
Prototyping and Early Validation of an Integrated, Electrochemical
and Mass Three-sensor Array for Dengue Detection
Ahmad Anwar Zainuddin
1,2
, Mohd Afiq Mohd Asri
1
, Cyril Guines
3
, Muhammad Zahid Zabedi
4
,
Khin Maung Htay
1
, Abdul Hakim Ab Rahim
1
, Matthieu Chatras
4
, Arnaud Pothier
4
,
Wing Cheung Mak
5
and Anis Nurashikin Nordin
1
1
Kulliyyah of Engineering, International Islamic University Malaysia, 53100 Gombak, Kuala Lumpur, Malaysia
2
Department of Computer Science and Engineering, School of Engineering, Manipal International University,
71800 Nilai, Negeri Sembilan, Malaysia
3
XLIM-UMR 7252, University of Limoges/CNRS, 87060 Limoges, France
4
3D Synapsis, 44a, Jalan Keluli AK7/AK, Taman Perindustrian Bukit Raja Selatan, 40000 Shah Alam, Selangor, Malaysia
5
Biosensors and Bioelectronics Centre, Department of Physics, Chemistry and Biology (IFM), Linkoping University,
58183 Linkoping, Sweden
Keywords: Dengue, Biosensor, Sensor Arrays, Integrated Electrochemical-Quartz Crystal Microbalance, Point-of-Care
Diagnostics.
Abstract: This paper presents the current progress towards a lab-on-chip biosensor for early dengue detection, consisting
of an integrated sensor with dual-function working electrode that enables in-situ measurements of both
electrochemical impedance spectroscopy (EIS) and quartz crystal microbalance (QCM) enclosed in a
miniaturized 3D-printed package equipped with electrical contacts and sample fluid delivery to the quartz
biosensor array. The sensors consist of an array of three 10 MHz IEQCM biosensors on a single quartz
substrate. Early validation is performed for future dengue sensing application. We report the design,
optimisation, and fabrication of the sensors, as well as early optimisation and validation of surface
bioconjugation of antibodies. This lab-on-chip has the potential to provide accurate dengue detection due to
its high sensitivity and dynamic range, as well as providing rapid and early dengue detection in point-of-care
settings.
1 INTRODUCTION
Biosensors are analytical devices that integrate
molecular recognition platforms with a
physicochemical transducer to produce a single
detection processing unit (Hong et al., 2012; Miserere
& Merkoçi, 2015). Rapid point-of-care (PoC)
biosensors are advantageous as they allow analyses to
be performed in the field (Hu et al., 2016; Lisowski
& Zarzycki, 2013; St John & Price, 2014).
Commercial screen printed electrodes have been
commonly employed by researchers for
electrochemical dengue detection due to their
simplicity, biocompatibility, cost-effectiveness,
disposability, and flexibility of integration (Cecchetto
et al., 2015; Parkash et al., 2014; Sinawang et al.,
2016). Apart from SPGE, quartz crystal microbalance
(QCM) has been used as a simple PoC device, which
offers shorter analysis time, real-time monitoring and
label-free detection (Omar & Fen, 2018). A 2017
study reported the detection of dengue NS1 antigens
using modified bacterial cellulose nanocrystals (CN)-
QCM (Pirich et al., 2017). Based on literature, while
QCM devices excel in sensitivity, electrochemical
sensors provide better selectivity.
Integration of these two mechanisms on a single
device have also been done in the form of an
electrochemical quartz crystal microbalance
(EQCM). These devices produce high accuracy of
measurements in biological and chemical systems
(Yang et al., 2015; Yu et al., 2009). Basic EQCM
sensors comprise of a quartz crystal microbalance
sensor (QCM), a counter electrode and a reference
electrode, integrated into a single measurement
platform. The advantage of these integrated sensors is
that it can measure both resonance frequency changes
and electrochemical reactions in a single platform.
204
Zainuddin, A., Asri, M., Guines, C., Zabedi, M., Htay, K., Rahim, A., Chatras, M., Pothier, A., Mak, W. and Nordin, A.
Prototyping and Early Validation of an Integrated, Electrochemical and Mass Three-sensor Array for Dengue Detection.
DOI: 10.5220/0010961500003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 1: BIODEVICES, pages 204-211
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

This is done by using the top electrode as both the
electrochemical working electrode and QCM
terminal. An early demonstration of combined
electrochemical and QCM sensors observed that the
an integrated sensor enabled cross-validation of the
measurement, increased the accuracy of detection,
and reduced false positives and false negatives (Yu et
al., 2009). However, existing EQCM systems are
limited to laboratory-only settings due to the
instruments’ large footprint and handling
complexities (Ashton, 2012; Srivastava et al., 2018).
We previously hypothesized that using dual-
function sensors could increase sensor sensitivity to
very low limits of detection, increase dynamic range
of disease quantification, and provide higher
diagnostic accuracy through cross-validation of
parallel measurement techniques (A. Zainuddin et al.,
2019). In this paper, we present our current progress
towards a rapid point-of-care (PoC) device for early
detection of dengue, based on integrated
electrochemical and mass biosensor. This PoC device
has an array of three identical sensors on a single
quartz substrate. Each sensor is a three-electrode
system which can perform in-situ measurements of
the electrode’s surface changes based on piezoelectric
and electrochemical transductions (Figure 1(a, b)).
