
Sovereignly Donating Medical Data as a Patient:
A Technical Approach
Florian Lauf
1a
, Hendrik Meyer zum Felde
2b
, Marcel Klötgen
1c
, Robin Brandstädter
3d
and Robin Schönborn
1e
1
Fraunhofer Institute for Software and Systems Engineering ISST, Dortmund, Germany
2
Fraunhofer Institute for Applied and Integrated Security AISEC, Garching near Munich, Germany
3
Fraunhofer Institute for Experimental Software Engineering IESE, Kaiserslautern, Germany
hendrik.meyerzumfelde@aisec.fraunhofer.de, robin.brandstaedter@iese.fraunhofer.de
Keywords: Medical Data Donation, Data Sovereignty, Patient Empowerment, Usage Control, International Data Spaces.
Abstract: Data is the new asset of the 21
st
century, and many new business models are based on data. However, data is
also needed in the medical research domain, such as in the procedure of applying new machine learning
methods for gaining new medical findings. Furthermore, the hurdle arises that medical data comprises
personal data, and thus, it requires particular care and protection. Hence, patients must consent to the data
donation process for general medical research but without selecting specific research projects. We argue that
patients must gain more influence in the data donation process to cover this lack of data sovereignty.
Therefore, we developed a concept and implementation empowering patients to make sovereign decisions
about donating their medical data to specific medical research projects. Our work comprises concepts of the
Medical Informatics Initiative, International Data Spaces, and MY DATA Control Technologies with new
specific elements combining these components. This approach of patient empowerment enables a new kind
of data sovereignty in the medical research domain.
1 INTRODUCTION
When considering the restriction of data usage and
access by an individual or company, then we enter the
scientific field of data sovereignty. Being sovereign
as an individual means being able to determine which
entities have access to one’s own data and how this
data may be processed. Furthermore, the current
regulation in Europe dictates that individuals must be
informed about storing and processing their personal
data (European Parliament and Council of European
Union, 2016). Additionally, individuals must
explicitly give consent to each specific usage of their
medical data. However, donating medical data for
cutting-edge research is essential. For instance, the
exploration of large amounts of data with machine
learning methods results in some completely new
a
https://orcid.org/0000-0003-0844-3722
b
https://orcid.org/0000-0002-5837-8730
c
https://orcid.org/0000-0003-4109-8641
d
https://orcid.org/0000-0001-8439-3697
e
https://orcid.org/0000-0001-7510-622X
research approaches (Specht-Riemenschneider &
Radbruch, 2021), but legal consent represents a
challenge (Ohmann et al., 2017).
The Medical Informatics Initiative provides a first
text-based template for patient consent forms
(Medical Informatics Initiative, 2020) that is based on
broad consent concepts (Bild et al., 2020; Caulfield &
Kaye, 2009; Sheehan, 2011), especially for medical
research, which serves as a step to simplify the
process of donating medical data. Therefore, patients
who want to donate their medical data to the medical
research can consent into these forms. Both medical
researchers and patients obtain clarity on how
personal medical data is permitted to be used for
further research due to the expressed information of
the consent forms. In this paper, we extend the broad
consent model and give patients additional freedom
Lauf, F., Felde, H., Klötgen, M., Brandstädter, R. and Schönborn, R.
Sovereignly Donating Medical Data as a Patient: A Technical Approach.
DOI: 10.5220/0010880800003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 5: HEALTHINF, pages 623-630
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved
623

in decision-making. We present a mechanism that
allows patients to decide individually for which
research projects their medical data will be donated.
Furthermore, to ensure that an individual’s choice
of whether data transfer is permitted or prohibited, we
enforce data use and access policies in a technical
manner using International Data Spaces technology
(IDSA, 2019). Using specific connectors, the related
architecture dictates the conditions of securely
transferring data for use and access. Thus, our
research objective comprises a linking of separated
concepts to design a trustworthy donation system for
medical data and individual data sovereignty. We
contribute to foster the involvement of patients in data
donating processes and propose a technical system to
realize this patient’s empowerment. Consequently,
we define the following research questions (RQ):
RQ1: How can patients be technically empowered to
donate their medical data sovereignly to selected
medical research projects?
RQ2: What are important components for an
adequate implementation of such a concept?
