
Tele-tDCS: A Novel Tele-neuromodulation Framework using
Internet of Medical Things
Samuel S. D. Herring
1
, M. A. Hannan Bin Azhar
1a
and Mohamed Sakel
2b
1
School of Engineering, Technology and Design, Canterbury Christ Church University, U.K.
2
East Kent Hospitals University, NHS Foundation Trust, Kent, U.K.
Keywords: tDCS, Neuromodulation, Parkinson’s, IoMT, Tele-health, Biomedical Device, e-Health.
Abstract: As part of the Internet of Medical Things (IoMT) within Biomedical Engineering, telehealth is an emerging
field. Due to the recent events surrounding COVID-19, it has become obvious that Telehealth treatments must
be developed as a means of protecting vulnerable patients in hospitals by reducing the need to visit and
therefore reducing risk to physicians. This paper investigates the feasibility of developing a non-invasive
remote neuro-stimulation system using internet-based transcranial Direct Current Stimulation (tDCS). A
hardware-based prototype tDCS device has been developed to be controlled using a remote command-line
interface over the internet. As a result, a physician can remotely set the parameters for the tDCS treatment
and monitor the treatment in real-time to ensure patient safety. In this study, the feasibility of a Tele-tDCS
system was investigated, as well as the capabilities a Tele-tDCS system should offer to patients.
1 INTRODUCTION
Telehealth is an evolving field, a part of the Internet
of Medical Things (IoMT) within Biomedical
Engineering. In current society, it is of growing
significance to develop such systems, as it allows
patients to be treated remotely by physicians.
However, models must be in place to ensure that the
treatments may be performed appropriately, taking
into consideration the security risks associated with
the IoMT (Hall et al., 2014). Recent events involving
COVID-19 make it clear that there is a need for
telehealth treatments to be developed because of the
benefits of protecting vulnerable patients by reducing
the need for visits to hospitals and other clinical
settings, and also reducing risk to physicians through
less physical patient contact (Smith et al., 2020).
Transcranial Direct Current Stimulation (tDCS)
has been used in neurorehabilitation for many years
to effectively increase or decrease mental function
and learning (Bucur et al., 2018) and it is considered
to safe and widely accepted (Bikson et al., 2016). The
use of tDCS for treating neurological disorders such
as Parkinson's disease and other movement-related
disorders has been considered in several studies
a
https://orcid.org/0000-0003-1190-6644
b
https://orcid.org/0000-0001-6749-5229
(Boggio et al., 2006; Lefaucheur et al., 2017). It has
been demonstrated that such treatments are effective
in a wide range of patients with neuro disorders,
where tDCS treatment has improved quality of life
(QoL) in patients who would otherwise suffer
significantly (Leite et al., 2014). In order to obtain the
desired results with tDCS systems, researchers
typically need to work with patients. This is because
these types of systems are required to precisely target
and focus on specific areas of the brain for stimulation
(Park et al., 2011). In addition, tDCS systems can be
very expensive, limiting their use to specialist units
with facilities where they exist (Zaghi et al., 2009).
Consequently, new approaches to performing tDCS
have been developed for improving patient outreach.
As an example, the methods mentioned by Sourav
(2017) are built on open source framework to provide
the same therapies, ultimately benefiting more
patients. However, such systems are still under
development and susceptible to certain limitations,
such as the accuracy of actual output currents and the
efficacy of the system.
As tDCS treatments require specialised clinician
supervision, treatment monitoring and delivery are
essential features of any novel tDCS system. By
84
Herring, S., Azhar, M. and Sakel, M.
Tele-tDCS: A Novel Tele-neuromodulation Framework using Internet of Medical Things.
DOI: 10.5220/0010882500003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineer ing Systems and Technologies (BIOSTEC 2022) - Volume 1: BIODEVICES, pages 84-93
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

providing remote and cloud-based services for such
treatment, more patients could be reached. This study
will explore how tDCS could be utilised by doctors
remotely using cloud-based applications. A system
like this must take into consideration legal and ethical
implications, including the need for safe testing and
development prior to clinical trials. As a result, these
automated remote solutions have to be secure and
must pose no risk to patient privacy or abuse or
misuse of tDCS treatments. In order to keep costs
down and make the device more affordable for
patients, this study will explore use of off-the-shelf
components in the hardware design.
The remainder of the paper is organised as
follows: Section 2 discusses the optimal conditions
reported in the literature for tDCS treatments, Section
3 discusses the effects of tDCS on patients from
previous studies, Section 4 presents the proposed
framework and use cases for evaluating the system,
Section 5 explains the development of the remote
software interface, Section 6 describes the hardware
components of the system, Section 7 reports the
results of the evaluation of the system, and Section 8
concludes the paper and suggests directions for future
work.
2 TRANSCRANIAL DIRECT
CURRENT STIMULATION
The tDCS is a non-invasive brain stimulation
technique, used to modulate the excitability of the
central nervous system in humans (Woods et al.,
2016). The aim of stimulating the central nervous
system is to change the discharge of neurons in the
brain. The effects of the altered neurons have effects
that may be potentially positive or negative for a
patient. There have been a number of studies
investigating optimum testing parameters for patients
undergoing a tDCS treatment. These parameters
include session durations (minutes), current doses
(mA) and session timelines. The aim is to discover the
optimum conditions to produce the greatest long-term
cognitive plasticity improvement (Fertonani et al.,
2014).
A study conducted by Bikson et al. (2009)
established the safety limits for tDCS treatments and
suggested the average treatment time to be 20
minutes, at a range of 5-30 minutes. The duration of
treatment depends on the neurophysician's
prescription for each session, as confirmed by Thair
et al. (2017). Therefore, any tDCS system being
developed would need to have the capability of
performing optimally throughout the treatment
duration, potentially 30 minutes (Bikson et al., 2009;
Thair et al., 2017). Additionally, studies have been
conducted to determine whether current tDCS doses
are both safe for patients and provide a sufficient level
of stimulation to see positive results. Research from
Parazzini et al. (2014) found that 1 mA had no brain-
stem interference, so is an appropriate dose for
prolonged tDCS treatment up to 30 minutes. An
earlier study by Parazzini et al. (2013) found a dose
below 2 mA did not affect the heart, indicating a safe
current range of 1 to 2 mA. Once again, a doctor
would prescribe a precise amount for the patient
(Parazzini et al., 2014; Parazzini et al., 2013).
