256.
The network can identify the non-AFib segments
of the ECG signal. For the complete signal
classification, a moving window (size: 5s, slide: 1s)
was conducted on the signal to classify each segment.
A majority voting was applied to avoid sudden
incorrect classification. Each time frame was covered
by 5 sliding windows, so the time frame is only
labeled AFib when more than 3 windows (segments)
were classified as AFib.
2.2.3 Stage II: Feature Extraction & ANN
In the testing phase, the use of a relatively simplified
DL network (with two layers BiLSTM and three
Conventional layers structure, such as Stage I) didn’t
perform well in the identification of paroxysmal or
persistent AFib. The loss didn’t go down and the
training accuracy remained at 69.15%, which means
the network was uncapable to learn. Deeper and
complex network structure were excluded to avoid
increasing the computation burden. Therefore,
manual features extraction was applied in the
classification stage, where entropy and standard
deviation of RR intervals (which are commonly used
as input features for classification) were selected. The
process of Stage II is shown in Figure 3(b).
R-peaks were extracted by Pan–Tompkins
algorithm (Pan and Tompkins, 1985). Five RR
intervals were clipped as a segment, and the entropy
and standard deviation were extracted from the
segments. Afterward, they were sent to the fully
connected layers to classify into non-AFib or AFib
segments. Similar to Stage I, a moving window (size:
5 intervals, slide: 1 interval) was also applied to
identify the whole signal as persistent or paroxysmal.
The entropy calculation is given by the equation:
E𝑅 =
P
𝑅
log P
𝑅
where E is the entropy of the segment, R
i
indicates
each RR interval length and P is the occurrence
probability.
The training sets were only persistent AFib
labelled (1), and paroxysmal AFib segments were
labelled according to the reference label. Because the
paroxysmal segments were approximately 30% of
persistent segments, and the non-AFib segments of
the paroxysmal are less. Therefore, a moving window
Paroxysmal Atrial Fibrillation Detection by Combined Recurrent Neural Network and Feature Extraction on ECG Signals
87
(size: 5 intervals, slide: 1 interval) was applied to
section more paroxysmal segments to balance the
data structure. The rest training settings were the
same as Stage I.
2.3 Evaluation Metrics
The validation accuracy of the two stages indicates
their capability to identify the small segments (within
windows). The overall performance of the algorithm
can be reflected by the score of the testing recordings.
In this paper, the CSPC2021 Challenge scoring
scheme is considered (Wang et al., 2021).
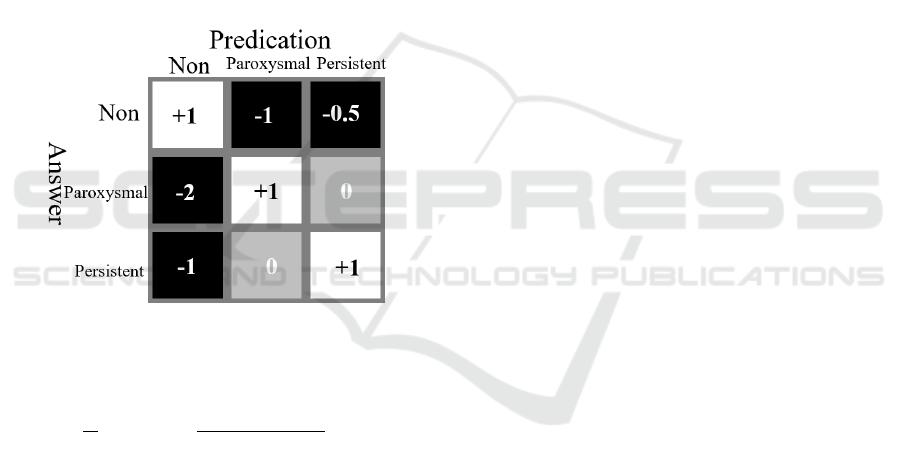
The score includes two parts: the first part (Ur)
classifies the AFib correctly, and the score matrix is
shown in Figure 4. The second part (Ue) is meant to
detect the AFib onsets. If the onsets and end of the
AFib episodes were detected within ±1 R-peak, Ue
+ 1, within ±2, Ue + 0.5.
Figure 4: The score matrix for part one.
