
Virtual Planning and Simulation of Coarctation Repair in Hypoplastic
Aortic Arches: Is Fixing the Coarctation Alone Enough?
Seda Aslan
1
, Xiaolong Liu
1
, Qiyuan Wu
1
, Paige Mass
2
, Yue-Hin Loke
2
,
Narutoshi Hibino
3
, Laura Olivieri
2
and Axel Krieger
1
1
Department of Mechanical Engineering, Johns Hopkins University, Baltimore, MD, U.S.A.
2
Division of Cardiology, Children’s National Hospital, Washington DC, U.S.A.
3
Section of Cardiac Surgery, Department of Surgery, The University of Chicago Medicine, Chicago, IL, U.S.A.
nhibino@surgery.bsd.uchicago.edu, axel@jhu.edu
Keywords:
Virtual Aorta Repair, Coarctation of Aorta, Transverse Arch Hypoplasia, Computational Fluid Dynamics.
Abstract:
Coarctation of aorta (CoA) is a congenital heart disease that may coexist with transverse arch hypoplasia
(TAH). Infants who suffer from both conditions are often treated only for CoA at the initial repair if the degree
of TAH is diagnosed as mild. In this study, we investigated the effect of virtually repairing the CoA of three
patients (n=3) who also suffer from TAH. We repaired the CoA by using virtual stents that were modeled based
on descending aorta diameters of the patients. Using computational fluid dynamics (CFD) simulations, we
investigated the changes in time-averaged wall shear stress (TAWSS) after the virtual repair and calculated the
peak systolic pressure drop (PSPD), which is the indicator of the performance of the repair. The magnitude of
TAWSS was reduced in the repaired CoA regions in all the patients. The PSPD was improved in two patients,
remaining above 20 mmHg in one of them. There was no significant change in PSPD for one patient after the
virtual repair. The results may potentially help clinicians to gain better insights into whether the CoA repair
alone in patients with existing TAH is sufficient.
1 INTRODUCTION
Coarctation of aorta (CoA) is a narrowing that usu-
ally occurs in the region distal to the left subclavian
artery. It is a congenital defect that affects approxi-
mately 2200 newborns every year in the United States
(Mai et al., 2019). Infantile CoA is often accompa-
nied by transverse arch hypoplasia (TAH). Different
from CoA, TAH involves a larger narrowed portion
of the aorta (Ma et al., 2017). The condition of TAH
can be severe and the treatment of TAH may require
surgical repair. CoA and TAH are associated with up-
per body hypertension (Kenny et al., 2011), left ven-
tricular dysfunction, and aortic aneurysm formation
(Schubert et al., 2020). Depending on the degree of
TAH, the clinicians may decide to repair only CoA
when the TAH is mild (Siewers et al., 1991).
TAH repair is a very extensive procedure that is
performed on bypass via a sternotomy to reconstruct
the aortic arch structure by using vascular patches or
grafts. Compared to TAH repair, the procedure to
repair CoA alone typically has less technical com-
plexity and does not require cardiopulmonary bypass.
The common procedures to treat CoA include surgical
techniques such as resection with end-to-end anasto-
mosis and tubular bypass grafts (Liu et al., 2020; Rao,
2020), and minimally invasive catheter-based tech-
niques such as balloon angioplasty and stent place-
ment (Alkashkari et al., 2019). Since the minimally
invasive techniques result in faster recovery and they
are cost-effective, they play a crucial role in treatment
of CoA (Kwon et al., 2014). Stenting has the advan-
tage of resisting re-coarctation better than balloon an-
gioplasty (Kwon et al., 2014) and became popular to
treat CoA. Whether the treatment is surgical or min-
imally invasive, coarctation repairs have a high rate
of success in short term and mid-term (Alkashkari
et al., 2019). A multi-center study showed that 98%
of the coarctation repairs reduced the peak systolic
pressure drop below 20 mmHg, which is the criteria
for intervention (Forbes et al., 2007; Rao, 2020). An-
other study focused on stent placement and showed
that it results in high survival rate and reduced pres-
sure drop as well as increased diameter in the CoA
region (Su
´
arez de Lezo et al., 2015).
The results of CoA repair can also be simulated
138
Aslan, S., Liu, X., Wu, Q., Mass, P., Loke, Y., Hibino, N., Olivieri, L. and Krieger, A.
Virtual Planning and Simulation of Coarctation Repair in Hypoplastic Aortic Arches: Is Fixing the Coarctation Alone Enough?.