Finally, the number of sessions needed to achieve
the optimum neurological and cognitive
improvement is also an integral part of the treatment.
Studies from Castillo-Saavedra et al. (2015) showed
that five sessions per week were the optimal number.
These results were mirrored by Loo et al. (2010) with
treatments lasting between two and eight weeks.
However, no further improvements were observed
after week six. In cases where the number of sessions
were exceeded, there was a risk of minor negative
effects on the patients (Loo et al., 2010). Therefore,
the platform must support a scheduling or control
mechanism to protect the patient in accordance with
the physician's instructions.
In order to prove any tDCS system is successful
in treating a patient, there have been randomised
sham tDCS studies, where the device suggests to the
patient that the system is providing the current to the
patient. However, in reality no current is administered
– this is called a sham or placebo tDCS trial (Palm et
al., 2013; Palm et al., 2012). While such studies
describe methodologies to perform sham tDCS trials,
they don’t discuss a device specific method that
would allow the hardware platform to automate the
process by providing both fake and real treatments to
the patients. Previous studies only suggest a random
cross-over mechanism during the middle of the trial
by swapping patients between sham or real tDCS
treatments (Palm et al., 2012). Therefore, further
investigations are needed to automate the integration
of placebo and real treatments into the hardware
platform.
3 EFFECTS OF tDCS ON
PATIENTS
Several studies have been conducted on the benefits
of tDCS for treating a variety of health conditions,
Tele-tDCS: A Novel Tele-neuromodulation Framework using Internet of Medical Things
85
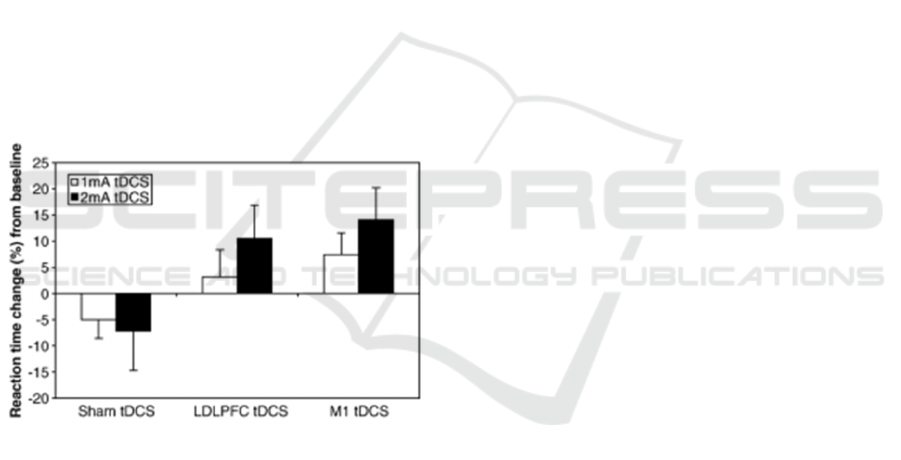
from relatively simple cognitive improvements
(cognitive neuroplasticity) to treating depression,
Parkinson's disease, dyslexia, and fibromyalgia with
the goal to improve patient QoL (Fregni et al., 2006;
Boggio et al., 2006). Figure 1 illustrates how tDCS
significantly improved cognitive reaction times of
patients with Parkinson's disease. It shows
improvement in both 1 mA and 2 mA doses. With up
to 15% increase in response time to some patients,
tDCS has the potential to improve QoL for many
patients. While the studies show direct improvements
in patients, they do not examine in detail the exact
varying parameters of treatment, even if this is a
relatively minor variation in current, due to varying
load resistance (through the patient's head), device
output voltage or overall power output. From patient
to patient, the head size and skull thickness will vary,
which means that there is a wide variance in head
resistance, with the average being 7560 +/- 4130
Ohm-cm (Law, 1993). To account for physiological
differences, additional studies need to be undertaken
to explore more precise parameter values, including
the variations in load output during tDCS treatments
for a wide range of patients.
Figure 1: Response time improvements from patient's with
Parkinson's Disease (Boggio et al., 2006).
As discussed previously, research has been
conducted to explore the suggested parameter ranges
for patients and define treatment durations (Bikson et
al., 2009; Bikson et al., 2016). Yet little is known
about the design of tDCS devices. Specifically, the
design decisions that have been made to ensure that
patients receive their treatment safely. This may
involve safety-net systems that work both
autonomously or manual overrides to stop incorrect
treatment current doses or durations. To put such a
system into the hands of a large and possibly
unrestricted group of patients, it must be explored
how the tDCS devices can be controlled with a high
degree of precision.
4 PROPOSED Tele-tDCS
FRAMEWORK
The cloud communication is an important element of
a Tele-tDCS platform to protect both patients and
physicians. Studies have demonstrated the use of
Tele-tDCS devices where patients are treated
remotely after receiving the specialist device, and
doctors work remotely with patients via video
conferencing (Cucca, et al., 2019). Although this
framework provided a mechanism for delivering a
patient's required dose in line with current tDCS
safety regulations and guidelines (Bikson et al., 2009;
Bikson et al., 2016), it did not provide a mechanism
for collecting real-time data about individual
treatment parameters or details regarding patient’s
conditions. Additionally, it does not discuss further
safety mechanisms that allow the device to deliver the
correct dose to the patient or the ability for the doctor
to remotely control it. In this paper, a novel IoMT
device is presented that facilitates bi-directional
communication between a patient's tDCS device held
remotely (such as at their home) and a physician's
software interface.
Figure 2 demonstrates the treatment process in the
Tele-tDCS framework. Each element of the process
is indicated with a change of colour in the figure. The
first phase (blue), is checking the device is online. If
confirmed, the Command Line Interface (CLI) of the
system provides the option to set the tDCS treatment
parameters. Once set, the treatment is considered
authorised and waits for the patient starting the
treatment through a button press on the device. The
confirmation triggers a start signal to be sent from the
tDCS device to the CLI. Once received, the CLI
enters a loop for the duration of the treatment, where
it regularly polls the tDCS device to collect the real-
time treatment values. This looping mechanism also
contains a treatment abort loop, which checks for
connection loss between the CLI and the device, as
well as checking to see whether the physician or
patient has stopped the treatment.
