
Estimating Use of Short-term Asthma Reliever Inhalers from
Electronic Prescription Records
Holly Tibble
1,2 a
, Aziz Sheikh
1,2,3 b
and Athanasios Tsanas
1,2,4
c
1
Usher Institute, University of Edinburgh, Edinburgh, U.K.
2
Asthma UK Centre for Applied Research, Edinburgh, U.K.
3
Health Data Research UK BREATHE Hub for Respiratory Health, University of Edinburgh, Edinburgh, U.K.
4
The Alan Turing Institute, London, U.K.
Keywords: Asthma, Inhaler, Reliever, Salbutamol, Electronic Health Records, Prescriptions.
Abstract: Asthma is a common chronic lung disease which can be effectively managed for most people through regular
use of inhaled controller therapy. Short-acting Beta-2 Agonists (symptom relievers; SABA) may also be
prescribed to be used as needed, however over-reliance may indicate poor symptom control. SABA usage can
be estimated from refill rate observed in prescribing records. This study was a secondary analysis of a Scottish
longitudinal dataset of linked primary and secondary care data. The aims of this study were to estimate the
mean inhaled SABA dose per day for people diagnosed with asthma in a large EHR database, and to examine
variation by demographic factors such as age, sex, and social deprivation. The prescriptions dataset contained
over 40 million prescriptions between 2009 and 2017. 1,987,119 asthma reliever prescription records were
identified (5% of all prescriptions), of which 97% were inhaled formulations. The Spearman correlation
coefficient between subsequent years of aggregated (median) daily estimated SABA from one person-year to
the next was 0.67. Higher median daily inhaled SABA amounts were statistically significantly associated
(Wilcoxon Rank-Sum test p-value<0.05) with being older, male, living in an area of higher deprivation, and
any non-inhaled SABA prescription.
1 INTRODUCTION
Asthma is a common chronic lung disease
characterised by inflammation of the airways and
hyper-responsiveness (sensitivity of the nerve
endings in the airways so they become easily
irritated) to stimuli including allergens, exercise, and
infections (World Health Organization, 2020).
Inflammation results in obstruction of the airways,
and can present as wheezing, chest tightness,
coughing and shortness of breath (American
Academy of Allergy Asthma & Immunology, 2020).
Asthma can be effectively managed for most
people through regular use of Inhaled Cortico-
Steroids (ICS) (Barnes, 1998; Barnes & Pedersen,
1993; Suissa, Ernst, Benayoun, Baltzan, & Cai,
2000), although additional therapies such as Long-
Acting Muscarinic Antagonists (LAMA) and
Monoclonal Antibody therapy (MAb) (British
a
https://orcid.org/0000-0001-7169-4087
b
https://orcid.org/0000-0001-7022-3056
c
https://orcid.org/0000-0002-0994-8100
Thoracic Society & SIGN, 2019; Global Initiative for
Asthma, 2019; Peters, Ferguson, Deniz, & Reisner,
2006) may be used in parallel for those with
insufficient control of their symptoms. Additionally,
most people are prescribed Short-Acting β-2 Agonist
(SABA) symptom reliever inhalers to be used as
needed (British Thoracic Society & SIGN, 2019;
Global Initiative for Asthma, 2019). SABA is a
bronchodilator (it opens the airways) which acts by
relaxing the muscles in the airways, with effects
lasting for around three to six hours (Ullman &
Svedmyr, 1988).
SABA may also be prescribed at higher doses in
non-inhaled formulations, including tablet, syrup,
injections or using a nebuliser (Pharmaceutical Press
Joint Formulary Committee, 2019a). Other reliever
medications are also available, such as
anticholinergics or theophylline (British Thoracic
Society & SIGN, 2019; Global Initiative for Asthma,
Tibble, H., Sheikh, A. and Tsanas, A.
Estimating Use of Short-term Asthma Reliever Inhalers from Electronic Prescription Records.
DOI: 10.5220/0010954700003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 4: BIOSIGNALS, pages 311-318
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
311

2019; Peters et al., 2006). These may be used in
conjunction with controller medications, such as
inhalers containing both an ICS and a Long-Acting β-
2 Agonist (LABA), components (O’Byrne et al.,
2018), as alternatives to SABA, or simply as
additional reliever therapy.
While use of SABA inhalers is encouraged to
relieve exacerbations, both mild and severe, regular
usage is an indicator that current therapy is not
sufficient to achieve symptom control. The 2021
Global INitiative for Asthma (GINA) guidelines
classify SABA over-use as three or greater 200-dose
SABA inhalers within a year (Reddel et al., 2021).
Estimating an individual’s SABA usage may flag
when a step-up in therapy may be required, or when
an individual is not sufficiently adherent to their
routine controller therapy (Chan et al., 2020). This
can be used identify those currently at higher risk of
adverse outcomes, including the need for
unscheduled care, morbidity, and mortality
(Canonica et al., 2021; Stanford, Shah, D’Souza,
Dhamane, & Schatz, 2012).