The paper is structured as follows. In Section 2, we
discuss related works that we have included in our
conceptual approach. Data sovereignty of citizens,
work of the Medical Informatics Initiative including
the SMITH Service Platform, and International Data
Spaces technology with embedded usage control are
the pillars of our research. We integrated these
previous works into our approach for donating
medical data. Hence, we describe our concept in
Section 3. Subsequently, the appropriate
implementation is presented in Section 4. Afterward,
in Section 5, we discuss our implemented concept.
Finally, we outline our research in Section 6 and point
out further research to create a comprehensive tool for
patients to donate their own medical data.
2 RELATED WORK
This section describes the works related to our
research into sovereign donation of medical data.
2.1 Data Sovereignty of Citizens
We understand data sovereignty as a subdomain of
digital sovereignty that puts the asset ‘data’ in the
spotlight (Adonis, 2019; Couture & Toupin, 2019;
Otto, 2016). Furthermore, we state that data
sovereignty of citizens is a means to comply with the
informational self-determination required by the
German legislator because the relevant data constitute
personal data (European Parliament and Council of
European Union, 2016; Steinmüller et al., 1972). In
addition, personal data comprises data that is created
by and about an individual (World Economic Forum,
2011). Overall, we interpret the term data sovereignty
as the knowledge and control of who can access an
individuals’ data and where this data is transferred
(Posch, 2017).
However, insights into the data sovereignty of
citizens show the lack of current solutions to share
one’s data in a self-determined way due to the
inadequate abilities of citizens to make sovereign
data-sharing decisions. However, the Digital Life
Journey describes the digitized lives of citizens and
addresses several areas being included in a holistic
approach of citizens’ data sovereignty (Meister &
Otto, 2019). Initial use cases such as the project
DaWID demonstrate how citizens can sovereignly
participate in data ecosystems with their own personal
data (Lauf et al., 2021).
2.2 Medical Informatics Initiative and
SMITH Service Platform
The Medical Informatics Initiative (MII) aims at
optimizing healthcare through providing
interoperable primary care data for clinical and
medical research (Semler et al., 2018), according to
the FAIR data principles (Wilkinson et al., 2016).
Each university hospital in Germany establishes a
Data Integration Center (DIC), thus ensuring
organizational, regulatory, and functional
prerequisites while addressing interoperability and
(re-)usability of data (Winter et al., 2018). During
their treatment, patients can give consent for their
data to be used in future research projects. Afterward,
researchers can find and identify patient-related data
and request data sets in a cross-organizational
workflow, the Data Use and Access (DUA) process,
thus addressing findability and accessibility of data.
The national commitment to a legal broad consent
is a fundamental achievement of the MII (Medical
Informatics Initiative, 2020), allowing for patient-
related data to be processed and used in a determined
and limited research context. Thus, the broad consent
forms the basis and the first level of agreement to
future secondary-purpose data usage given by each
patient. Yet, it does not support transparency and
consenting to specific research projects or a
horizontal or vertical selection of data sets by
patients. Therefore, the DUA process provides a
second level of consent (Klötgen et al., 2021),
realized as a vicarious and project-specific agreement
based on individual regulations of the DICs.
HEALTHINF 2022 - 15th International Conference on Health Informatics
624

The SMITH consortium develops the SMITH
Service Platform (SSP), which provides common use
cases and user interfaces (UI) for all connected DICs,
such as the DUA process. Researchers submit a
project-specific data usage proposal through the SSP,
and the Data Use and Access Committee (UAC) of
each involved DIC decides whether the requested
data may be provided for the specific research project.
In the end, the researcher and each DIC conclude a
project-specific contract, allowing the requested data
sets to be provided by the SSP. The DUA process is
realized as a distributed process with a central process
management (Klötgen et al., 2021), providing tasks
for the necessary process control and integration of
DIC’s subprocesses, components, and actors.
In order to manage consents and digital identities,
pseudonyms, and their relations, the MII consortia
establish Trusted Third Parties (TTP) as essential
building blocks of data processing workflows,
including protection, pseudonymization, and
anonymization of data. Many DICs will integrate the
‘generic Informed Consent Administration Service’
(gICS) as a tool to manage patients’ broad consents
(Rau et al., 2020). In this context, gICS allows
requesting data sets of all consenting patients and it
can be integrated into the real-time data processing
tasks of a DIC. Yet, gICS does not support patients in
constraining specific data donations for selected
medical research projects.
2.3 International Data Spaces
Numerous technologies exist that are capable of
transferring sets of data to a remote consumer. But
when it comes to organizational requirements, such
as privacy regulations, security requirements, and
legal contracting behind the technical process, the
scientific landscape becomes rather scarce on options.