This system is packaged in a portable unit for
deployment at patient-side or in the field (Figure 1(c,
d)). We envision this sensor to achieve ultralow limit
of detection, dual-sensing cross-validation capability,
portable size, short sample-to-analysis time, and
parallelization of multiple assays.
2 DEVICE DESIGN,
FABRICATION AND
VA L I D AT I O N
2.1 Device Concept and Operation
The biosensor has two measurement mechanisms,
namely mass and electrochemical sensing. The mass
sensor, which is known as quartz crystal
microbalance (QCM) are widely used as a non-
destructive method to measure the changes in surface
mass due to adsorption process, based on changes in
the resonance frequency (Deng et al., 2018). The
QCM consists of electrodes on the top and bottom
(BE) sides of thin AT-cut quartz substrate. The
electrochemical biosensor consists of a three-
electrode system, which are working electrode (WE),
counter electrode (CE) and reference electrode (CE).
These electrodes are implemented as a planar device
on top of thin AT-cut quartz substrate. This leads to
the working electrode (top) having dual functions as
electrochemical and QCM sensor in a single chip
device. The quartz crystal substrate (diameter, d
q
=
14 mm) consisted of an array of three 10 MHz
integrated electrochemical quartz crystal
microbalance (IEQCM) sensors as shown in Figure
1(a). The centre-to-centre distance of QCM
electrodes was set to s = 6 mm to minimize frequency
interference (A. Zainuddin et al., 2019). Figure 1(b)
illustrates a two-electrode system consisting of WE
and CE for both the cross-section and top view of the
integrated electrochemical and QCM biosensor. The
electrochemical sensor is placed within a well, which
is made by silicone gasket to contain the liquid. Gold
(Au) was selected for the working and counter
electrodes as it is an inert (noble) electrode that has
high resistance to oxidation (Pereira et al., 2011;
Serafín et al., 2011) and has unique covalent bonding
characteristics with thiol-based self-assembly
monolayers (SAM) (A. A. Zainuddin et al., 2016) that
enables simple antibody immobilization. The
variables d
WE
, w
CE
, g and h
q
indicate the diameter of
working electrode, width of counter electrode, gap
between electrodes and quartz thickness,
respectively. To minimize the interference of electric
field during electrochemical measurements and
higher current density across electrodes, the w
CE
and
g were set to 1000 µm and 70 µm, respectively. The
device is then interfaced with measuring
instrumentation with a 3D-printed custom enclosed
sensor packaging (Figure 1(c)). This 3D-printed
packaging was developed for a single quartz crystal
substrate with diameter of 14 mm.
2.2 IEQCM Sensor Fabrication
Sensor fabrication was performed at the XLIM
Circuits Technology Center cleanroom in XLIM
Research Institute, Université de Limoges. IEQCM
sensors consists of 3 top/working electrodes (WE), a
common counter electrode (CE), and 3 bottom
electrodes (BE). The sensors were fabricated on a 14
mm (Ø) 168 µm thick AT-cut quartz crystal
piezoelectric substrate (Great Microtama Industries,
Indonesia) using standard lift-off lithography.
Fabrication begins with WE and CE on the top
side of quartz, followed by the fabrication of BE at
the bottom side of quartz. First, the substrate was
cleaned with piranha solution, 30% H
2
SO
4
, 5%
H
2
O
2
). Then, it was rinsed in ethanol and deionized
water (DI) and dried in N
2
stream. Subsequently, the
substrate was pre-baked at 120 °C for 300 s. The
image reversal photoresist (Merck AZ5214E,
Prototyping and Early Validation of an Integrated, Electrochemical and Mass Three-sensor Array for Dengue Detection
205
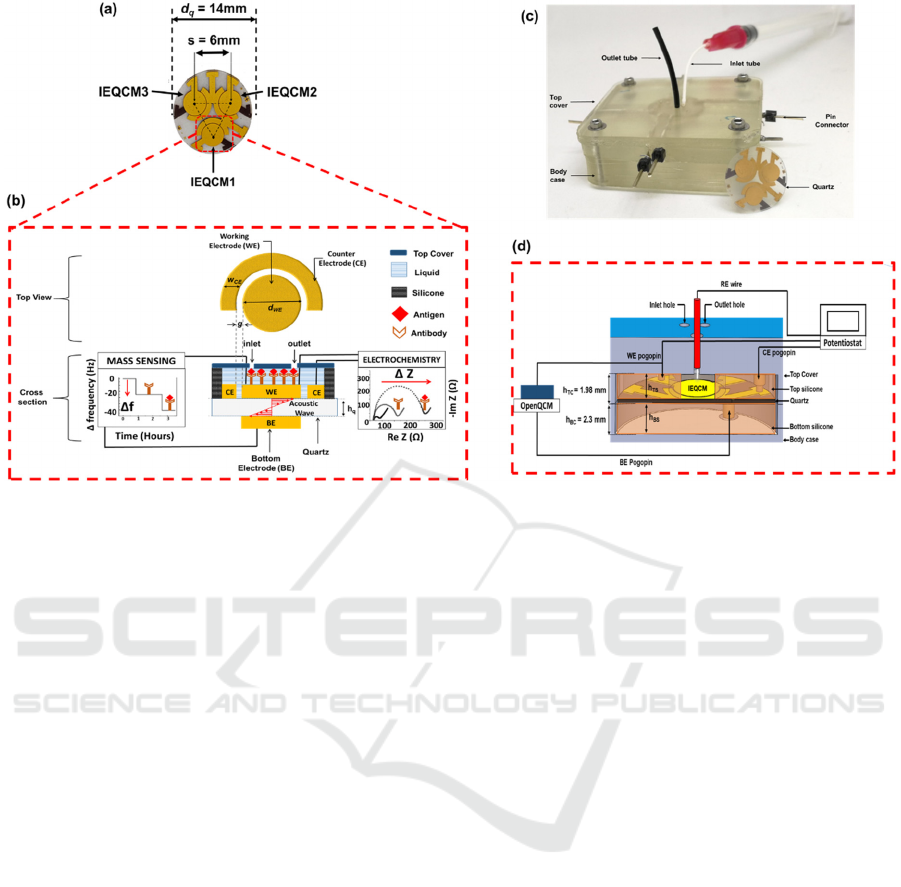
Figure 1: (a) Top view of the fabricated IEQCM sensor. (b) Conceptual illustration of the functional units of the integrated