Electronic Health Records (EHRs) can be used in
pragmatic observational and intervention studies of
asthma (Varsano, Segev, & Shitrit, 2017). SABA
usage can be estimated from prescribing records by
recording the rate of SABA cannister refill. While
previous studies have reported reliever use estimated
from prescribing records, the methodology has not
been sufficiently described for reproducibility or
validated. For example, many studies measure SABA
use averaged over a year (Blakey et al., 2017;
Disantostefano, Boudiaf, Stempel, Barnes, &
Greening, 2016; FitzGerald, Tavakoli, Lynd, Al
Efraij, & Sadatsafavi, 2017; Lugogo et al., 2021;
Makhinova, Barner, Richards, & Rascati, 2015;
Stanford et al., 2012), however this leads to
substantial delay in the detection of increased use.
The aims of this paper were to: (1) estimate the
mean inhaled SABA dose per day for people
diagnosed with asthma in a large EHR database, and
(2) examine the trends of mean inhaled SABA dose
with regards to consistency over time and variation
by demographic factors such as age, sex, and social
deprivation.
2 METHODS
2.1 Data
The Asthma Learning Healthcare System (ALHS)
dataset was created to develop and validate a
prototype learning healthcare system for asthma
patients in Scotland, in which patient data are used to
generate a continuous loop of knowledge-generation,
evidence based clinical practice change, and change
assessment/validation (Soyiri et al., 2018). Over half
a million patients from 75 general practices in
Scotland were recruited, with primary care records
linked to national accident and emergency, hospital
and mortality datasets using the Scottish health
identification number known as the Community
Health Index (CHI) (Tibble et al., 2019). The
prescriptions dataset contained 41,432,295 valid
prescription records for 671,298 individuals dated
between January 31
st
, 2009, to March 31
st
, 2017.
2.2 Reliever Medication Processing
To identify asthma medications, the medication’s
name was searched for “SALBUTAMOL” or any of
the brand names listed in Table 1 (extracted from the
British National Formulary Version 80; September
2020 (Pharmaceutical Press Joint Formulary
Committee, 2019a)).
Reliever medications with non-inhaled
formulations (tablets, injections, or nebulising
solution) were identified. For inhaled medications,
the mean inhaled SABA dosage per day was
estimated from the dosage, the quantity prescribed,
and the dates between prescriptions.
The medication strength (micrograms of
salbutamol in each dose unit, e.g., inhaler puff) was
then extracted by searching the medication name for
any of the values 95, 100, 200, or 400, followed by
“MICRO” or “MCG” (with or without a preceding
space). The volume of the inhaler was estimated by
searching the medication name for 60, 100, 120, or
200, following by “DOSE” (with or without a
preceding space or hyphen). The most common pack
sizes per brand and strength were then examined so
that the modal value could be imputed.
The 99.9
th
percentile of the dispensed quantity of
inhalers in a single prescription was calculated to be
used as the upper threshold for outliers, such as
recording the prescription of a 200-dose inhaler
mistakenly as the prescription of 200 inhalers. In such
cases, the recorded quantity was replaced with the
value 1 (a single inhaler).
Finally, multiple prescriptions of inhaled
salbutamol collected on the same day (multiple
prescriptions for single inhaler units rather than a