The International Data Spaces (IDS) provide an
ecosystem for sharing data, which aims to cover all
the issues previously mentioned (IDSA, 2019). The
IDS Association (IDSA) provides standardized
policy negotiation and attested state-of-the-art
security guarantees, and it aims to provide usage
control for shared data. The IDS infrastructure has the
goal of letting data providers remain in control and
keep the ownership of their data even after the data
has been released to consuming parties.
The IDS consist of divisions, which focus on
sharing data for a certain domain, such as the Medical
Data Space. This medical-specific data space allows
scientists to share and regulate data being relevant for
1
https://camel.apache.org/ (last accessed: 2021/10/27)
health studies. Furthermore, participants
communicate via IDS Connectors (IDSA, 2019),
which serve as components for transferring data
among each other. These connectors are typically
attested using Trusted Platform Modules or Trusted
Execution Environments. Most IDS Connectors
contain Apache Camel
1
, which is an open-source
message routing framework (IDSA, 2019). Apache
Camel has more than 200 different protocol adapters
allowing to transform incoming and outgoing
messages across protocol boundaries. As a security
mechanism, each implementation of an IDS
Connector must regularly pass a certification, which
determines the level of trust and security achieved. In
the context of IDS, three different security levels exist
to handle ordinary communication as well as highly
confidential data flows. The set of minimal trust
levels and security requirements which need to be
guaranteed by an IDS Connector is defined in a so-
called IDS Policy. IDS Connectors must mutually
agree on IDS Policies which contain requirements for
security standards and rules for processing data flows.
Furthermore, IDS Policies result in so-called IDS
Contracts after successful negotiation. This
negotiation is a specific IDS process to deposit IDS
Contracts with IDS Policies on the recipient side
(Hosseinzadeh et al., 2020).
2.4 Usage Control
Usage control is a research field that deals with the
extension of traditional access control to enforce rules
on data even after their release. Eitel et al. gave a
suitable definition for the perspective of this topic:
“[Usage control] is about the specification and
enforcement of restrictions regulating what must
(not) happen to data. Thus, usage control is concerned
with requirements that pertain to data processing
(obligations) rather than data access (provisions).
Usage control is relevant in the context of intellectual
property protection, compliance with regulations, and
digital rights management.” (Eitel et al., 2021)
The idea of usage control was first formalized by
Park and Sandhu with their model of UCON-ABC,
which stands for usage control with definitions for
authorizations, obligations, and conditions (Park &
Sandhu, 2004; Sandhu & Park, 2003). By that time,
classical access control was the prevalent paradigm,
but it was unable to enforce access rights beyond the
first provision of a user’s access rights. For instance,
one of the first implementations of usage control in a
distributed system was proposed by Pretschner
Sovereignly Donating Medical Data as a Patient: A Technical Approach
625

(Pretschner et al., 2006). Afterward, the UCON-ABC
was incrementally improved, implemented numerous
times, and equipped with more expressive policy
languages such as XACML and additional
extensions, as it is the case for a tool like the MY
DATA Control Technology
2
. IDSA also deals with
usage control, defines standardized rules and a
corresponding policy language for implementing
such usage control mechanisms (Bader et al., 2020;
Eitel et al., 2021; Hosseinzadeh et al., 2020). For
instance, the previously mentioned MY DATA
Control Technology can be used to enforce the rules
in an IDS Connector.
3 CONCEPT
MII’s DUA process incorporates two levels of
agreement to data usage. Acting as the data owner, a
citizen provides a broad consent on the first level of
agreement, thus approving the usage of medical data
for the research in general. This enables researchers
to find patients’ data managed by the specific DIC for
usage within data use projects. Acting as the data
provider, the UAC of a DIC approves a researcher’s
data usage proposal and agrees or disagrees to the
usage of each patient’s data vicariously on the second
level of agreement. Due to the lack of transparency
and influence for citizens regarding specific data use
projects, we added a third level of agreement enabling
citizens to constrain the usage of their data in the
context of specific data use projects and thus,
strengthening patient empowerment and data
sovereignty. These three levels are represented in
Figure 1 and form the starting point of our work.
Our concept comprises components and systems
of the SMITH project such as SSP and DICs on the
one hand, and IDS technology such as IDS
Connectors and embedded usage control on the other
hand. Hence, in our concept, we combine existing
technologies from SMITH and IDS to create a portal
focusing on the participation of patients.
Figure 1: Levels of Agreement.