electrochemical and mass biosensor. (c) Custom 3D-printed enclosed sensor interfacing device. (d) Cross-sectional view of
biosensor packaging. The height of top silicone area, h
tc
was optimally set to 1.98 mm to prevent leakage. Pogo pins are used
to form electrical contacts to the IEQCM quartz wafer. Laser-cut silicone (top and bottom) is used as hermetic gaskets and
wells. Each unit of IEQCM has three holes with radius of 400 µm corresponding to the sample inlet and outlet, and a port for
an external reference electrode (RE). The QCM measurements were done using a portable openQCM (Novatech, Italy) which
displayed measurement of frequency versus time. The electrochemical measurements were done using three electrodes (WE,
CE and RE), which were carried out using a potentiostat (Autolab PGSTAT128N).
MicroChemicals GmbH, Germany) was applied by
spin-coating to a resulting thickness of 1.5 µm and
baked at 105 °C for 60 s. Pattern was transferred onto
the resist (exposure time: 3.5 s) using a Karl Suss
MJB-3 or MJB-4 mask aligner. Prior to full exposure,
the substrate was baked at 120 °C for 60 s, followed
by the full exposure photolithography about 20 s.
After the photoresist removal process with MF-26,
Ti/Au electrode (20 nm/200 nm thick) was deposited
using E-Beam evaporator (PLASSYS MEB 300).
Finally, the release process (lift-off) was done with
acetone to strip the remaining photoresist and define
the electrodes. The same procedures were repeated to
realize the BE at the bottom side of quartz substrate.
The position of the BE relative to the WE are aligned
with assistance of alignment markers on the top side
of the quartz.
The disposable silver/silver chloride (Ag/AgCl)
RE was fabricated through bleach immersion. Silver
wire with diameter of 100 µm and minimum length
of 3 cm was chosen so that it can be fitted into the
reference electrode inlet port in the packaging
module. The silver wire is first soldered to copper
wired cables. The pure silver wire (99% Ag) is then
immersed into undiluted household bleach (NaClO,
40 mg mL
−1
) for up to 5 min (da Silva et al., 2014).
All the wire interconnects will be described in the
next section.
2.3 Sensor Packaging
A 3D-printed custom enclosure for the IEQCM
biosensor was fabricated in this work. This sensor
packaging would enable integration of electrical
contacts and sample fluid delivery to the IEQCM
sensor array. The packaging unit weighed 50 g,
measured 80 mm × 32 mm × 12.4 mm (l × w × h),
and was fabricated from a rapid 3D clear resin
(Monocure 3D, New South Wales, Australia) formed
using using a WDi7 DLP stereolithographic 3D
printer (3D Synapsis, Malaysia). Print setting for the
biosensor packaging is 50 microns per layer, with
cure time of 22.4 s per layer. Printed units were rinsed
using isopropyl alcohol and cleaned in an ultrasonic
bath (Crest Ultrasonics Corp., NJ) to remove resin
residuals, and post-processed under commercial nail
UV lamp to cure underexposed coatings.
This packaging consists of two parts (top cover
and body case). In the top cover, there are three
different regions which correspond to three IEQCM
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
206

sensors in this system. Each region has three holes
with radius of 400 µm corresponding to sample inlet
and outlet, and a port for the external reference
electrode. The top cover contains a slot with height
h
TC
= 1.98 mm to contain the silicone gasket. A
circular silicone gasket (thickness h
TS
= 2 mm,
diameter 14 mm) is laser cut with three holes of
diameter 5.5 mm as wells to contain liquid samples
under test, which corresponds to the three IEQCM
sensors in this system. This matching of h
TC
and h
TS
was optimized over several iterations to prevent
sample leakages across the silicone gasket-top cover
interface (see Figure 1(d)). Silicone was selected to
ensure hermetic sealing and prevent mechanical
stress on the quartz crystal.