single prescription for multiple units) were condensed
into a single prescription.
SERPICO 2022 - Special Session on Diagnostic, Prognostic, and Phenotyping Models from Mined Administrative Healthcare Data
312
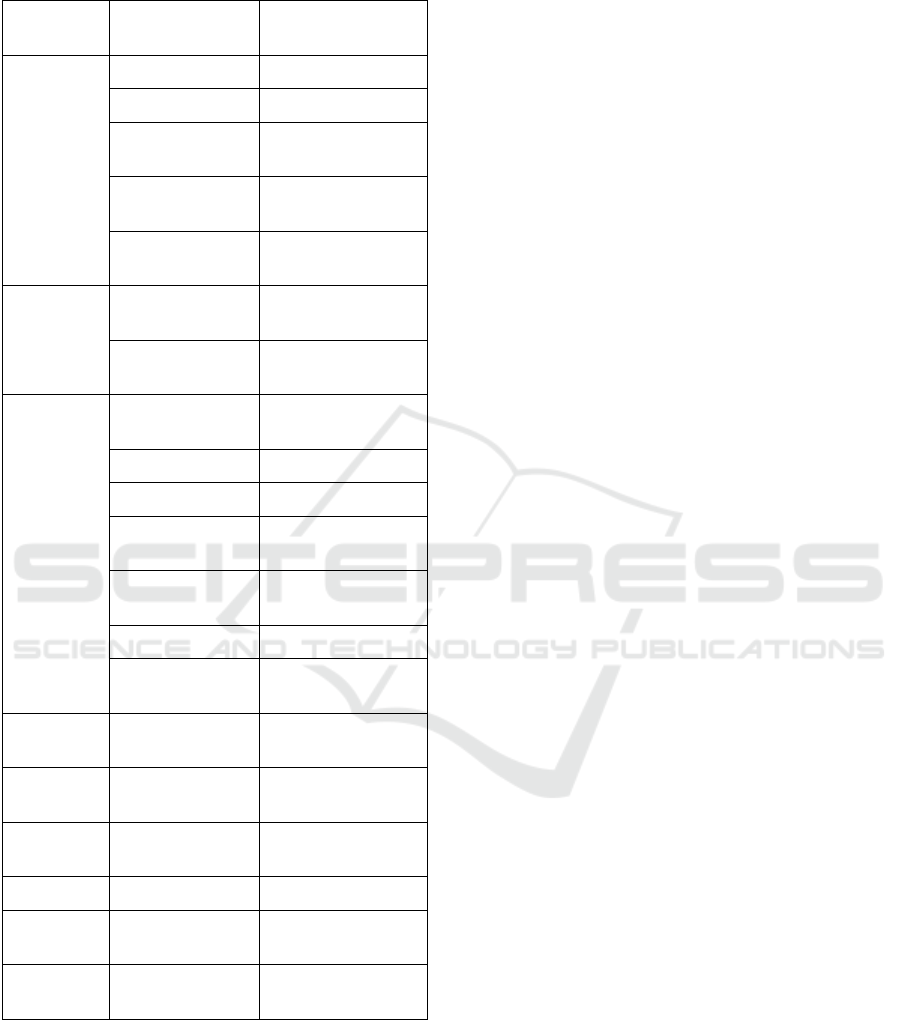
Table 1: Salbutamol brand names, formulations, and
strengths.
Brand
Names
Formulation
Medication
Strength
Generic
Tablet 2mg, 4mg
Oral Solution 2mg/5ml
Pressurised
Inhaler
100mcg
Inhalation
Powder
100mcg, 200mcg
Nebulising
Solution
2.5mg/2.5ml,
5mg/2.5ml
Salamol
Pressurised
Inhaler
100mcg
Nebulising
Solution
5mg/2.5ml
Ventolin
Infusion
Ampoules
5mg/5ml
Injection 500mcg/1ml
Oral Solution 2mg/5ml
Pressurised
Inhaler
100mcg
Inhalation
Powder
200mcg
Nebules 2.5mg, 5mg
Nebulising
Solution
5mg/1ml
Airomir
Pressurised
Inhaler
100mcg
Salbulin
Inhalation
Powder
100mcg
AirSalb
Pressurised
Inhaler
100mcg
Ventmax Capsule 4mg, 8mg
Asmasal Inhalation
Powder
95mcg
Pulvinal
Salbutamol
Inhalation
Powder
200mcg
2.3 Analysis Plan
The asthma reliever prescription record identification
and cleaning process was reported. The number of
inhaled SABA cannisters prescribed per person-year
(in which at least one was prescribed) and time
between prescriptions was summarised.
The mean SABA dosage daily was then estimated
as the prescribed quantity of inhalers multiplied by
the medication strength in micrograms and the
volume of the inhaler unit, and then divided by the
number of days until the next prescription.
The deviation in estimates of daily inhaled SABA
usage by person and by person-year were estimated,
and the Spearman correlation coefficient between an
individual’s average from one year to the next was
evaluated.
The differences in inhaled SABA use associated
with non-inhaled salbutamol use and demographic
factors (age, sex, and deprivation) were investigated
using Wilcoxon Rank Sum tests. For the non-binary
variables (age and deprivation), increments of ordinal
categories were assessed in a pairwise manner. The
Scottish Index of Multiple Deprivation (SIMD) is a
composite geographic-level measure incorporating
income, employment, education, health, access to
services, crime and housing (Scottish Government
National Statistics Publications, 2016), reported here
in quintiles.
3 RESULTS
3.1 Reliever Medication Identification
Of the 41,432,295 valid prescription records,
1,987,119 (4.8%) were identified as salbutamol
products. 68,265 (3.4%) of the prescriptions were
identified as relating to non-inhaled formulations.
For 65.4%, no brand name was listed, indicating
either that it was a generic, or that the recorded
information was incomplete. 27.1% of records were
for Ventolin, and the remaining 7.6% had other
recorded brands.
1,918,854 of the prescriptions related to inhaled
salbutamol, of which 79.4% were unbranded, 9.4%
were Ventolin, 9.2% were Salamol, and 2.6% were
Airomir (remaining 0.4% of prescriptions were for
other brands). The most commonly prescribed
strength was 100mcg (94.1% of prescriptions),
followed by 200mcg (5.4%), 95mcg (0.5%), and
400mcg (<0.1%). Asmasal was the most common
brand at 95mcg (44.5%), and 55.5% were generic.
100% of the 400mcg prescriptions were generic.
The number of doses per cannister was not
recorded in the prescription data for 84.7% of
prescriptions. Table 2 shows the most common
number of doses that could be extracted from each
brand and strength, the percentage of extractable
records with that value, and the number of records for
which no value was extractable.