For the prototype system a publicly available
secure platform, Particle Cloud (Particle, 2020), was
used as the data hub for the framework. However,
clinic's private cloud system would be the obvious
choice when the device is manufactured after the
validation stage for trust and security reasons. Particle
have released a white paper that contains a security
checklist for all applications on their network
(Particle, 2020). API requests sent between the device
and the remote software interface utilise a 2048-bit
TLS certificate which uses HTTPS as a required
protocol.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
86
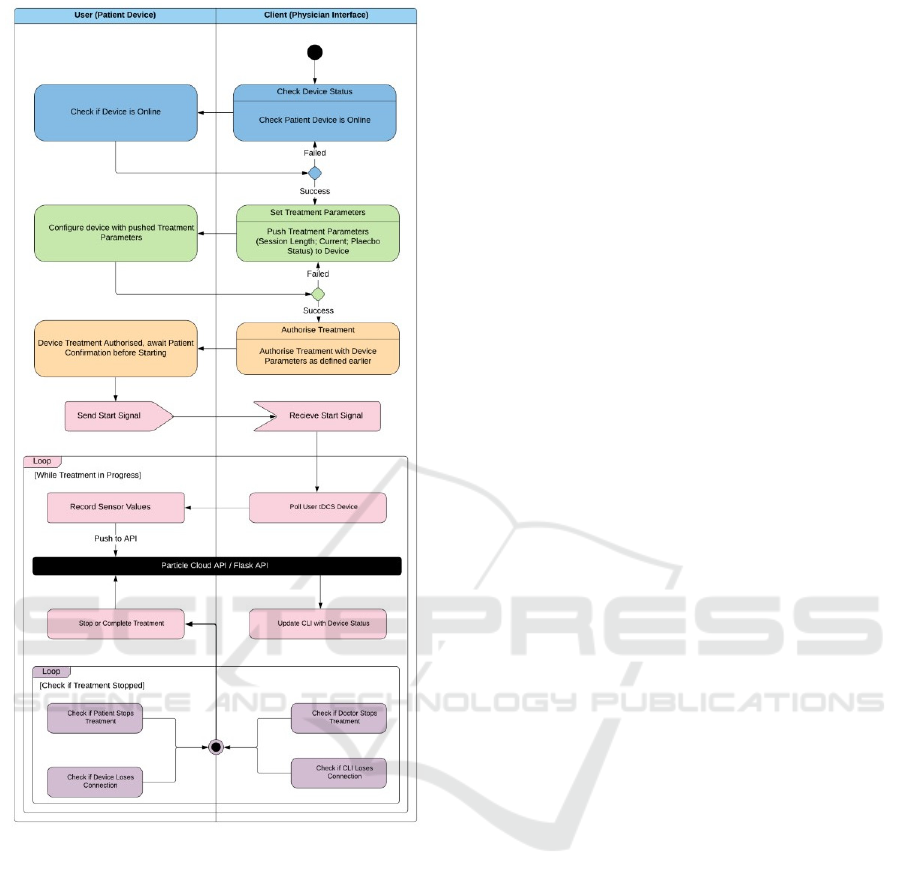
Figure 2: Tele-tDCS data flow diagram.
In addition to the secure API calls, the device uses
the OAuth 2.0 Standard for secure device login when
creating tokens for User to Client Communications.
These standards used for the proposed prototype
IoMT Tele-tDCS system ensure that all data on the
device, inbound and outbound are secure and users
are not at risk being compromised by unauthorised
actors during a treatment. In the future, as medical
needs evolve, this framework allows for more
functional safety-oriented Tele-treatments, in
contrast to closed proprietary systems that cannot be
modified (Cucca et al., 2019). In addition to
architectural descriptions for secure operational
features, following use cases were considered to
evaluate system’s behaviour from user’s point of
view.
4.1 Use Case 1: Set Treatment
Parameters
The first use case of the framework is the ability to set
treatment parameters in the tDCS device. There are
three variables of interest: session length, current
status, and placebo status. In this scenario, the doctor
will access the client and define these parameters
within the acceptable safety ranges for tDCS
treatments. In this case, they stand for 1-60 minutes,
1-2 mA, and True/False for duration, current state,
and placebo status, respectively. This range falls
within the standard practice guidelines for tDCS
treatments (Bikson et al., 2009; Bikson et al., 2016;
Thair et al., 2017). Consequently, only appropriate
treatment values will be allowed to be entered via the
physician's interface while parsing the inputs at the
point of data entry.
4.2 Use Case 2: Monitor Treatment
Progress in Real-time
Another use case of the framework is to record sensor
data in real-time during treatments. The objective is
to emulate the ability of a doctor to be part of a
patient's care in real-time. Monitoring can be
performed for all treatment parameters as well as
incoming parameters from sensors using the I2C
Protocol (SparkFun, 2020).
4.3 Use Case 3: Patient Treatment
Safety Mechanisms
Safeguarding patients is one of the most important
aspects of Telehealth devices. Tele-tDCS systems
must ensure patient safety throughout the treatment
period (Riggs et al., 2018). Although there are
systems that provide remote monitoring so that a
physician can monitor the patient before tDCS
delivers the dose, this remote monitoring does not
provide the ability to abort a treatment by physicians
remotely if necessary. Similarly, the patient or
caregiver may need to end the treatment at any time.
The proposed Tele-tDCS device will implement this
through a regular polling mechanism between the
physician's CLI and the user's tDCS device. This is a
passive safety mechanism that can be operated
manually; however, real safety mechanisms should be
a combination of active and passive safety
mechanisms. The proposed tDCS device overcomes
these flaws by providing active safety systems on-
board that enable tracking and monitoring of the
output current and other treatment parameters. A
parameter deviation will immediately stop the dose
Tele-tDCS: A Novel Tele-neuromodulation Framework using Internet of Medical Things
87
from being delivered to the patient, and an alert will
immediately be sent to the physician’s CLI.