For the (lower) body case, a circular silicone
(thickness h
BS
= 2 mm, diameter 14 mm) was used to
support the quartz substrate, placed inside a slot with
height h
BC
= 2.3 mm. For electrical contact to the
sensors, gold coated pogo pins (2.80 mm; Harwin
Asia Pte Ltd, Singapore) were used due to its spring-
loaded contacts that produced low mechanical stress
on the quartz crystal surface. The pogo pins are
distributed in radial symmetry (9 for top unit, 3 for
bottom unit) to reduce mechanical stress over the
quartz wafer. The top and bottom silicone gaskets are
laser cut with holes that align with the pogo pins. The
pogo pins are wired through the case by fine copper
wires (100 µm diameters) and soldered to pin headers
outside the case, for connections to external
instrumentation. The top cover and the body case are
then clamped together with bolts and nuts.
2.4 Surface Modification on IEQCM
Figure 3 shows the surface modification and
immobilization process for the NS1 immunosensors.
Bio-functionalization of mixed self-assembled
monolayers (mix-SAMs) on gold working electrode
surface was performed to immobilize the anti-NS1
IgG antibodies (ab138696, Abcam, Cambridge, UK).
Before the mix-SAMs process, the bare IEQCM
electrodes were cleaned with piranha solution, rinsed
with a large amount of deionised water and dried with
air blower pump. Polydimethylsiloxane (PDMS, 10:1
precursor-curing agent ratio; Sylgard 184, Dow
Corning) slabs with punched liquid wells was used as
a reversible mask to limit bio-functionalization
reactions (SAM, EDC/NHS, Glycine) specifically to
the working electrode.
The alkanethiols mix-SAMs was formed on the
electrodes by immersing these electrodes for 24 h at
25°C in a mixed solution containing 1 mM
11-
mercaptoundecanoic acid (for covalent anti-NS1
attachment) and 1 mM 6-mercaptohexanol (6COH) in
ethanol. Alkanethiol SAM is formed on the WE due
to formation of gold-thiol bonds. After the mix-SAMs
preparation, the electrodes were washed with excess
anhydrous ethanol and deionized water to remove any
unbound molecules. It was followed by immersion of
the WE in an aqueous solution containing 0.4 M N-
(3-dimethylaminopropyl)-N-ethylcarbodiimide
(EDC) and 0.1 M N-hydroxysuccinimide (NHS) for
40 min, to activate the carboxylic acid terminated-
group on the modified electrodes for NS1 antibody
attachment. The electrodes were then washed with
excess amounts of phosphate-buffered saline (PBS,
pH 7.4) and dried with air blower pump. It was
followed by the incubation of 50 µL of anti-NS1
solution (1 µg mL
−1
in PBS) onto the electrodes
surface. Following this incubation for 1 h, the
remaining NHS esters were deactivated by addition
of a 1 M ethanolamine solution for 5 min and the
surface was thoroughly rinsed with deionized water.
Finally, the modified mix-SAMs-NHS/EDC-anti-
NS1 electrodes were immersed in 5 mM glycine
solution (in PBS) for 30 min to block non-specific
binding sites.
2.5 QCM and EIS Validation
Early validation was performed with endpoint
measurements (i.e., not in continuous time) at each
surface modification step. The measurement setup for
the lab-on-chip biosensor is shown in Figure 1(d).
Initially, the QCM measurements were done using a
portable openQCM (Novatech, Italy) which
displayed measurement of frequency versus time. The
frequency changes were monitored by injecting 100
µL of phosphate-buffered saline (PBS) to the inlet
and outlet of the packaging biosensor. Following
injection of PBS into the chamber, the measurement
is left to stabilize, and the last 200 s of the
measurements is averaged to obtain frequency change
values corresponding to the surface modification step.
The electrochemical measurements were
performed on an Autolab PGSTAT128N potentiostat
(Metrohm AG, Switzerland). In this work, 40 µl of 5
mM ferri-ferrocyanide ([Fe(CN)
6
]
3-/4−
) in PBS were
used as redox probe (K
3
[Fe(CN)
6
], Sigma Aldrich, St.
Louis, MO). EIS measurements were recorded via
AC potential of 5 mV amplitude in the frequency
range from 1 MHz to 0.01 Hz at the optimized
oxidation peak potential of 0.2 V and plotted over a
Nyquist plot.