The overall score (U) is calculated by:
𝑈=
1
𝑁
𝑈𝑟
+
𝑀𝑎
𝑚𝑎𝑥
𝑀𝑟
,𝑀𝑎
×𝑈𝑒
3 RESULTS
For Stage I, the validation sets achieved 90.14%
accuracy to classify the non-AFib and AFib segments
with a specificity of 93.65% and sensitivity of
84.82%, respectively. The result indicated that Stage
I could identify the non-AFib segments well but may
miss some AFib segments. However, it wasn’t an
issue for the whole signal because the majority voting
and the appropriate threshold can improve the overall
performance and remedy the sensitivity. In the testing
phase, a 2.5% threshold was set which means if less
than 2.5% of the signal is classified as AFib, the
overall signal will be regarded as non-AFib. By this
approach, the accuracy of non-AFib signals
classification could be increased approximately from
92.62% to 96%. Theoretically, raising the threshold
can improve the non-AFib accuracy on validation to
almost 100%, but it will lose its sensitivity and
generalization.
For Stage II, on the validation sets, it did the
accuracy of 92.56% with a specificity of 86.24% and
sensitivity of 95.77% to classify non-AFib and AFib
segments on the AFib signals. The result showed that
Stage II might tend to classify the healthy segments
into AFib segments. However, because of the
considered two stages design, non-AFib signals have
been excluded before Stage II; thus, it won’t affect the
overall classification performance. It will only affect
the detection of the onset of the AFib.
During the testing recordings, the two-stages
method achieved 2.0953 overall mark, including
0.8714 Ur and 1.4039 Ue. It showed a satisfying
performance on the classification, while the onset
detection can be improved. Furthermore, the total
neural network is only about 1.6 MB in Matlab
(coding in Python can be smaller, approximately 500
k.), which is possible to use on a personal laptop or
embedded device.
4 DISCUSSION
This study aimed to design an algorithm using NNs
to detect paroxysmal AFib and make the computing
load small enough for a portable embedded ECG
device. This is done because patients typically neglect
paroxysmal AFib due to its intermittent
characteristics and lack of appropriate databases. In
this study, a two-stage algorithm was designed using
the CSCP2021 database, and it proofed its capability
to classify the AFib segments and onsets on the
validation sets.
Firstly, the use of a two-stage method rather than
one NN will be justified. Before the training, our
preconceived thought on paroxysmal AFib was like
intermittent non-AFib and AFib waveforms in the
ECG signals. However, it is not, or at least the
BiLSTM or Conventional Neural Network (CNN)
cannot easily learn it. For non-AFib or AFib segments
from the non/persistent AFib signals, the network in
Stage I can learn in a very short time within one
epoch, while the segments from paroxysmal could not
regress, and the loss didn’t go down (training
accuracy also stuck at 69.15%, which is
approximately equal to the data proportion). This may
indicate that the paroxysmal AFib may hold
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
88

pathological characteristics even in the healthy
episodes and using a simplified network cannot
classify the non-AFib or AFib episodes. There is no
doubt that using the deeper neural network with
complex structure, such as adding lots of CNN layers
and attention layers, will learn the difference. Still, it
will make the computing load quite extensive, which
is contrary to the original intention. Therefore, a
second phase was included for the detection of the
paroxysmal onset.
Secondly, the use of Stage II to finish the whole
classification task is tested. However, the
performance was not satisfying due to the
oversensitivity of the Stage II network and its trend to
identify the segment as AFib. Besides, feature
extraction relies greatly on reliable and accurate R
peak detection. When the signal has massive motion
artefacts, the failed R peak detection will cause an
error in the algorithm. This is another advantage of
the two-stage structure.
Thirdly, there is still room for the improvement of
the overall performance. In the blind test of the
challenge, the overall mark is decreased from 2 to
approximately 1.7. This result showed that the
generalization needs to be improved, especially in
Stage II. Currently, only two features were used while
adding more features might be a solution to improve
the algorithm. Besides, appropriate window length
may also affect the result. Currently, a 5s window on
Stage I and five intervals on Stage II are used. Longer
window length may provide more information,
especially on the feature extraction of Stage II. Short
duration cannot maximize the feature difference.
5 CONCLUSIONS
This study proposed a two-stage neural network
algorithm that can detect paroxysmal AFib and its
onsets. For performance, it can achieve 90.14% and
92.56% accuracy on non-AFib and AFib segments
classification respectively in the two stages, got
2.0953 overall mark on our testing sets. As few
researches have focused on paroxysmal AFib
detection using NNs, the finding of this study will
provide knowledge for the further researches in this
area. In the meantime, the proposed method also
holds the advantage of a small computing load,
making it possible for embedded ECG devices.
ACKNOWLEDGEMENT
This work has been funded in part from KFAS,
Kuwait Foundation for Advancement of Sciences,
project no. CN20-13EE-01.
REFERENCES
January, C. T., Wann, L. S., Alpert, J. S., Calkins, H.,
Cigarroa, J. E., Cleveland, J. C., ... & Yancy, C. W.