2
https://www.dataspaces.fraunhofer.de/de/software/usage-
control/mydata.html (last accessed: 2021/10/19)
Figure 2: Concept Overview.
Figure 2 shows an overview of our concept, and the
demonstrated scenario follows a chronological
sequence with 14 steps, marked in the illustration.
Firstly, patients agree in the MII broad consent to
donate their data for all interested medical research
projects at the doctor’s appointment, as described
before in the first level of agreement, and, in addition,
they gain personal login details for the patient portal
(0). After that, data is available, and researchers use
the SMITH Marketplace to provide a data usage
proposal containing a data query that is needed for the
appropriate research (1). The Data Sharing Services
component, acting as the central repository for data
use projects and connecting component for multiple
DICs, manage all workflow based interactions
between involved actors and provide all tasks
included in the DUA process (2). When a task
addresses a DIC, its Data and Metadata Transfer Unit
(DMT) is notified (3), which retrieves the necessary
information and triggers a local subprocess. Thus, the
UAC is able to manage and provide the individual
evaluation of a data usage proposal (4). When the data
use project is accepted, the Data Sharing Services
provide data provision tasks to the involved DICs in
the same way, and the requested data sets are sent to
the provider app within the IDS Connector instance
(5). Based on the research project conditions, an IDS
Policy is created (6) and negotiated with the
researcher’s IDS Connector (7). After the project
policy is deployed, patients may intervene. The
implemented patient portal shows project information
and corresponding data processing to the patients (8),
HEALTHINF 2022 - 15th International Conference on Health Informatics
626
so the involved patients gain insights into the
requesting projects and can decide to accept or to
reject the requested data transfer, which forms the
third level of agreement (9). The default setting
represents consent to the requesting project by using
the MII broad consent. If a patient rejects the data
transfer for a selected project, no data is sent to the
related researcher’s IDS Connector, and, as a result,
the researcher will not receive data from the declining
patient. Otherwise, after a period the requested data is
filtered using the IDS Policy (10) and subsequently
securely sent (11). Within the IDS Connector of the
researcher, data is also checked using the IDS Policy,
for instance, by checking valid time intervals for
usage and, after that verification, forwarded to the
consumer app (12). Hence, IDS mechanisms support
the enforcement of patient choices but transferred
data cannot be withdrawn by patients. After a defined
period for rejection, patient choices are final due to
the required researchers’ planning dependability.
Finally, the consumer app will display the requested
data to the researcher if the check of the IDS Policy
was valid (13).
Our objective is to improve the ability of patients
to be sovereign in their data donations for medical
research. By using the IDS Connectors, a technology
focusing on fair data sharing and sovereign
participants was chosen to achieve this objective.
Patients can intervene in the transfer of data for
specific research projects. Hence, patients can choose
which projects they want to support with their data.
This approach answers RQ1. A detailed description
of the used components is presented in the following
Section 4, answering to RQ2.
4 IMPLEMENTATION
A main objective of our work regarding usage control
is to empower patients to keep control of their
personal medical data. Another objective is to
consider the time span of requesting research projects
and to impose a time constraint on the visibility of the
data on the researcher’s side as an obligation, which
must be automatically enforced. Thus, data usage
control affects several steps in the process of
transferring data. First, a DIC checks the
authorization for accessing the patient data. This was
already implemented on DICs by UAC and is not part
of our implementation. After that and before sending
the data, the consent or refusal of patients is
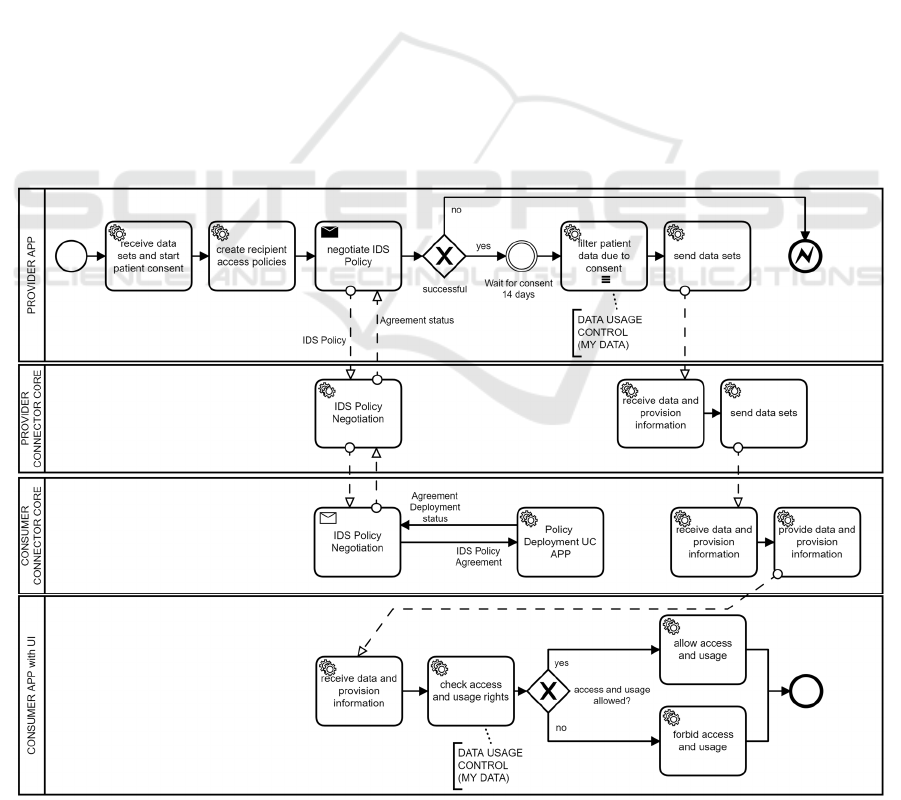
Figure 3: Business Process Model of Implementation.