Prototyping and Early Validation of an Integrated, Electrochemical and Mass Three-sensor Array for Dengue Detection
207
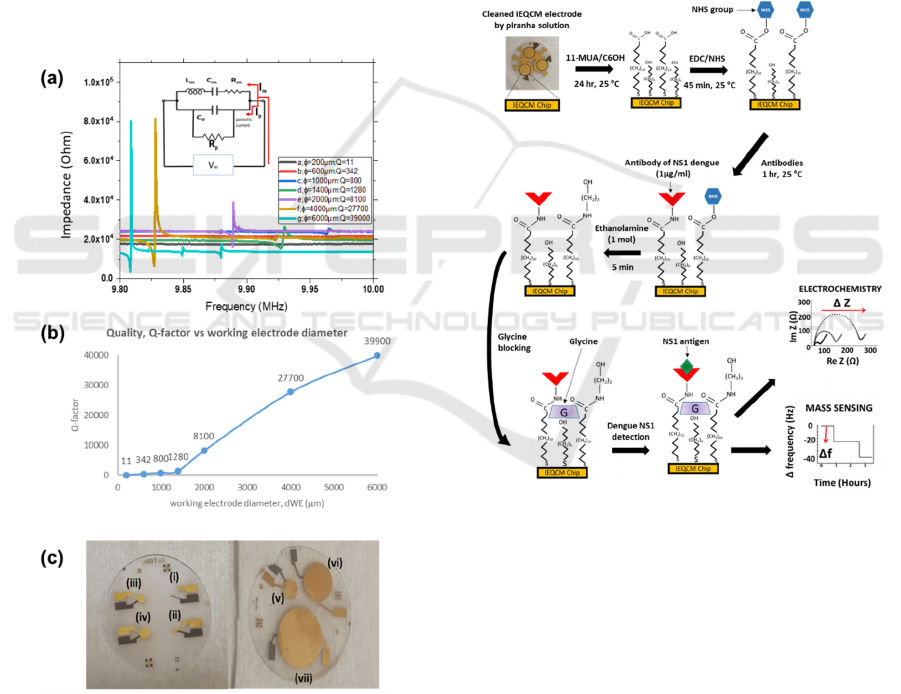
3 RESULTS AND DISCUSSIONS
3.1 Optimization of QCM Sensors
It was previously observed that quartz thickness of
168 µm with resonance frequencies of 10 MHz was
used for biosensing (Pirich et al., 2017), and was also
chosen for this work. The working (WE) and bottom
(BE) electrodes’ diameter were selected based on an
optimal Q-factor resolution. This Q-factor
corresponds to a greater mechanical energy stored by
the resonator. Figure 2(a) indicates the measurements
of resonance frequency for the working electrode of
7 varying diameters ranging from 200 μm to 6000
μm. Figure 2(c) shows the corresponding fabricated
QCM sensors. From the results, all diameters of
QCM, d
we
showed a fundamental resonance
frequency peak close to f
o
= 10 MHz.
Figure 2: (a) Measurement of resonance frequency and Q-
factor value at different diameter of QCM sensors. (Inset)
A modified BVD equivalent circuit, where Rp was to
represent an additional value for compensating parasitic
capacitance. (b) Plot of Q-factor versus working electrode
diameter, d
WE
. (c) Fabricated QCM sensors for WE
diameters (i) 200 µm, (ii) 600 µm, (iii) 1000 µm, (iv) 1400
µm, (v) 2000 µm, (vi) 4000 µm, (vii) 6000 µm.
According to the classical Butterworth-van-Dyke
(BVD) circuit, the RLC parameters of the QCM
equivalent circuit can be divided into motional
components, R
m
, L
m
and C
m
, as shown in Figure 2(a)
(inset). These motional components are derived from
the resonance operation of the QCM and an additional
parallel static capacitor (C
0
). The motional resistance
(R
m
)
represents energy loss at resonance frequency.
The motional impedance (L
m
) and motional
capacitance (C
m
) represent the vibrating mass and
coupling coefficient, respectively. C
0
contributes to
the dielectric energy storage because the oscillation
crystal is established in between the two electrodes
(A. A. Zainuddin et al., 2018). R
p
was added to
represent an additional value for compensating
parasitic capacitance. Taking these factors into
Figure 3: The modification and immobilization process for
the NS1 immunosensor design. The mixed thiol-SAM
structures, in which 11-mercaptoundecanoic acid serves as
a linker layer for anti-NS1 antibodies attachment, and
6-mercaptohexanol functioned as spacers, is constructed
onto a gold electrode surface. It is followed by the standard
EDC/NHS amine conjugation which enables Anti-NS1
immobilization on this gold electrode. Glycine is used to
block nonspecific sites. Samples containing dengue NS1
antigen is then introduced on the sensor interface. The
faradaic impedance measurements are carried out using a
redox probe in solution [Fe(CN)
6
]
3−/4−
. When NS1 antigens
are bound to anti-NS1 antibodies, the change of impedance
is measured by EIS through approximation of the diameter
of the semi-circle of Re (Z), R
ct
. The change of frequency
is also evaluated from the reduction in resonance frequency
of the propagated acoustic wave through the surface of the
quartz using QCM.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
208
account, widening the diameter of working electrode
may enhance the Q-factor value. This is due a high
resistance R
m
, which caused significant reduction of
current, I
m
flowing through the RLC equivalent
circuit of the QCM sensor. Therefore, a weak
resonance frequency was observed due to the
existence of a high parasitic current, I
p
. This I
p
was
forced by the electric actuation signal flowing
through the QCM parasitic capacitance C
0
. Thus, this
I
p
caused small I
m
flowing through the RLC
equivalent circuit of the QCM (Waszczuk et al.,
2011). Consequently, these smaller diameters (200
µm to 1400 µm) are not suitable to be used in the
QCM in liquid sensing due to low Q factor.