5 REMOTE INTERFACE
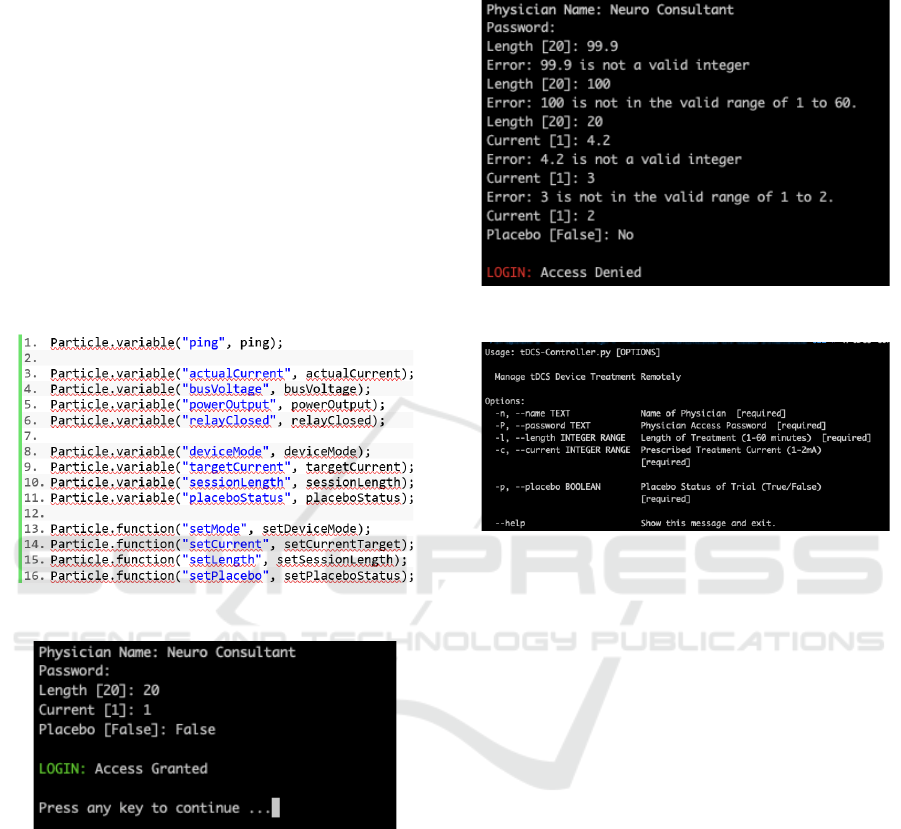
Remote CLI was developed to provide a reliable
connection to the Particle Platform using secure API
requests and OAuth tokens. Among the variables
stored on this platform are the treatment parameters
(Current, Session Length, Placebo Status) as well as
the device status and a selection of other relevant
variables. At device start-up configuration, a particle
class specific to each device is called (as shown in
Figure 3), revealing its status. These variables are
made publicly accessible via the Particle Class.
Figure 3: Particle microcontroller setup code.
Figure 4: Tele-tDCS physician's CLI login.
Using the Click Library, the CLI was written in
Python 3.7. The library provides all the necessary
error handling for parameters (Pallets, 2020). In order
to run the CLI, the physician will need to install a
Python 3.7 emulator and Click, with all its
dependencies installed. To use the Tele-tDCS
System, the physician connects to the tDCS
Controller's CLI as shown in Figure 4. This interface
allows the physician to define the tDCS treatment
parameters within the expected safe ranges. The
system also checks input data to ensure the requested
parameters are within an acceptable range and
suggests help texts where incorrect parameters have
been entered (Figure 5).
Figure 5: System checks incorrect inputs.
Figure 6: Help page option for each of the treatment.
In the CLI, parameters will be passed to the
device that are safe for the majority of patients,
although the final parameter values are determined by
the physician. The system also includes a doctor's
name, as well as a password access to the system to
be used for both security and for logging treatment
information. In the event of any ambiguities, or if the
physician is using the utility for the first time, they
may refer to the CLI's inbuilt help page which defines
the system's permitted parameters, as shown in Figure
6, to allow successful setting of the treatment
parameters
6 Tele-tDCS DEVICE
HARDWARE
6.1 Tele-tDCS Device Circuit
In Figure 7, the detailed breadboard schematic of the
proposed Tele-tDCS device circuit is shown. Power
for the current dose is supplied by a Li-ion battery,
which powers both the microcontroller of the Particle
Argon as well as the Adafruit Power Boost 1000B IC.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
88
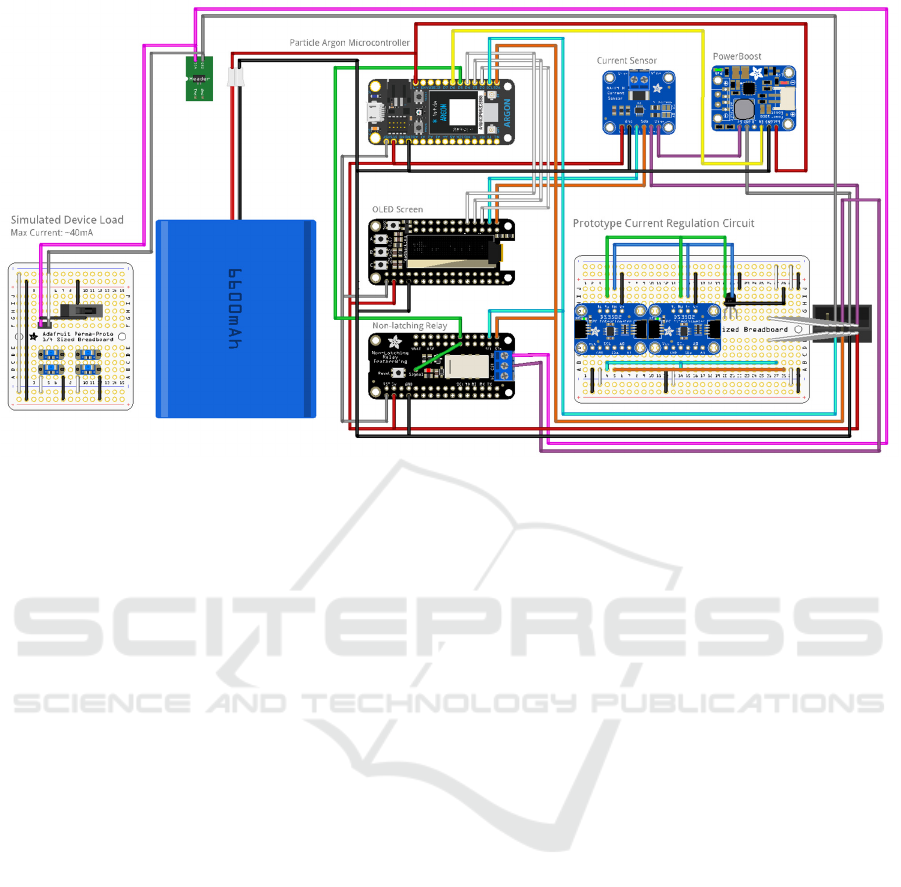
Figure 7: Tele-tDCS graphical breadboard schematic.