Estimating Use of Short-term Asthma Reliever Inhalers from Electronic Prescription Records
313
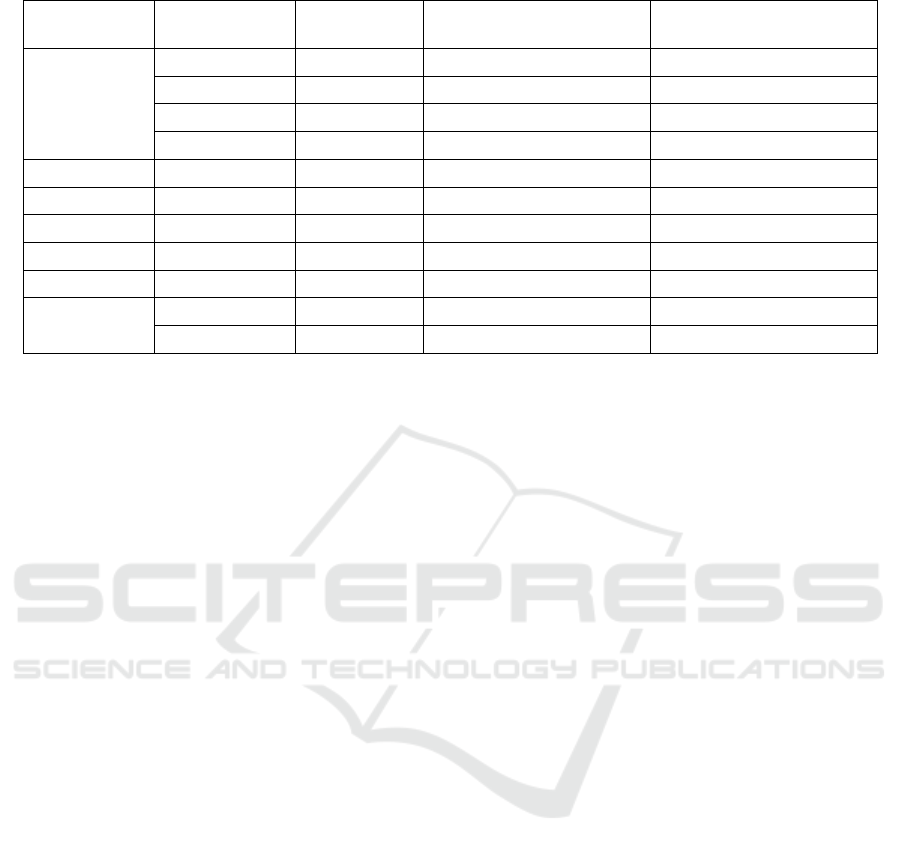
Table 2: The most common doses per cannister extracted by brand name and strength from inhaled salbutamol prescriptions.
Brand
Strength
(micrograms)
Doses per
Cannister
Percentage of prescriptions
with this value extracted
Percentage of prescriptions
no value extractable
Generic /
Brand not
listed
95 200 100% 48.9%
100 200 100% 84.9%
200 60 64.8% 64.9%
400 200 100% 98.1%
Airomir 100 200 100% 77.7%
Asmasal 95 200 99.8% 81.5%
Pulvinal 200 100 98.0% 89.0%
Salamol 100 200 100% 84.4%
Salbulin 100 200 100% 96.9%
Ventolin
100 200 100% 90.6%
200 60 99.0% 89.8%
Consequently, Pulvinal was imputed as 100
doses, Ventolin and generic (or brand not listed)
200mcg as 60 doses, and all other brand-strengths as
200 doses. In total, 94.8% of prescriptions were
estimated to have 200 doses, 4.9% to have 60 doses,
and 0.3% to have 100 doses.
Less than 0.01% of records had missing quantity,
and 0.07% had quantity above the calculated outlier
threshold of 4 inhalers. After imputing these records
to have a single unit quantity, the quantity of cannisters
prescribed was estimated to be 1 for 50.1% of
prescriptions, 2 for 48.5%, 3 for 0.8%, and 4 for 0.6%.
Finally, multiple prescriptions of single inhaled
salbutamol cannisters collected on the same day were
condensed into a single prescription, resulting in
1,882,586 person-days.
3.2 Inhaled Salbutamol Use
In years containing at least one inhaled SABA
prescription, a median of 3 cannisters were obtained
(range 1 – 262, interquartiles 1-6). This was equal to
a median of 420 doses obtained each year (range 1-
52400, interquartiles 200-1200). Using the GINA
criteria (Reddel et al., 2021), a median of 66.7% of
each individual’s person-years could be classed as
periods of over-use (range 11.1% - 100%,
interquartiles 37.5% - 88.9%).