Sovereignly Donating Medical Data as a Patient: A Technical Approach
627

considered in the data sets. Subsequently, IDS
Policies are exchanged between IDS Connectors that
include the duration of the project so that no data will
be displayed after this time span. Figure 3 shows the
process that implements our concept.
For our prototypal implementation, we use
Trusted Connectors as a specialization of IDS
Connectors (Schütte et al., 2018), which are open
source and feature a high trust level. A Trusted
Connector is a runtime environment. Its core
component serves as a gateway for inbound and
outbound network requests and communication
between user apps running on it, for instance,
consumer and provider apps (Schütte et al., 2018).
Furthermore, MY DATA Control Technology is
integrated using Camel Interceptors
1
. A Camel
Interceptor is an integration pattern of the Apache
Camel framework with the purpose of interrupting the
original flow of messages and applying various
actions to the messages and data. In addition, we have
implemented connector apps on both the provider’s
side and the consumer’s side, whose functionality is
described in the following.
Starting on the provider’s side, see top left of
Figure 3, the provider app fetches the requested data
sets from the DIC’s storage. Those date sets are
expressed in HL7 FHIR
2
format, where a FHIR
Bundle
3
acts as a project, and FHIR Conditions
4
contained in a FHIR Bundle represent the medical
data of specific patients. Subsequently, patients can
view the available projects and their included own
medical data for donation in a UI within the patient
portal. Patients withdraw their consent for specific
projects or retain their consent by broad consent. The
authentication of patients in the patient portal is based
on the patient login details which they received at the
doctor’s appointment before. Furthermore, the IDS
Contracts with policies, which are generated by our
prototype, are ‘negotiated’ with the recipient over the
period of use, matching the requested duration of the
project. Patients have 14 days to withdraw their
consent. The prototype implements this mechanism
with a time-based event. After that, the provider
transforms the originally fetched data sets, filtering
out the medical data of patients who decided to
withdraw their consent. As next step, the provider
transmits the altered data sets —only after successful
IDS Contract negotiation—via the provider’s core
1
https://camel.apache.org/components/3.11.x/eips/intercep
t.html (last accessed: 2021/10/27)
2
https://www.hl7.org/fhir/ (last accessed: 2021/10/27)
3
https://www.hl7.org/fhir/bundle.html (last accessed:
2021/10/28)
component of Trusted Connector to the consumer’s
core component of another Trusted Connector.
The altered data sets are now arriving on the
consumer’s side. Since the data sets possess a unique
identifier as an attribute of the FHIR Bundle, they can
be referenced by the IDS Policies. Before the data is
passed to the consumer app, where the researcher will
be able to view the potentially altered data sets
tabularly in a UI, MY DATA Control Technologies
check the corresponding time-based rules for the
project. The rules are also checked before each
display in the UI, so the data will only be visible
within the consumer app for the duration of the
project and can also no longer be transferred within
the IDS Connector. In addition, the flow of messages
in the connector is defined by so-called Camel
Routes
5
. On these Camel Routes, the Camel
Interceptor is applied to control the flow of messages.