It is important to note that the microcontroller's
power supply and the power boost supply are fully
isolated. This ensures that as the load from the
microcontroller changes in usage, there will not be
any potential interference with the patient's DC load.
The current dose the patient requires is of low
amplitude, so an acceptable error range is very small,
with the prototype having a minimum resolution of ±
0.1mA. The microcontroller can control the Power
Boost by pulling the ‘EN’ pin low from the D6 pin;
this can be seen as a yellow line in Figure 7.
Therefore, in placebo scenarios, the power can be
turned off without the patient’s knowledge, removing
potential bias in the trials.
Upon powering up the Power Boost, its 5V output
is directly connected to the current sensor (via the Vin
pin), allowing the microcontroller to monitor the
current output from the Power Boost. Sensor readings
are then polled by the system through the SCL and
SDA pins. These allow sensor data to be shared via
an I
2
C Protocol connection between the two ICs. Vout
from the current sensor connects to Vin from the
current regulation prototype circuit, which provides a
set current value out to the patient.
In Figure 7, the purple connection indicates the
Vin pin connection. One of the circuit's active safety
mechanisms is implemented through a Non-Latching
Relay, which acts as a safety barrier between the
patient and the device. There are two features in
this,one controlled by the microcontroller and the
other controlled by the non-latching relay. First, if a
deviation occurs from the prescribed treatment
parameters, the microcontroller will immediately
cease administering the treatment. Also, the relay
shares Vcc (power lines) with the microcontroller. As
a result, if the microcontroller loses power, the relay
will automatically open, stopping any dose from
being delivered to the patient.
6.2 Current Regulator Circuit
In comparison to other tDCS systems (Sourav et al.,
2017), the current regulation circuit provides a
variable current that can be digitally controlled in
real-time. By knowing the precise current
measurement, the microcontroller dynamically
adjusts the digital potentiometers wiper values to
provide the required current to the patient. Through a
dynamic adjustment process the current sensor
calculates the required resistance needed from the
Digital Potentiometers (Digi Pots) for the LM344
Current Regulator. The logic to dynamically adjust
the wiper values is shown in Figure 8, where first the
sensor for current is being read and then compared
with the target value. If the actual current is greater
than the target value, then the Digi Pot wiper value is
increased.
Figure 9 shows the Digi Pots with the required
connections for LM344. The default values of the
Digi Pots were 64 and 32, respectively. After
experimentation, these values were found to provide
the required target current of 1mA. Using two Digi
pots in series, resistance values were varied for the
LM344 Circuit Regulator. The values of 64 and 32
Tele-tDCS: A Novel Tele-neuromodulation Framework using Internet of Medical Things
89
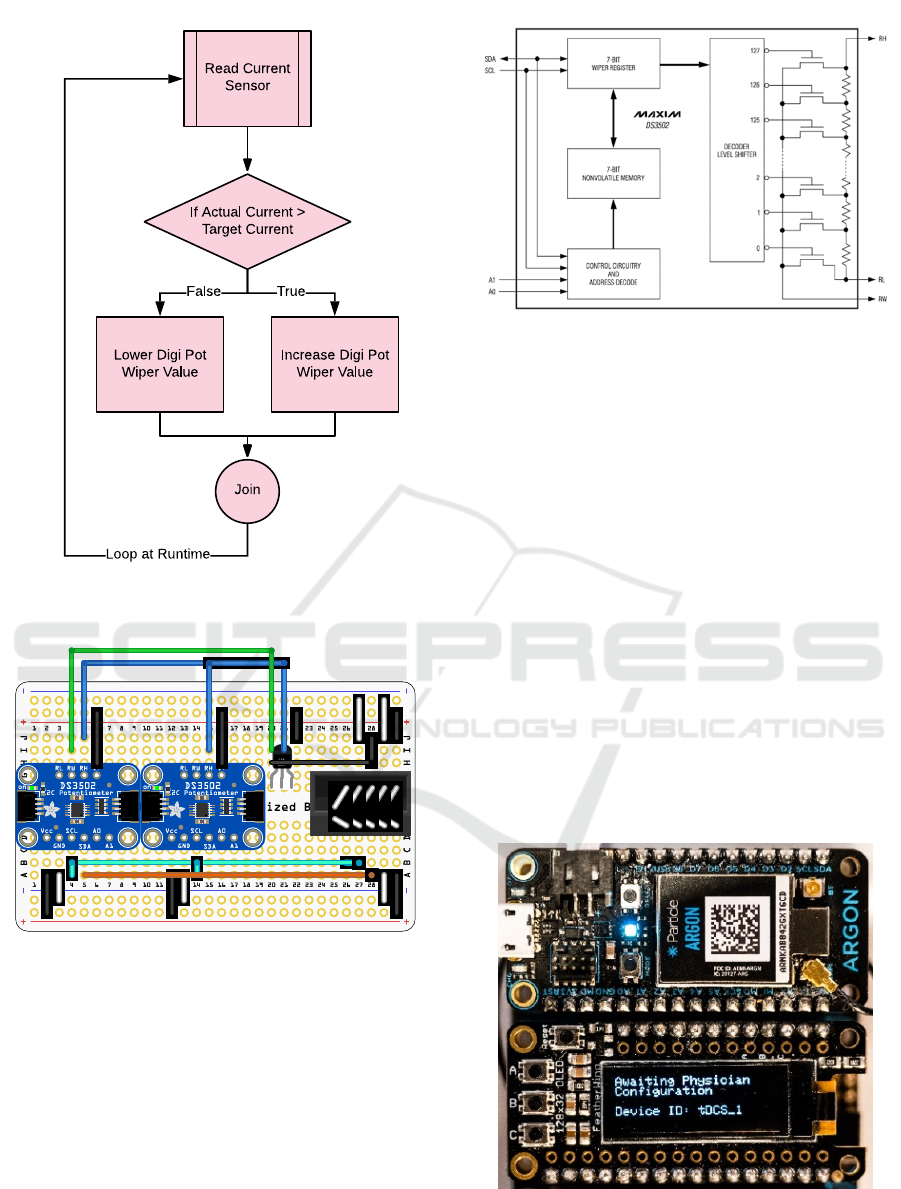
Figure 8: Dynamic adjustment of Wiper values.