For each individual’s last prescription during the
study period (n=149,621), the duration that the
prescription lasted for could not be calculated (for
29.3% of individuals, this was their only prescription
in the study period). For the remaining prescriptions,
the median time until a repeat was filled was 48 days
(interquartiles 27 and 93 days), with a range of 1 to
2981 days.
The median estimated inhaled salbutamol amount
was 124.2mcg per day, with interquartiles of 45.2 and
360.4mcg. The range of observed daily estimates was
4.1 to 200,000mcg, with the latter being roughly
equivalent to 8 full inhalers being consumed on a
single day (and thus clearly erroneous). Outlier
values were observed when high amounts were
obtained and then a subsequent refill was made
shortly after. For example, there were 7618 (0.4%)
cases in which the estimated daily use was equal to
the amount dispensed, as another prescription was
obtained the very next day.
The InterQuartile Range (IQR) for the estimated
inhaled salbutamol amount was 315.2mcg. There
was less variation within each individual’s estimates,
with a median within-person IQR of 82.0. The
variation within a person was roughly equal to the
variation within a person-year, however, with a
median within-person-year IQR of 93.7. The
Spearman correlation for an individual’s median
estimate from one year to the next was 0.67.
The median daily inhaled salbutamol amount
increased across age categories: 77.5mcg in the under
18s, 88.9mcg in the 18-35s, 136.4mcg in the 36-55s,
180.2mcg in the 56-75s, and 202.0mcg in the those
aged over 75 (Wilcoxon Rank Sum test p<0.001 for
each age group increment’s pairwise difference). The
median amount also increased consistently from
highest SIMD quintile (least deprived) to lowest, at
97.3mcg, 106.4mcg, 121.6mcg, 141.8mcg, and
159.4mcg, respectively (p<0.001 for each quintile
increment’s pairwise difference). Finally, the median
daily amount was higher for men (135.1mcg) than
women (115.6mcg, p<0.001). This finding was
consistent across age groups, as shown in Figure 1.
SERPICO 2022 - Special Session on Diagnostic, Prognostic, and Phenotyping Models from Mined Administrative Healthcare Data
314
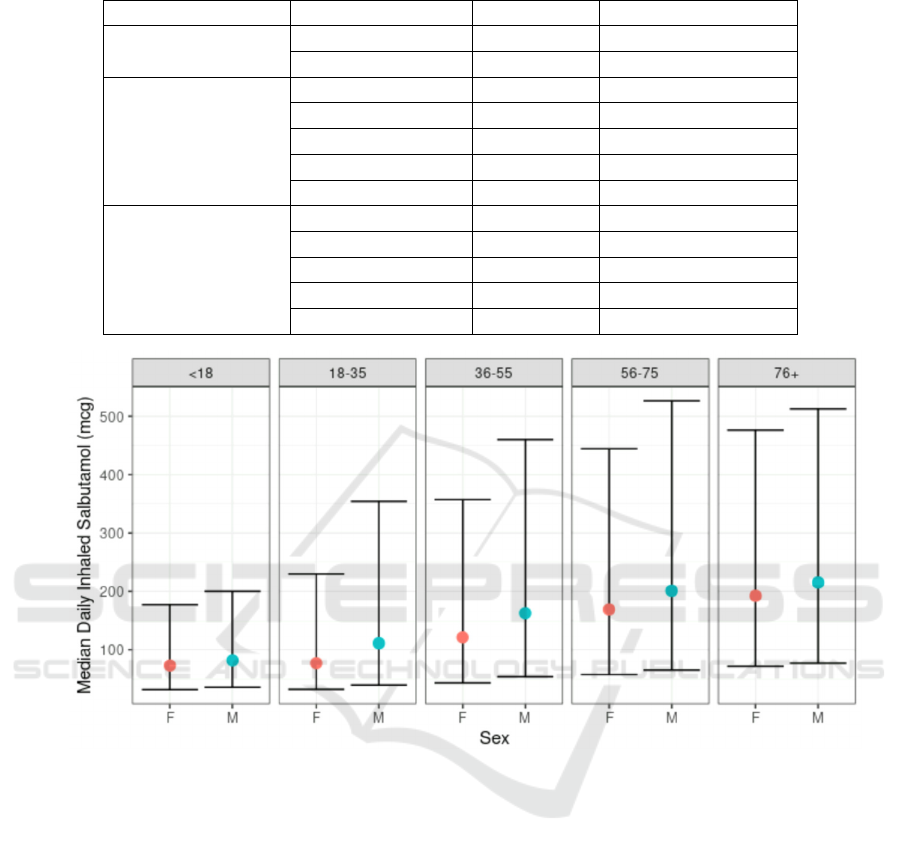
Table 3: Median and Interquartile Range of Daily Inhaled Salbutamol (micrograms) by demography.