(2014). 2014 AHA/ACC/HRS guideline for the
management of patients with atrial fibrillation: a report
of the American College of Cardiology/American Heart
Association Task Force on Practice Guidelines and the
Heart Rhythm Society. Journal of the American
College of Cardiology, 64(21), e1-e76.
Kornej, J., Börschel, C. S., Benjamin, E. J., & Schnabel, R.
B. (2020). Epidemiology of atrial fibrillation in the 21st
century: novel methods and new insights. Circulation
research, 127(1), 4-20.
Zhou, X., Ding, H., Wu, W., & Zhang, Y. (2015). A real-
time atrial fibrillation detection algorithm based on the
instantaneous state of heart rate. PloS one, 10(9),
e0136544.
De Giovanni, E., Aminifar, A., Luca, A., Yazdani, S.,
Vesin, J. M., & Atienza, D. (2017, September). A
patient-specific methodology for prediction of
paroxysmal atrial fibrillation onset. In 2017 Computing
in Cardiology (CinC) (pp. 1-4). IEEE.
Pourbabaee, B., Roshtkhari, M. J., & Khorasani, K. (2018).
Deep convolutional neural networks and learning ECG
features for screening paroxysmal atrial fibrillation
patients. IEEE Transactions on Systems, Man, and
Cybernetics: Systems, 48(12), 2095-2104.
Annavarapu, A., & Kora, P. (2016). ECG-based atrial
fibrillation detection using different orderings of
Conjugate Symmetric–Complex Hadamard Transform.
International Journal of the Cardiovascular Academy,
2(3), 151-154.
Rizwan, A., Zoha, A., Mabrouk, I. B., Sabbour, H. M., Al-
Sumaiti, A. S., Alomainy, A., ... & Abbasi, Q. H.
(2020). A review on the state of the art in atrial
fibrillation detection enabled by machine learning.
IEEE reviews in biomedical engineering, 14, 219-239.
Liu, C., Oster, J., Reinertsen, E., Li, Q., Zhao, L., Nemati,
S., & Clifford, G. D. (2018). A comparison of entropy
approaches for AF discrimination. Physiological
measurement, 39(7), 074002.
Kalidas, V., & Tamil, L. S. (2019). Detection of atrial
fibrillation using discrete-state Markov models and
Random Forests. Computers in biology and medicine,
113, 103386.
Xiong, Z., Stiles, M. K., & Zhao, J. (2017, September).
Robust ECG signal classification for detection of atrial
fibrillation using a novel neural network. In 2017
Computing in Cardiology (CinC) (pp. 1-4). IEEE.
Paroxysmal Atrial Fibrillation Detection by Combined Recurrent Neural Network and Feature Extraction on ECG Signals
89

Petmezas, G., Haris, K., Stefanopoulos, L., Kilintzis, V.,
Tzavelis, A., Rogers, J. A., ... & Maglaveras, N. (2021).
Automated atrial fibrillation detection using a hybrid
CNN-LSTM network on imbalanced ECG datasets.
Biomedical Signal Processing and Control, 63, 102194.
Ping, Y., Chen, C., Wu, L., Wang, Y., & Shu, M. (2020,
June). Automatic detection of atrial fibrillation based
on CNN-LSTM and shortcut connection. In Healthcare
(Vol. 8, No. 2, p. 139). Multidisciplinary Digital
Publishing Institute.
Michaud GF, Stevenson WG. (2021) Atrial Fibrillation. N
Engl J Med. , 384(4):353-361.
Wang, X., Ma, C., Zhang, X., Gao, H., Clifford, G., & Liu,
C. Paroxysmal Atrial Fibrillation Events Detection
from Dynamic ECG Recordings: The 4th China
Physiological Signal Challenge 2021.
Graves, A., Fernández, S., & Schmidhuber, J. (2005,
September). Bidirectional LSTM networks for
improved phoneme classification and recognition. In
International conference on artificial neural networks
(pp. 799-804). Springer, Berlin, Heidelberg.
Liu, G., & Guo, J. (2019). Bidirectional LSTM with
attention mechanism and convolutional layer for text
classification. Neurocomputing, 337, 325-338.
Pan, J., & Tompkins, W. J. (1985). A real-time QRS
detection algorithm. IEEE transactions on biomedical
engineering, (3), 230-236.
Lee, E., Choi, E. K., Han, K. D., Lee, H., Choe, W. S., Lee,
S. R., ... & Oh, S. (2018). Mortality and causes of death
in patients with atrial fibrillation: a nationwide
population-based study. PLoS One, 13(12), e0209687.
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
90