In summary, our implementation points out that a
close dovetailing between technology and contracts is
required. The IDS provide data exchange
mechanisms that guarantee a high level of policy
enforcement. These components are relevant for a
valid implementation of our concept, which answers
RQ2. Furthermore, our added elements, such as the
patient portal and the integration of three levels of
agreement, are also part of an answer to RQ2.
5 DISCUSSION
Our concept and implementation demonstrate
sovereign data donation in medical research.
Furthermore, our work provides benefits for all
participants in the data donation process. The results
could also be beneficial for future work towards a
European strategy for data spaces, since IDS provides
a potential foundation of Gaia-X (Otto et al., 2021).
Firstly, we show an opportunity for patients to
donate their medical data sovereignly by combining
existing technology with additional elements. This
result answers RQ1, defined in Section 1. Further,
using our patient portal leads to a sovereign patient
empowerment and fosters trust in donating medical
data. Providing patients with choices of specific
research projects for their medical donation
strengthens patients’ trust because of the secure
4
https://www.hl7.org/fhir/condition.html (last accessed:
2021/10/28)
5
https://camel.apache.org/manual/routes.html (last
accessed: 2021/10/28)
HEALTHINF 2022 - 15th International Conference on Health Informatics
628

implementation and enforcement of their individual
choices in a technical way by our developed system.
Secondly, our approach is based on data in HL7
FHIR format. Therefore, our work is interoperable
with several other existing medical tools and systems,
using the same international standard. Hence, our
work contributes to a comprehensive data availability
due to the interconnection of numerous data sources,
such as DIC or other medical and clinical data
storages. The implementation is based on components
by MII, SMITH, IDS, and MY DATA Control
Technologies, so we point out an interplay of these
different components. Therefore, by adding specific
new components, our concept implementation
approach responds to RQ2.
Finally, extending IDS technology with aspects
involving citizens moves the rather industrial focus
further to a more general application. So far, IDS have
been used mostly in corporate and scientific contexts.
Since our approach describes a patient embedding,
citizens can participate in data ecosystems from now
on. It must be noted that citizens do not use their own
IDS Connectors, but they can interact with a portal
allowing them to adjust data donation flows. These
settings are transformed into machine-readable
policies that are embedded into the IDS Connectors
of DICs and researchers. This procedure enables
citizens to participate in data donation in particular
and in data sharing processes in general. However,
there are still limitations on data usage for analysis
purposes in external systems. The policy enforcement
no longer exists if data leaves the IDS Connector, but
this does not relieve data consumers of their legal
obligations to comply with the contract. However,
there are two promising approaches enabling
technical enforcement. On the one hand, the
development of special IDS applications with
appropriate analysis functions embedded into the
Connector, and on the other hand, the extension of
existing applications with data usage mechanisms.
6 CONCLUSION AND OUTLOOK
We conceptualized and implemented an initial
approach, empowering patients to make sovereign
data-donating decisions. For this objective, we
combined the MII broad consent concept with
components from the IDS and MY DATA Control
Technologies to create new opportunities for patients
to control the use of their data. Our concept is based
on patients’ broad consent given during medical
treatment. Broad consent allows donating medical
data for medical research, but patients cannot choose
specific research projects. We argue that patients
must become more involved in the data donation
process for medical research. To this end, we
developed a system empowering patients to make
sovereign decisions about donating their medical data
to specific medical research projects. As a result, we
contribute to sovereign medical data donation
considering individual patients. Additionally, with
our system based on industrial technologies such as
IDS and MY DATA Control Technologies, we
contribute to (industrial) data ecosystems considering
not only companies but also individuals’ preferences.
Since we developed an initial prototype, our
research is limited in terms of application in common
practice. Further research should validate our
prototype with patients donating medical data and
researchers requesting medical data. Furthermore,
our approach can further enhance patients’ data
sovereignty, for instance, by enabling patients to
select specific data types for their donation to specific
medical research projects.