Factor Level Median IQR
Sex
Male 135.14 48.54 - 387.10
Female 115.61 42.64 – 387.10
Age Group
Under 18 77.52 33.78 – 190.00
18-35 88.89 34.42 – 277.78
36-55 136.36 47.17 – 394.74
56-75 180.18 60.42 – 476.19
Over 75 202.02 73.26 – 487.80
Scottish Index of
Multiple Deprivation
1 (Most deprived) 159.36 52.22 – 444.44
2 141.84 48.43 – 408.16
3 121.58 45.05 – 357.14
4 106.38 41.11 – 312.50
5 (Least deprived) 97.32 39.45 – 270.27
Figure 1: Boxplot of the Daily Inhaled Salbutamol (micrograms), stratified by Age Group and Sex. The central dot denotes
the median value, and the error bars denote the interquartile range.
3.3 Non-inhaled Salbutamol Use
5799 people had at least one prescription for non-
inhaled salbutamol (tablets, injections, or nebulising
solution) during the study period, of whom 5058 also
had inhaled salbutamol (87.2%). Conversely, only
3.4% of individuals with prescriptions for inhaled
salbutamol also had non-inhaled salbutamol
prescriptions. Those with at least one prescription for
non-inhaled salbutamol had a median of three such
prescribing events, with interquartiles 1 to 13 (range
1 to 254).
The median daily inhaled salbutamol amount was
lower (120.5mcg, interquartiles 44.3 to 354.0mcg) in
person-years without non-inhaled salbutamol
prescribed, compared to years in which at least one
prescription for non-inhaled salbutamol was filled
(496.9mcg, interquartiles 200.0 to 975.6mcg,
Wilcoxon Rank Sum test p<0.001). Indeed, it was
also higher in those with any non-inhaled salbutamol
prescribed during the study period (384.6mcg,
interquartiles 143.4 to 800.0mcg) compared to those
with none (117.0mcg, interquartiles 43.3 to
344.8mcg, p<0.001).
4 DISCUSSION
4.1 Principal Findings
5% of prescriptions were identified as salbutamol-
based asthma symptom reliever medications, of
which 97% were inhaled formulations. In years
containing at least one inhaled SABA prescription, a
Estimating Use of Short-term Asthma Reliever Inhalers from Electronic Prescription Records
315

median of 3 cannisters were obtained. Using the
GINA threshold of 600-doses (Reddel et al., 2021), a
median of 66.7% of each individual’s person-years
could be classed as periods of over-use (range 11.1%
- 100%, interquartiles 37.5% - 88.9%). The median
time until a repeat was filled, for people with more
than one SABA prescription, was 48 days.
The median estimated daily inhaled salbutamol
amount was 124.2mcg (interquartiles 45.2 and
360.4mcg). The median of the interquartile range for
daily estimates within a person-year was 82.0mcg,
and there was moderate-strong correlation between
the median from one person-year to the next
(Spearman correlation coefficient = 0.67).
In a series of pairwise Wilcoxon Rank Sum tests,
higher median daily inhaled salbutamol was found to
be significantly associated with being older, male, or
living in an area of higher deprivation. The median
daily inhaled salbutamol amount was higher in the
3.4% of individuals with any non-inhaled salbutamol
prescribed during the study period. For these
individuals, the median daily inhaled salbutamol was
higher in years in which non-inhaled salbutamol was
also prescribed.
4.2 Results in Context
Many previous studies in the literature estimated
daily inhaled SABA use over the duration of a year
(Blakey et al., 2017; Disantostefano et al., 2016;
FitzGerald et al., 2017; Lugogo et al., 2021;
Makhinova et al., 2015; Stanford et al., 2012). In this
study, we found that substantial variation within a
year was commonplace, with a median within-
person-year interquartile range of 89.3mcg. Studies
such as Stanford et al. (2012), Blakey et al. (2017) ,
and Fitzgerald et al. (2017) have successfully
demonstrated that SABA use in the previous year is a
predictive factor for incidence of asthma attacks.
Assuming that SABA over-reliance is a contributory
factor to this increased risk, rather than simply acting
as a marker for increased asthma symptoms or
reduced symptom control, the ability to detect short-
term changes in SABA use may facilitate more timely
detection of increased risk in order to aid intervention.
Another common way of utilising asthma
symptom reliever inhalers for assessing symptom
control is to calculate the ratio of symptom controller
to reliever medication prescriptions. This may be
either as the number of prescriptions or cannisters
themselves (Baltrus et al., 2017; Lieu, Capra,
Quesenberry, Mendoza, & Mazar, 1999) or the ratio
of inhaled corticosteroid (typically converted to
budesonide equivalent as some medications such as
Ciclesonide are double potency) to salbutamol in
micrograms (Gonem, Cumella, & Richardson, 2019).
Finally, this study only used inhaled salbutamol
for the primary analyses, with prescriptions of other
formulations (including solutions for nebuliser
devices) evaluated as a potential risk modifier. Other
formulations may be prescribed for very young
children or those for whom inhaled therapy cannot be
used reliably, or in the case of acute exacerbation
(Pharmaceutical Press Joint Formulary Committee,
2019b). Other studies, such as Stanford et al.
converted nebulised doses to the equivalent inhaled
doses (Stanford et al., 2012), however the use of non-
inhaled formulations has been identified previously
as a strong risk predictor for adverse outcomes. For
example, Paris et al. found a 21.6 times increase in
hazard ratio for asthma-related hospitalisation for
every daily nebulised salbutamol use (Paris et al.,
2008).