The QCM diameters of 2000 µm, 4000 µm and
6000 µm corresponds to motional resistances
R
m
= 1.51 kΩ, R
m
= 315 Ω and R
m
= 76.10 Ω
respectively. These R
m
values correspond to higher
Q-factors (Figure 2(b)), which is necessary for
sensing application in fluids. The highest Q-
factorvalue (39000) with the resonance frequency of
9.83 MHz is produced at diameter of 6000 μm.
However, d
WE
= 4000 µm was selected for our
biosensor since it showed an optimal size with
adequate Q-factor value (27700) while also allowing
geometric fit into a radial array of 3 identical sensors
on a single 14 mm quartz crystal substrate.
3.2 Electrochemical and Mass
Detection for Validation of Surface
Bio-Functionalization
Dengue is a mosquito-borne viral disease caused by
the dengue virus, which is prevalent in most tropical
and subtropical regions. Dengue disease is endemic
in more than 100 countries from South East Asia,
Western Pacific, Eastern Mediterranean, Africa to
America (Cheah et al., 2014). About 400 million
people are infected per year, of which 25% are
symptomatic (febrile illness) and 75% are
asymptomatic (Flores & O’Neill, 2018; Messina et
al., 2015), resulting in an estimated 500,000 recorded
cases of Dengue Hemorrhagic Fever (DHF) and
25,000 deaths occur per annum (Low et al., 2017).
Early detection of dengue infection is very important
for epidemiological strategies, clinical management
and administering appropriate treatment to the
patients. The urgency for early dengue detection
presents a suitable test case for our biosensor.
Electrochemical impedance spectroscopy (EIS) is
an electrochemical detection method that estimates
impedance or current changes in electrode surface
owing to surface reactions (Prakash et al., 2012).
Deposition of antigens and antibodies on working
electrode impede current flow to the electrode and
electrolyte system. The use of Randles circuit can be
implemented as a simple model to portray a solution
resistance (R
S
), a double layer capacitor or constant
phase element (CPE) and a charge transfer resistance
(R
CT
). The value of CPE and R
CT
are measured to
show the changes in capacitance of electrode surface
(Berggren et al., 1999). Warburg impedance (W)
contribution to the overall impedance is negligible in
our system, and thus excluded from the Randles
circuit model used in our data acquisition (Figure
4(b)).
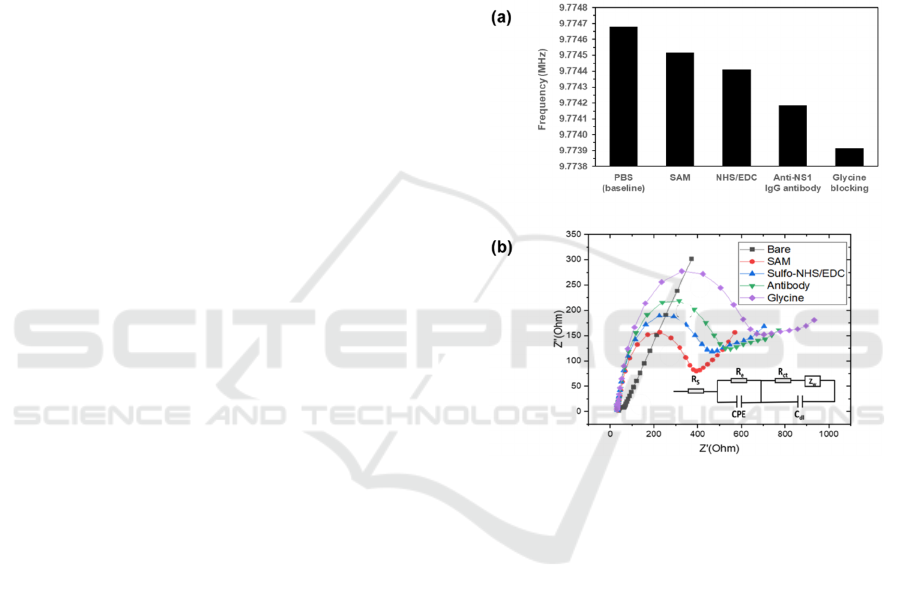
Figure 4: Change of frequency (a) and Nyquist impedance
diagrams (b) of a surface functionalized IEQCM, layer-by-
layer: bare electrode, SAM, NHS/EDC, dengue anti-NS1
capture antibody, and blocking agent glycine. Surface
modification experiments were performed with n = 1.
The step-by-step monitoring of the surface
functionalization of the WE are performed by EIS and
QCM. Figure 4(a, b) shows the frequency change of
the QCM measurement and Nyquist diagrams of EIS
measurement following surface functionalization
steps of the IEQCM: bare electrode (Au), SAM,
SAM-EDC/NHS, SAM-EDC/NHS-antibody, SAM-
EDC/NHS-antibody-glycine. In Figure 4(a), it can be
observed that the resonant frequency is reduced for
every phase of the functionalization steps, which
corresponds to the added mass onto the surface of the
bare gold electrode. The stepwise reduction of
resonant frequency during each phase confirms the
completion of each immobilization step. This is also
confirmed by the increasing impedance (denoted by
R
CT
i.e., the diameter of the half circle) as indicated
Prototyping and Early Validation of an Integrated, Electrochemical and Mass Three-sensor Array for Dengue Detection
209

by the Nyquist plot from the EIS measurement in
Figure 4(b). The bare IEQCM exhibited a low
resistance, suggesting a fast electron process of
potassium ferrocyanide in PBS to the electrode
surface.