Figure 9: Current regulator breadboard schematic.
are set to DS3502_0 and DS3502_1, respectively.
They are set within the 7-bit wiper register, seen in
the datasheet of the Digi Pots (Figure 10). This allows
the device an approximate current output of 1±
0.5mA, calculated through trials with the device.
However, these Digi Pots would also allow for a
higher current should the tDCS treatment require
alternative current values.
Figure 10: DS3502 datasheet (Maxim, 2009).
Once the device is turned on, during
configuration, it is possible to configure it to reach a
more precise current output within 0.1mA by
adjusting the Digi Pot Wiper Values. Changing wiper
values would take less than five seconds,
Alternatively, wiper settings can be adjusted for
specific current values during calibration, such that
during operation the device skips the wiper setting
time to deliver the same current.
6.3 Tele-tDCS Device Interface
Upon turning on the Tele-tDCS device, it will
perform basic configuration and setup, which
includes connecting to the Wi-Fi network, or other
available web platforms in the vicinity. The system
will then enter an "Awaiting Physician
Configuration" mode, shown in Figure 11, when it
awaits for the treatment parameters to be sent from
the physician's CLI (as shown in Figure 12).
Figure 11: Tele-tDCS device interface.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
90
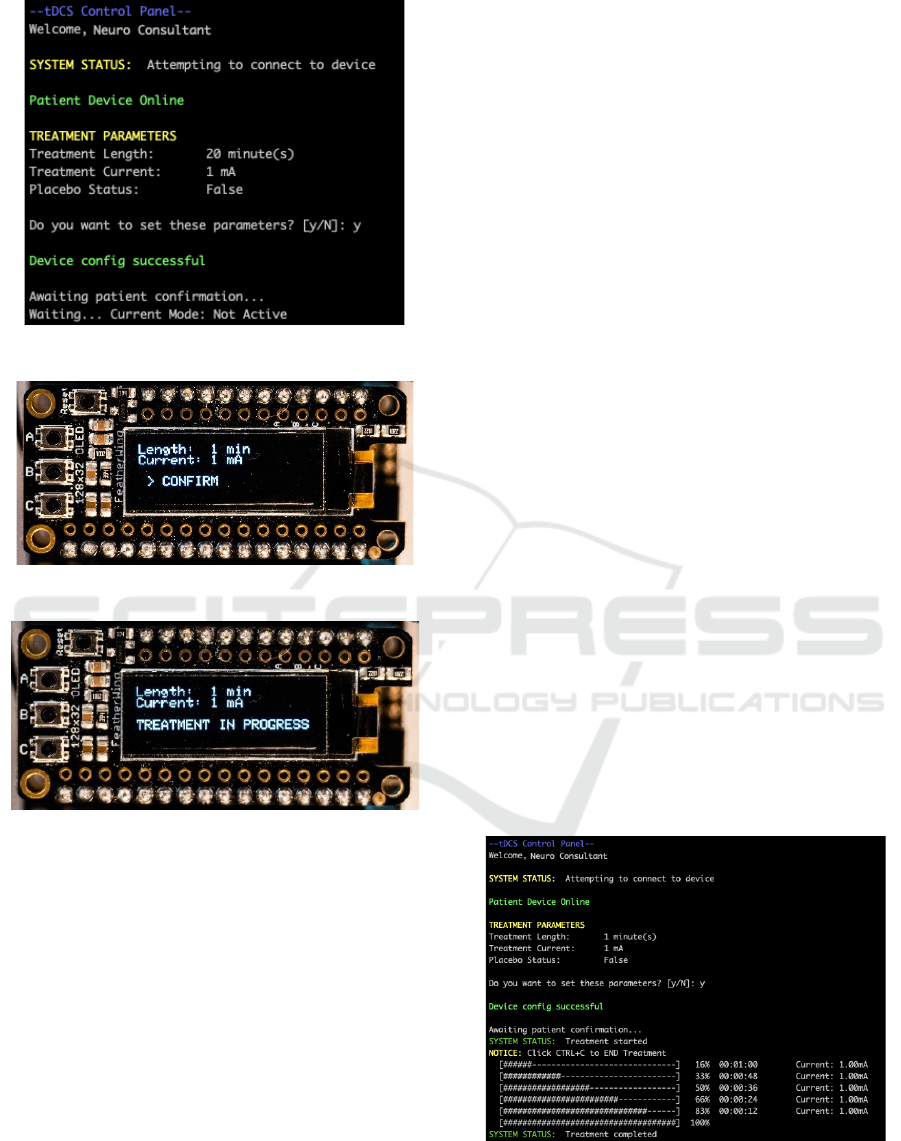
Figure 12: CLI setting device's treatment parameters.
Figure 13: CLI prompting the user to confirm.
Figure 14: Device status during treatment.
The RGB Status LED is shown in Figure 11 as
being a blue colour, indicating that the device is
connected to the Particle Cloud. Once the parameters
have been pushed by the physician using CLI control
panel (Figure 12), the patient should have the final
confirmation to start the treatment when they are
ready. Figure 13 demonstrates device interface
prompting the user to
confirm the treatment
parameters and begin the treatment. Once confirmed,
Tele-tDCS Device Interface shows the user the
'TREATMENT IN PROGRESS' in Figure 14.