One limitation of the assessment of inhaled
salbutamol use from prescription records is that
prescription refills do not perfectly correspond to
medication usage. This is a limitation of this type of
analysis for any type of medication, however for
reliever medications it may be more common. For
example, individuals may wish to have multiple
inhalers at different locations, and may also be more
likely to misplace inhalers due to their irregular use.
Estimating daily inhaled SABA use over multiple
refills may mitigate this affect somewhat, as periods
of overlap of inhalers may be evened out.
5 CONCLUSIONS
In this study, we have estimated the median inhaled
SABA dose per day and the proportion of individuals
with GINA-defined SABA over-use from our
Scottish cohort, and identified factors associated with
higher inhaled SABA use: increased age, male sex,
higher deprivation, and history of non-inhaled SABA
prescription.
REFERENCES
American Academy of Allergy Asthma & Immunology.
(2020). Asthma Defined. Retrieved November 25,
2020, from https://www.aaaai.org/conditions-and-
treatments/conditions-dictionary/asthma
Baltrus, P., Xu, J., Immergluck, L., Gaglioti, A., Adesokan,
A., & Rust, G. (2017). Individual and county level
predictors of asthma related emergency department
visits among children on Medicaid: A multilevel
SERPICO 2022 - Special Session on Diagnostic, Prognostic, and Phenotyping Models from Mined Administrative Healthcare Data
316

approach. Journal of Asthma, 54(1), 53–61.
https://doi.org/10.1080/02770903.2016.1196367
Barnes, P. J. (1998). Efficacy of inhaled corticosteroids in
asthma. Journal of Allergy and Clinical Immunology,
102(4), 531–538. https://doi.org/10.1016/S0091-
6749(98)70268-4
Barnes, P. J., & Pedersen, S. (1993). Efficacy and Safety of
Inhaled Corticosteroids in Asthma. American Review of
Respiratory Disease, 148(4_pt_2), S1–S26.
https://doi.org/10.1164/ajrccm/148.4_Pt_2.S1
Blakey, J. D., Price, D. B., Pizzichini, E., Popov, T. A.,
Dimitrov, B. D., Postma, D. S., Thomas, M. (2017).
Identifying Risk of Future Asthma Attacks Using UK
Medical Record Data: A Respiratory Effectiveness
Group Initiative. Journal of Allergy and Clinical
Immunology: In Practice, 5(4), 1015–1024.
https://doi.org/10.1016/j.jaip.2016.11.007
British Thoracic Society, & SIGN. (2019). British guideline
on the management of asthma (2019 Edition).
Canonica, G. W., Paggiaro, P., Blasi, F., Musarra, A.,
Richeldi, L., Rossi, A., & Papi, A. (2021). Manifesto on
the overuse of SABA in the management of asthma:
new approaches and new strategies. Therapeutic
Advances in Respiratory Disease, 15,
175346662110425. https://doi.org/10.1177/175346662
11042534
Chan, A. H. Y., Katzer, C. B., Horne, R., Haughney, J.,
Correia de Sousa, J., Williams, S., & Kaplan, A. (2020).
SABA Reliance Questionnaire (SRQ): Identifying
Patient Beliefs Underpinning Reliever Overreliance in
Asthma. Journal of Allergy and Clinical Immunology:
In Practice, 8(10), 3482-3489.e1. https://doi.org/
10.1016/j.jaip.2020.07.014
Disantostefano, R. L., Boudiaf, N., Stempel, D. A., Barnes,
N. C., & Greening, A. P. (2016). The frequency of, and
adherence to, single maintenance and reliever therapy
instructions in asthma: A descriptive analysis. Npj
Primary Care Respiratory Medicine, 26(May), 1–5.
https://doi.org/10.1038/npjpcrm.2016.38
FitzGerald, J. M., Tavakoli, H., Lynd, L. D., Al Efraij, K.,
& Sadatsafavi, M. (2017). The impact of inappropriate
use of short acting beta agonists in asthma. Respiratory
Medicine, 131, 135–140. https://doi.org/10.1016/
j.rmed.2017.08.014
Global Initiative for Asthma. (2019). Pocket Guide for
Asthma Management and Prevention 2019. Retrieved
from www.ginasthma.org.
Gonem, S., Cumella, A., & Richardson, M. (2019). Asthma
admission rates and patterns of salbutamol and inhaled
corticosteroid prescribing in England from 2013 to
2017. Thorax, 74, 705–706. https://doi.org/10.1136/
thoraxjnl-2018-212723
Lieu, T. A., Capra, A. M., Quesenberry, C. P., Mendoza, G.