4 CONCLUSIONS AND FUTURE
WORKS
We report our progress in the development and
validation of an integrated electrochemical quartz
crystal microbalance (IEQCM) sensor arrays with
mixed-SAM bio-recognition layer developed for
point-of-care dengue detection. We demonstrated that
this sensor could perform both as an electrochemical
sensor and as a QCM sensor, enabling future
developments for diagnostic cross-validation on a
single platform. The biosensor is very promising with
regards to its potential use at the point-of-care. The
small footprint of the device, coupled with the
portable openQCM instrumentation, allows a high
degree of portability. While it is currently used with
an AutoLab PGSTAT128N potentiostat which is too
bulky for use in point-of-care settings, open-source
portable potentiostats (Hoilett et al., 2020) may allow
integration and development of portable and fully-
automated IEQCM dual sensing devices in the near
future. The sensor also allows to some degree of
parallelized analysis due to its triple arrayed
electrodes, further improving its capabilities for
point-of-care use. Ongoing works on this project
include development of miniaturized potentiostat for
a portable integrated instrument, improved sensor
fabrication process for integrated reference electrode
and larger scale manufacturing, and validation works
using real dengue patient samples.
ACKNOWLEDGEMENTS
This project is funded by the Swedish Research
Council (2014-4254), the Malaysian Ministry of
Higher Education under Fundamental Research Grant
Scheme (FRGS15-217-0458), and the French
National Research Agency under the Investments for
the Future program (ANR-10-LABX-0074-01
Sigma-LIM). Dr Sheroz Khan (IIUM) facilitated the
international collaborative effort. Dr Rosminazuin Ab
Rahim (IIUM) facilitated the logistics for funding.
We thank Dr Raihan Othman and Dr Shahrul Razi
Meskon (IIUM) for access to and training for the
AutoLab potentiostat.
REFERENCES
Ashton, S. J. (2012). Differential Electrochemical Mass
Spectrometry. In Design, Construction and Research
Application of a Differential Electrochemical Mass
Spectrometer (DEMS) (pp. 9–27). Springer, Berlin,
Heidelberg. https://doi.org/10.1007/978-3-642-30550-
4_2
Berggren, C., St\a alhandske, P., Brundell, J., & Johansson,
G. (1999). A feasibility study of a capacitive biosensor
for direct detection of DNA hybridization.
Electroanalysis, 11(3), 156–160.
Cecchetto, J., Carvalho, F. C., Santos, A., Fernandes, F. C.
B., & Bueno, P. R. (2015). An impedimetric biosensor
to test neat serum for dengue diagnosis. Sensors and
Actuators B: Chemical, 213, 150–154. https://doi.org/
10.1016/j.snb.2015.02.068
Cheah, W. K., Ng, K. S., Marzilawati, A. R., & Lum, L. C.
(2014). A review of dengue research in malaysia. The
Medical Journal of Malaysia, 69, 59–67.
da Silva, E. T. S. G., Miserere, S., Kubota, L. T., &
Merkoçi, A. (2014). Simple On-Plastic/Paper Inkjet-
Printed Solid-State Ag/AgCl Pseudoreference
Electrode. Analytical Chemistry, 86(21), 10531–10534.
https://doi.org/10.1021/ac503029q
Deng, F., Chen, W., Wang, J., & Wei, Z. (2018).
Fabrication of a sensor array based on quartz crystal
microbalance and the application in egg shelf life
evaluation. Sensors and Actuators B: Chemical, 265,
394–402. https://doi.org/10.1016/j.snb.2018.03.010
Flores, H. A., & O’Neill, S. L. (2018). Controlling vector-
borne diseases by releasing modified mosquitoes.
Nature Reviews Microbiology, 16(8), 508–518.
https://doi.org/10.1038/s41579-018-0025-0
Hoilett, O. S., Walker, J. F., Balash, B. M., Jaras, N. J.,
Boppana, S., & Linnes, J. C. (2020). KickStat: A Coin-
Sized Potentiostat for High-Resolution Electrochemical
Analysis. Sensors, 20(8), 2407. https://doi.org/10.3390/
s20082407
Hong, P., Li, W., & Li, J. (2012). Applications of
aptasensors in clinical diagnostics. Sensors, 12(2),
1181–1193.
Hu, J., Cui, X., Gong, Y., Xu, X., Gao, B., Wen, T., Lu, T.
J., & Xu, F. (2016). Portable microfluidic and
smartphone-based devices for monitoring of
cardiovascular diseases at the point of care.
Biotechnology Advances, 34(3), 305–320.
Lisowski, P., & Zarzycki, P. K. (2013). Microfluidic paper-
based analytical devices (μPADs) and micro total
analysis systems (μTAS): Development, applications
and future trends. Chromatographia, 76(19–20), 1201–
1214.
Low, J. G. H., Ooi, E. E., & Vasudevan, S. G. (2017).