As the
treatment starts, the real-time monitoring also begins,
and the device will push the sensor readings to the
Particle Cloud API, ready to be received by the CLI.
7 EVALUATION OF THE
SYSTEM
Experiments were conducted to evaluate the system
for all three use cases outlined in the framework,
described in Section 4. A dummy load was used to
test the operation and functionality of the Tele-tDCS
system without any human presence. This was done
to verify the circuit functionality and safety. Upon
receiving ethical approval in the future, human trials
will be carried out.
In the first use case, framework's ability to
manage tDCS treatment parameters was tested. This
test was necessary to ensure usability of the system,
as well as simplicity and clarity of use. Furthermore,
it is important to verify that the communication
between the physician and the device is in real-time,
without any delays that could impair the safe
administration of doses or stop treatments in the event
of problems. Experiment was conducted to test
whether the parameters of a simulated treatment,
which are tDCS session length, current state and
placebo status, could be delivered to the tDCS device
successfully. The tDCS device screen showed
‘Awaiting Physician Configuration”. The three
parameters were then set, and once this was
confirmed the treatment was able to commence, and
the GUI stated, ‘Treatment in Progress’. Once the
current was administered over the prescribed time the
GUI stated, ‘Treatment Stopped’. The GUI then
confirmed ‘Treatment Completed.’ Each of these
stages required confirmation from the Physician’s
CLI. The physician’s interface ensured that only
appropriate treatment ranges were used by parsing the
parameters at the point of data entry.
Figure 15: CLI monitoring Live a simulated treatment.
Figure 15 shows demo of the treatment phase,
which also shows the status when the treatment was
Tele-tDCS: A Novel Tele-neuromodulation Framework using Internet of Medical Things
91
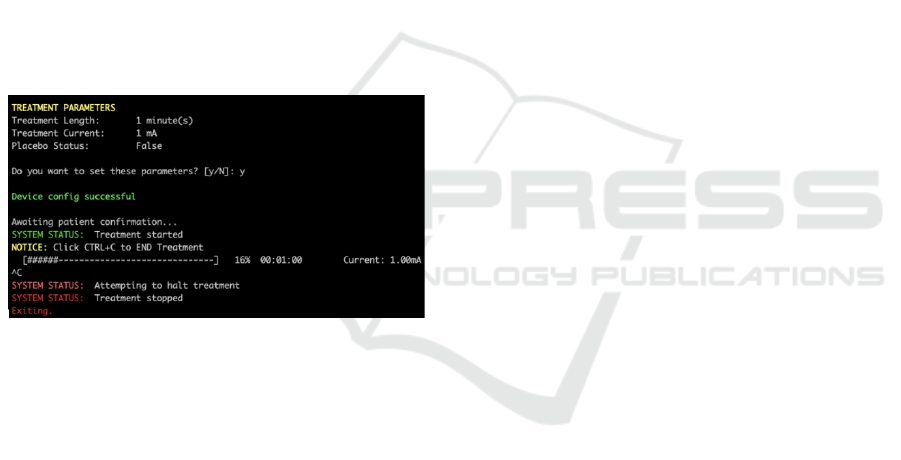
completed. The interface provided a clear and concise
information to the tDCS device remotely throughout
the experiment’s simulated treatment process.
Additionally, the ability to stop the treatment at any
time by the tDCS device was very simple through a
button push. In real use, some patients may find the
technology stressful, so it has been kept simple to
minimise difficulties and to aid physicians in
reassuring and guiding patients during remote
consultations.
In Use Case 2, the treatment progress was
monitored in real time. The device was tested to
ensure that the tDCS device’s treatment parameters
were continuously monitored, including any
additional parameters that can be sent to the device
from a sensor using the I
2
C Protocol. The
experimental use of the CLI and device indicated that
a doctor could have the same level of control that can
be achieved during face to face contact. The systems
provided constant feedback from the device regarding
its status both operationally and regarding its
treatment output.
Figure 16: Treatment being halted by the CLI.
Final experiment was conducted to testify the
third use case of the framework to examine the safety
of the device. The remote monitoring in this instance
would allow the physician to monitor the patient in
real time. It was possible to abort a treatment anytime
either by the remote CLI or by using tDCS device’s
own control. Figure 16 demonstrates how inputs
from the CLI (in real, use by a doctor) can halt the
treatment taking control of the device remotely. The
treatment can be also aborted from the device and this
was implemented using a regular polling mechanism
between the physician’s CLI and the tDCS device.
When the treatment was halted, not only the power
boost was turned off, but also the relay was opened,
preventing any residual power within the circuit from
reaching to the simulated electrical load acting as a
patient’s head. In the case of deviations from a set
current value during the treatment, onboard tracking
systems could alert the remote CLI. Overall, this
experiment demonstrated a sound safety mechanism
of the hardware during simulated treatments.
8 CONCLUSIONS
While the device has been shown to function, further
testing and ethical approval must be obtained before
it can be used for human trials. At present, there is a
short time delay for the device to calculate the
resistance values needed for the Digi Pots to provide
the required current output. With enhanced PCB-
based prototypes, future research should aim for
faster current adjustments almost instantly. Current
ranges can be extended outside the 1-2mA range and
preliminary investigations have shown that this is
possible using the prototype model.
The prototype should be further refined in the
future, so that it can be used successfully in clinical
trials and can also provide a more seamless
experience for physicians. An NFC smart card system
should be supported on the device interface so that
physicians can access the device using an institution's
smart card system to configure treatment parameters
for patients. Moreover, the CLI should be extended
to automatically log the treatment data. These logs
can then be used to generate patient reports that can
be uploaded to a healthcare system, such as the
advanced Patient Administration System (SystemC,
2020). In the long run, the CLI should be developed
into a graphical mobile or web-based application,
enabling users to engage in tele-neurorehabilitation in
a more convenient, efficient, and secure manner.
REFERENCES
Bikson, M., Datta, A., Elwassif, M. (2009). Establishing
safety limits for transcranial direct current stimulation.
Clinical Neurophysiology, 120(6), pp. 1033-1034.
Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J,
Adnan T, et al. (2016). Safety of transcranial direct
current stimulation: evidence based update 2016. Brain
Stimulation; 9: 641 – 61. https://doi.org/10.