REFERENCES
Adonis, A. A. (2019). Critical Engagement on Digital
Sovereignty in International Relations: Actor
Transformation and Global Hierarchy. Global: Jurnal
Politik Internasional, 21(2), 262–282. https://doi.org/
10.7454/global.v21i2.412
Bader, S., Pullmann, J., Mader, C., Tramp, S., Quix, C., Müller,
A. W., Akyürek, H., Böckmann, M., Imbusch, B. T., Lipp,
J., Geisler, S., & Lange, C. (2020). The International Data
Spaces Information Model – An Ontology for Sovereign
Exchange of Digital Content. In J. Z. Pan (Ed.), Lecture
Notes in Computer Science: Vol. 12507. The Semantic Web
- ISWC 2020: 19th International Semantic Web Conference
(Vol. 12507, pp. 176–192). Springer.
https://doi.org/10.1007/978-3-030-62466-8_12
Bild, R., Bialke, M., Buckow, K., Ganslandt, T., Ihrig, K., Jahns,
R., Merzweiler, A., Roschka, S., Schreiweis, B., Stäubert,
S., Zenker, S., & Prasser, F. (2020). Towards a
comprehensive and interoperable representation of consent-
based data usage permissions in the German medical
informatics initiative. BMC Medical Informatics and
Decision Making, 20(1), 103. https://
doi.org/10.1186/s12911-020-01138-6
Caulfield, T., & Kaye, J. (2009). Broad Consent in Biobanking:
Reflections on Seemingly Insurmountable Dilemmas.
Medical Law International, 10(2), 85–100.
https://doi.org/10.1177/096853320901000201
Couture, S., & Toupin, S. (2019). What does the notion of
"sovereignty" mean when referring to the digital? New
Media & Society, 21(10), 2305–2322. https://doi.org/
10.1177/1461444819865984
Eitel, A., Jung, C., Brandstädter, R., Hosseinzadeh, A., Bader,
S., Kühnle, C., Birnstill, P., Brost, G., Gall, M., Bruckner,
Sovereignly Donating Medical Data as a Patient: A Technical Approach
629

F., Weißenberg, N., & Korth, B. (2021). Usage Control in
the International Data Spaces. Dortmund Berlin.
International Data Spaces Association.
European Parliament and Council of European Union. (2016).
Regulation (EU) 2016/679. https://eur-lex.europa.eu/legal-
content/EN/TXT/ ?uri=OJ:L:2016:119:TOC
Hosseinzadeh, A., Eitel, A., & Jung, C. (2020). A Systematic
Approach toward Extracting Technically Enforceable
Policies from Data Usage Control Requirements. In
Proceedings of the 6th International Conference on
Information Systems Security and Privacy (ICISSP 2020)
(pp. 397–405). https://doi.org/
10.5220/0008936003970405
IDSA. (2019). Reference Architecture Model: Version 3.0 |
April 2019. International Data Spaces Association.
https://internationaldataspaces.org/use/reference-
architecture/
Klötgen, M., Fiege, E., & Houta, S. (2021). Concept and
Implementation of Data Usage Proposal Process Based on
International Standards in SMITH. Studies in Health
Technology and Informatics, 278, 171–179. https://
doi.org/10.3233/SHTI210066
Lauf, F., Scheider, S., Meister, S., Radic, M., Herrmann, P.,
Schulze, M., Nemat, A. T., Becker, S. J., Rebbert, M.,
Abate, C., Konrad, R., Bartsch, J., Dehling, T., & Sunyaev,
A. (2021). Data Sovereignty and Data Economy—Two
Repulsive Forces? Position Paper. Dortmund. Fraunhofer
Institute for Software and Systems Engineering ISST.
https://doi.org/ 10.24406/isst-n-634865
Medical Informatics Initiative. (2020). Template text for patient
consent forms. https://www.medizininformatik-
initiative.de/en/template-text-patient-consent-forms
Meister, S., & Otto, B. (2019). Digital Life Journey: Framework
for a self-determined life of citizens in an increasingly
digitized world (basic research paper). Dortmund.
Fraunhofer Institute for Software and Systems Engineering
ISST. https://doi.org/10.24406/ ISST-N-559377
Ohmann, C., Banzi, R., Canham, S., Battaglia, S., Matei, M.,
Ariyo, C., Becnel, L., Bierer, B., Bowers, S., Clivio, L.,
Dias, M., Druml, C., Faure, H., Fenner, M., Galvez, J.,
Ghersi, D., Gluud, C., Groves, T., Houston, P., . . .
Demotes-Mainard, J. (2017). Sharing and reuse of
individual participant data from clinical trials: Principles
and recommendations. BMJ Open, 7(12), e018647.
https://doi.org/10.1136/bmjopen-2017-018647
Otto, B. (2016). Digitale Souveränität: Beitrag des Industrial
Data Space. Munich. Fraunhofer Institute for Software and
Systems Engineering ISST. https://
doi.org/10.13140/RG.2.2.35125.68321
Otto, B., Rubina, A., Eitel, A., Teuscher, A., Schleimer, A. M.,
Lange, C., Stingl, D., Loukipoudis, E., Brost, G., Böge, G.,
Pettenpohl, H., Langkau, J., Gelhaar, J., Mitani, K.,
Hupperz, M., Huber, M., Jahnke, N., Brandstädter, R.,
Wessel, S., & Bader, S. (2021). Gaia-X and IDS. Berlin.