Current Status of Dengue Therapeutics Research and
Development. The Journal of Infectious Diseases
,
215(suppl_2), S96–S102. https://doi.org/10.1093/
infdis/jiw423
Messina, J. P., Brady, O. J., Pigott, D. M., Golding, N.,
Kraemer, M. U. G., Scott, T. W., Wint, G. R. W., Smith,
D. L., & Hay, S. I. (2015). The many projected futures
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
210

of dengue. Nature Reviews Microbiology, 13(4), 230–
239. https://doi.org/10.1038/nrmicro3430
Miserere, S., & Merkoçi, A. (2015). Microfluidic
Electrochemical Biosensors: Fabrication and
Applications. In Lab-on-a-Chip Devices and Micro-
Total Analysis Systems (pp. 141–160). Springer.
http://link.springer.com/chapter/10.1007/978-3-319-
08687-3_6
Omar, N. A. S., & Fen, Y. W. (2018). Recent development
of SPR spectroscopy as potential method for diagnosis
of dengue virus E-protein. Sensor Review, 38(1), 106–
116.
Parkash, O., Yean, C., & Shueb, R. (2014). Screen Printed
Carbon Electrode Based Electrochemical
Immunosensor for the Detection of Dengue NS1
Antigen. Diagnostics, 4(4), 165–180. https://doi.org/
10.3390/diagnostics4040165
Pereira, S. V., Bertolino, F. A., Fernández-Baldo, M. A.,
Messina, G. A., Salinas, E., Sanz, M. I., & Raba, J.
(2011). A microfluidic device based on a screen-printed
carbon electrode with electrodeposited gold
nanoparticles for the detection of IgG anti-
Trypanosoma cruzi antibodies. Analyst, 136(22), 4745–
4751.
Pirich, C. L., de Freitas, R. A., Torresi, R. M., Picheth, G.
F., & Sierakowski, M. R. (2017). Piezoelectric
immunochip coated with thin films of bacterial
cellulose nanocrystals for dengue detection. Biosensors
and Bioelectronics, 92, 47–53.
Prakash, S., Pinti, M., & Bhushan, B. (2012). Theory,
fabrication and applications of microfluidic and
nanofluidic biosensors. Phil. Trans. R. Soc. A,
370(1967), 2269–2303.
Serafín, V., Agüí, L., Yáñez-Sedeño, P., & Pingarrón, J. M.
(2011). A novel hybrid platform for the preparation of
disposable enzyme biosensors based on poly (3, 4-
ethylenedioxythiophene) electrodeposition in an ionic
liquid medium onto gold nanoparticles-modified
screen-printed electrodes. Journal of Electroanalytical
Chemistry, 656(1), 152–158.
Sinawang, P. D., Rai, V., Ionescu, R. E., & Marks, R. S.
(2016). Electrochemical lateral flow immunosensor for
detection and quantification of dengue NS1 protein.
Biosensors and Bioelectronics, 77, 400–408.
https://doi.org/10.1016/j.bios.2015.09.048
Srivastava, J., Kushwaha, A., & Singh, M. (2018).
Imprinted Graphene-Starch Nanocomposite Matrix-
Anchored EQCM Platform for Highly Selective
Sensing of Epinephrine. Nano, 13(11), 1850131.
https://doi.org/10.1142/S179329201850131X
St John, A., & Price, C. P. (2014). Existing and emerging
technologies for point-of-care testing. The Clinical
Biochemist Reviews, 35(3), 155.
Waszczuk, K., Piasecki, T., Nitsch, K., & Gotszalk, T.
(2011). Application of piezoelectric tuning forks in
liquid viscosity and density measurements. Sensors and
Actuators B: Chemical,
160(1), 517–523.
Yang, Y., Tu, Y., Wang, X., Pan, J., & Ding, Y. (2015). A
label-free immunosensor for ultrasensitive detection of
ketamine based on quartz crystal microbalance.
Sensors, 15(4), 8540–8549.
Yu, L., Huang, Y., Jin, X., Mason, A. J., & Zeng, X. (2009).
Ionic liquid thin layer EQCM explosives sensor.
Sensors and Actuators B: Chemical, 140(2), 363–370.
Zainuddin, A. A., Nordin, A. N., Ab Rahim, R., & Mak, W.
C. (2016). Modeling of a novel biosensor with
integrated mass and electrochemical sensing
capabilities. Biomedical Engineering and Sciences
(IECBES), 2016 IEEE EMBS Conference On, 420–425.
http://ieeexplore.ieee.org/abstract/document/7843485/
Zainuddin, A. A., Nordin, A. N., Rahim, R. A., Ralib, A. A.
M., Khan, S., Guines, C., Chatras, M., & Pothier, A.
(2018). Verification of Quartz Crystal Microbalance
Array using Vector Network Analyzer and OpenQCM.
Indonesian Journal of Electrical Engineering and
Computer Science, 10(1), 84–93. https://doi.org/
10.11591/ijeecs.v10.i1.pp%p
Zainuddin, A., Nordin, A., Asri, M., Rahim, R., Guines, C.,
Chatras, M., Pothier, A., & Mak, W. (2019).
Development of Integrated Electrochemical–
QuartzCrystal Microbalance Biosensor Arrays:
Towards Ultrasensitive, Multiplexed and Rapid Point-
of-Care Dengue Detection: Proceedings of the 12th
International Joint Conference on Biomedical
Engineering Systems and Technologies, 220–227.
https://doi.org/10.5220/0007523802200227
Prototyping and Early Validation of an Integrated, Electrochemical and Mass Three-sensor Array for Dengue Detection
211