R., & Mazar, M. (1999). Computer-based models to
identify high-risk adults with asthma: Is the glass half
empty or half full? Journal of Asthma,
36(4), 359–370.
https://doi.org/10.3109/02770909909068229
Lugogo, N., Gilbert, I., Tkacz, J., Gandhi, H., Goshi, N., &
Lanz, M. J. (2021). Real-world patterns and
implications of short-acting β2-agonist use in patients
with asthma in the United States. Annals of Allergy,
Asthma and Immunology, 126(6), 681-689.e1.
https://doi.org/10.1016/j.anai.2021.01.024
Makhinova, T., Barner, J. C., Richards, K. M., & Rascati,
K. L. (2015). Asthma Controller Medication
Adherence, Risk of Exacerbation, and Use of Rescue
Agents Among Texas Medicaid Patients with Persistent
Asthma. Journal of Managed Care & Specialty
Pharmacy, 21(12), 1124–1132. https://doi.org/
10.18553/jmcp.2015.21.12.1124
O’Byrne, P. M., FitzGerald, J. M., Bateman, E. D., Barnes,
P. J., Zhong, N., Keen, C., Reddel, H. K. (2018).
Inhaled Combined Budesonide–Formoterol as Needed
in Mild Asthma. New England Journal of Medicine,
378(20), 1865–1876. https://doi.org/10.1056/NEJMo
a1715274
Paris, J., Peterson, E. L., Wells, K., Pladevall, M.,
Burchard, E. G., Choudhry, S., Williams, L. K. (2008).
Relationship between recent short-acting β-agonist use
and subsequent asthma exacerbations. Ann Allergy
Asthma Immunol., 101(5), 482–487. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC3624763/pdf/nihms412728.pdf
Peters, S. P., Ferguson, G., Deniz, Y., & Reisner, C. (2006).
Uncontrolled asthma: A review of the prevalence,
disease burden and options for treatment. Respiratory
Medicine, 100, 1139–1151. https://doi.org/10.1016/
j.rmed.2006.03.031
Pharmaceutical Press Joint Formulary Committee. (2019a).
British National Formulary Version 80. (BMJ Group
and Pharmaceutical Press, Ed.) (80th ed.). London.
Pharmaceutical Press Joint Formulary Committee. (2019b).
Salbutamol. In British National Formulary Version 80.
Reddel, H. K., Bacharier, L. B., Bateman, E. D., Brightling,
C. E., Brusselle, G. G., Buhl, R., Boulet, L.-P. (2021).
Global Initiative for Asthma (GINA) Strategy 2021 –
Executive Summary and Rationale for Key Changes.
American Thoracic Society. https://doi.org/
10.1164/rccm.202109-2205pp
Scottish Government National Statistics Publications.
(2016). Introducing The Scottish Index of Multiple
Deprivation 2016. Retrieved from https://www.gov.
scot/Resource/0050/00504809.pdf
Soyiri, I. N., Sheikh, A., Reis, S., Kavanagh, K., Vieno, M.,
Clemens, T., Simpson, C. R. (2018). Improving
predictive asthma algorithms with modelled
environment data for Scotland: an observational cohort
study protocol. BMJ Open, 8, e23289. https://doi.org/
10.1136/bmjopen-2018-023289
Stanford, R. H., Shah, M. B., D’Souza, A. O., Dhamane, A.
D., & Schatz, M. (2012). Short-acting β-agonist use and
its ability to predict future asthma-related outcomes.
Annals of Allergy, Asthma and Immunology, 109(6),
403–407. https://doi.org/10.1016/j.anai.2012.08.014
Suissa, S., Ernst, P., Benayoun, S., Baltzan, M., & Cai, B.
(2000). Low-Dose Inhaled Corticosteroids and the
Prevention of Death from Asthma. New England
Journal of Medicine, 343(5), 332–336. https://doi.org/
10.1056/NEJM200008033430504
Estimating Use of Short-term Asthma Reliever Inhalers from Electronic Prescription Records
317

Tibble, H., Tsanas, A., Horne, E., Horne, R., Mizani, M.,
Simpson, C. R., & Sheikh, A. (2019). Predicting asthma
attacks in primary care: protocol for developing a
machine learning-based prediction model. BMJ Open,
9(7), e028375. https://doi.org/10.1136/BMJOPEN-
2018-028375
Ullman, A., & Svedmyr, N. (1988). Salmeterol, a new long
acting inhaled Beta-2 adrenoceptor agonist: comparison
with salbutamol in adult asthmatic patients. Thorax,
43(May), 674–678.
Varsano, S., Segev, D., & Shitrit, D. (2017). Severe and
non-severe asthma in the community: A large electronic
database analysis. Respiratory Medicine, 123, 131–139.
https://doi.org/10.1016/j.rmed.2016.12.017
World Health Organization. (2020). WHO | Asthma:
Definition.
SERPICO 2022 - Special Session on Diagnostic, Prognostic, and Phenotyping Models from Mined Administrative Healthcare Data
318