International Data Spaces Association.
https://doi.org/10.5281/zenodo.5675897
Park, J., & Sandhu, R. (2004). The UCON ABC usage control
model. ACM Transactions on Information and System
Security, 7(1), 128–174. https://doi.org/
10.1145/984334.984339
Posch, R. (2017). Digital sovereignty and IT-security for a
prosperous society. In H. Werthner & F. van Harmelen
(Eds.), Informatics in the Future (pp. 77–86). Springer.
https://doi.org/10.1007/978-3-319-55735-9_7
Pretschner, A., Hilty, M., & Basin, D. (2006). Distributed usage
control. Communications of the ACM, 49(9), 39–44.
https://doi.org/10.1145/1151030.1151053
Rau, H., Geidel, L., Bialke, M., Blumentritt, A., Langanke, M.,
Liedtke, W., Pasewald, S., Stahl, D., Bahls, T., Maier, C.,
Prokosch, H.‑U., & Hoffmann, W. (2020). The generic
Informed Consent Service gICS®: Implementation and
benefits of a modular consent software tool to master the
challenge of electronic consent management in research.
Journal of Translational Medicine, 18(1), 287.
https://doi.org/ 10.1186/s12967-020-02457-y
Sandhu, R., & Park, J. (2003). Usage control: A vision for next
generation access control. In International Workshop on
Mathematical Methods, Models, and Architectures for
Computer Network Security. Symposium conducted at the
meeting of Springer.
Schütte, J., Brost, G., & Wessel, S. (2018). Der Trusted
Connector im Industrial Data Space. Garching. Fraunhofer
Institute for Applied and Integrated Security AISEC.
https://www.aisec.fraunhofer.de/
content/dam/aisec/Dokumente/Publikationen/Studien_Tec
hReports/deutsch/IDS-Paper_Datensouveraenitaet.pdf
Semler, S. C., Wissing, F., & Heyder, R. (2018). German
Medical Informatics Initiative. Methods of Information in
Medicine, 57(S 01), e50-e56. https://doi.org/
10.3414/ME18-03-0003
Sheehan, M. (2011). Can Broad Consent be Informed Consent?
Public Health Ethics, 4(3), 226–235. https://
doi.org/10.1093/phe/phr020
Specht-Riemenschneider, L., & Radbruch, A. (2021).
Datennutzung und -schutz in der Medizin: Forschung
braucht Daten. Deutsches Ärzteblatt, 118(27-28), 1359-
1361. https://www.aerzteblatt.de/int/ article.asp?id=220270
Steinmüller, W., Lutterbeck, B., Mallmann, C., Harbot, U.,
Kolb, G., & Schneider, J. (1972). Grundfragen des
Datenschutzes. In Anlage zu BT-Drucks. VI/3826.
Wilkinson, M., Dumontier, M., Aalbersberg, I. J., Appleton, G.,
Axton, M., Baak, A., Blomberg, N., Boiten, J.‑W., Da Silva
Santos, L., Bourne, P., Bouwman, J., Brookes, A., Clark, T.,
Crosas, M., Dillo, I., Dumon, O., Edmunds, S., Evelo, C.,
Finkers, R., . . . Mons, B. (2016). The FAIR Guiding
Principles for scientific data management and stewardship.
Scientific Data.
Winter, A., Stäubert, S., Ammon, D., Aiche, S., Beyan, O.,
Bischoff, V., Daumke, P., Decker, S., Funkat, G., Gewehr,
J. E., Greiff, A. de, Haferkamp, S., Hahn, U., Henkel, A.,
Kirsten, T., Klöss, T., Lippert, J., Löbe, M., Lowitsch, V., .
. . Löffler, M. (2018). Smart Medical Information
Technology for Healthcare (SMITH). Methods of
Information in Medicine, 57(S 01), e92-e105.
https://doi.org/10.3414/ME18-02-0004
World Economic Forum. (2011). Personal Data: The
Emergence of a New Asset Class. Geneva, CH. http://
www3.weforum.org/docs/WEF_ITTC_PersonalDataNew
Asset_Report_2011.pdf
HEALTHINF 2022 - 15th International Conference on Health Informatics
630