1016/j.brs.2016.06.004.
Boggio, P. S., et al. (2006). Effects of transcranial direct
current stimulation on working memory in patients with
Parkinson's Disease. Journal of Neurological Sciences,
Volume 249, pp. 31-38.
Bucur M, Papagno C. (2018). A systematic review of
noninvasive brain stimulation for post-stroke
depression. J Affect Disord. 238:69–78.
https://doi.org/10.1016/j.jad.2018.05.026.
Castillo-Saavedra, L., et al. (2015). Clinically Effective
Treatment of Fibromyalgia Pain With High-Definition
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
92

Transcranial Direct Current Stimulation: Phase II
Open-Label Dose Optimization. The Journal of Pain,
17(1), pp. 14-26.
Cucca, A., Sharma, K., Agarwal, S., Feigin, A. S. (2019).
Tele-monitored tDCS rehabilitation: feasibility,
challenges and future perspectives in Parkinson’s
disease. Journal of NeuroEngineering and
Rehabilitation, 16(20).
Fertonani, A., Brambilla, M., Cotelli, M., Miniussi, C.
(2014). The timing of cognitive plasticity in
physiological aging: a tDCS study of naming. Frontiers
in Aging Neuroscience, Volume 6, p. 131.
Fregni, F., Boggio, P. S., Nitsche, M. A., Rigonatti, S. P.
(2006). Cognitive Effects of Repeated Sessions of
Transcranial Direct Current Stimulation in Patients
with Depression. Depression and Anxiety, Volume 23,
pp. 482-484.
Hall, J. L., McGraw, D. (2014). For Telehealth to Succeed,
Privary and Security Risks Must Be Identified and
Addressed. Health Affairs, 33(2).
Law, S. K. (1993). Thickness and resistivity variations over
the upper surface of the human skull. Brain
Topography, Volume 6, pp. 99-109.
Lefaucheur, JP., et al. (2017). Evidence-based guidelines
on the therapeutic use of transcranial direct current
stimulation (tDCS). Clinical Neurophysiology, 128(1),
pp. 56-92.
Leite, J., Gonçalves, O. F., Carvalho, S. (2014). Facilitative
effects of bi-hemispheric tDCS in cognitive deficits of
Parkinson disease patients. Medical Hypotheses, 82(2),
pp. 138-140.
Loo, C. K., et al. (2010). A double-blind, sham-controlled
trial of transcranial direct current stimulation for
the treatment of depression. Journal of
Neuropsychopharmacology, Volume 13, pp. 61-69.
Maxim (2009). Maxim High-Voltage, NV, I2C POT
(DS3502). [Online] Available at:
https://web.archive.org/web/20190610042255/https://d
atasheets.maximintegrated.com/en/ds/DS3502.pdf
[Accessed 9/12/2021].
Pallets (2020). Click. [Online] Available at:
https://web.archive.org/web/20200519204909/https://c
lick.palletsprojects.com/en/7.x/ [Accessed 9/12/2021].
Palm, U., et al. (2013). Evaluation of Sham Transcranial
Direct Current Stimulation for Randomized, Placebo-
Controlled Clinical Trials. Brain Stimulation, 6(4), pp.
690-695.
Palm, U., Schiller, C., Fintescu, Z., Obermeier, M. (2012).
Transcranial direct current stimulation in treatment
resistant depression: A randomized double-blind,
placebo-controlled study. Brain Stimulation, Volume 5,
pp. 242-251.
Parazzini, M., et al. (2014). Modelling the electric field and
the current density generated by cerebellar transcranial
DC stimulation in humans. Clinical Neurophysiology,
125(3), pp. 577-584.
Parazzini, M. et al.
(2013). Numerical Estimation of the
Current Density in the Heart During Transcranial Direct
Current Stimulation. Brain Stimulation, 6(3), p. 2013.
Park, J.-H.et al. (2011). A Novel Array-Type Transcranial
Direct Current Stimulation (tDCS) System for Accurate
Focusing on Targeted Brain Areas. IEEE Transactions
on Magnetics, 47(5), pp. 882-885.
Particle (2020). Security Checklist for The Internet of
Things. [Online] Available at: https://cdn2.hubspot.net/
hubfs/2833873/White%20Papers/security-3.pdf
[Accessed 9/12/2021].
Riggs, A., Patel, V., Paneri, B., Portenoy, R. K. (2018). At-
Home Transcranial Direct Current Stimulation (tDCS)
With Telehealth Support for Symptom Control in
Chronically-Ill Patients With Multiple Symptoms.
Frontiers in Behavioral Neuroscience, Volume 12, p.
93.
Smith, A. C. et al. (2020). Telehealth for global
emergencies: Implications for coronavirus disease 2019
(COVID-19). Journal of Telemedicine and Telecare,
0(0), pp. 1-5.
Sourav, S., Rahman, A., Mamun, A. A., Alamgir, F. M.
(2017). Standard Transcranial Direct Current
Stimulation (tDCS) Model. Int. Journal of Computer
Networks and Communications Security, 5(12), pp.
264-270.
SparkFun, (2020). I2C. [Online] Available at:
https://web.archive.org/web/20200515151719/https://l
earn.sparkfun.com/tutorials/i2c/all [Accessed
9/12/2021].
SystemC, (2020). PAS. [Online] Available at:
https://web.archive.org/web/20200515205709/https://
www.systemc.com/solutions/epr/pas/ [Accessed
9/12/2021].
Thair, H., Holloway, A. L., Newport, R., Smith, A. D.
(2017). Transcranial Direct Current Stimulation
(tDCS): A Beginner's Guide for Design and
Implementation. Front Neurosci, Volume 11, p. 641.
Woods, A. J. et al. (2016). A technical guide to tDCS, and
related non-invasive brain stimulation tools. Clinical
Neurophysiology, 127(2), pp. 1031-1048.
Zaghi, S., Heine, N., Fregni, F. (2009). Brain stimulation
for the treatment of pain: A review of costs, clinical
effects, and mechanisms of treatment for three different
central neuromodulatory approaches. Journal of Pain
Management, 2(3), pp. 339-352.
Tele-tDCS: A Novel Tele-neuromodulation Framework using Internet of Medical Things
93