DOI: 10.5220/0010842600003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 3: BIOINFORMATICS, pages 138-143
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
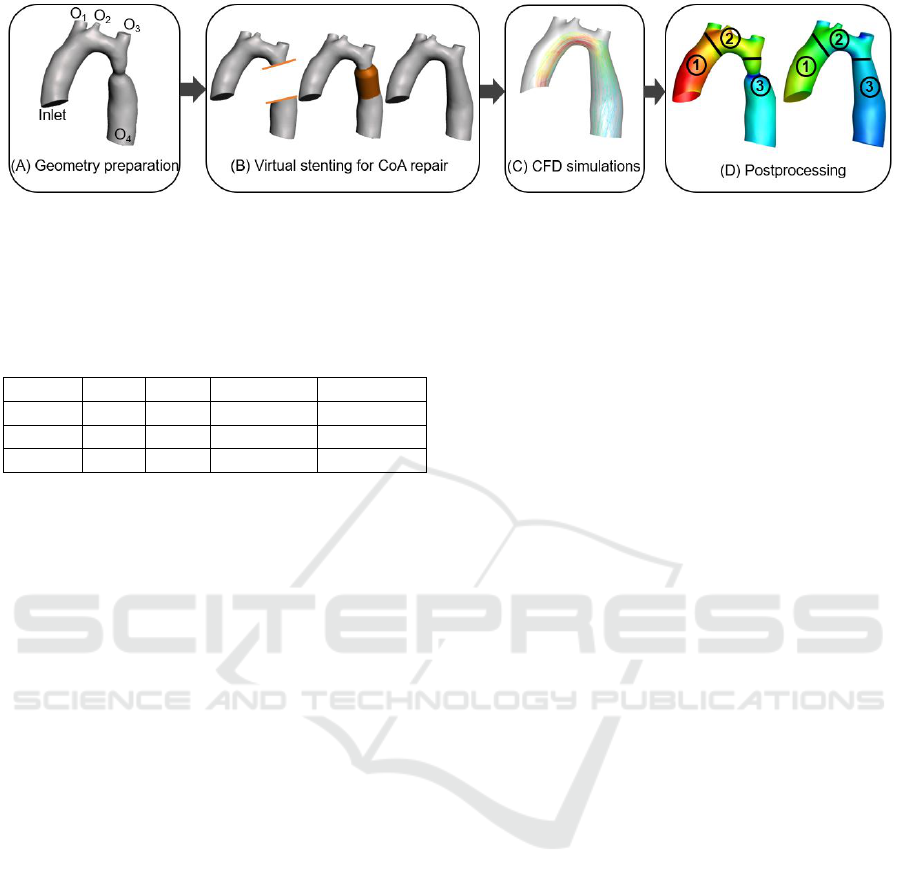
Figure 1: Schematic workflow of repairing CoA for patients with both CoA and TAH. (A) patient data acquisition and
geometry preparation. (B) from left to right: separation of CoA region from the rest of the aorta, virtual stent placement, and
final geometry after CoA repair. (C) performing CFD simulations. (D) postprocessing the results in the region of interest.
Table 1: Patients’ demographics. BSA: body surface area
(m
2
), D
CoA
: diameter of coarctation area, D
Ao
: diameter of
ascending aorta, D
Arch
: diameter of aortic arch.
BSA Age D
CoA
/D
Ao
D
Arch
/D
Ao
Case 1 1.27 9 0.43 0.67
Case 2 0.9 8 0.51 0.55
Case 3 0.38 9 mo 0.48 0.51
and analyzed using computational fluid dynamics
(CFD) models. Combined with imaging, CFD can
calculate not only pressure drop but also wall shear
stress (WSS) which is the most studied flow param-
eter since the extremely high or low WSS is known
to cause adverse effects on aorta walls (Dux-Santoy
et al., 2020). CFD studies showed that WSS and
peak systolic pressure drop (PSPD) were improved
after CoA repair (Schubert et al., 2020; Goubergrits
et al., 2015). However, even after a successful re-
pair of coarctation, long-term complications may oc-
cur (Torok et al., 2015; Quennelle et al., 2015). Espe-
cially, an existing, untreated TAH can cause late sys-
temic hypertension (Quennelle et al., 2015). There-
fore, utilizing CFD to predict the outcomes of CoA
repair in patients who also suffer from TAH may help
clinicians to make decisions on treatment method.
In this study, we investigated whether the CoA
repair with untreated TAH can provide satisfactory
hemodynamic results. We identified three represen-
tative patient cases (n=3) with both CoA and TAH
at different degrees. CoAs of the three patients were
virtually treated using stents that were designed with
commercially available stent dimensions. We per-
formed CFD simulations to predict the pressure drop
and TAWSS (time-averaged wall shear stress) of re-
paired aortas and analyzed the improvements. Our
findings indicated that the efficacy of only repair-
ing CoA varies in different patients. The results of
this study showed that the hemodynamics predicted
by CFD simulations in treatment planning stage may
provide guidance for making clinical decision on
whether to leave TAH untreated when repairing CoA.
2 METHODS
Figure 1 demonstrates the workflow that we followed
in this study. The four steps include: (A) patient data
acquisition and geometry preparation, (B) stent cre-
ation and virtual repairs of CoA, (C) CFD simulations
of native and virtually repaired CoA geometries, and
(D) postprocessing results. The details of each step
were provided in the remainder of this section.
2.1 Patient Data Acquisition and
Geometry Preparation
For this study, we selected three patients (n=3) who
were diagnosed with CoA and TAH (based on the z-
scores (Lopez et al., 2017)) with available Magnetic
Resonance Imaging (MRI) data and non-invasive
pressure measurements from arm and leg. The pul-
satile flow rates in ascending and descending aortas
were measured at the cross-sectional planes by 2-
D phase-contrast cardiac magnetic resonance (CMR)
imaging of each patient. The patients’ demographics
are provided in Table 1. We included the ratios of
the diameters of the arch and CoA to ascending aorta
(Ao) to provide the severity of TAH and CoA.
The data were acquired as part of and Institutional
Review Board (IRB) approved retrospective study.
Segmentation of images was performed using Mim-
ics software (Materialise, Leuven, Belgium) to cre-
ate three-dimensional (3-D) models of native aortas
that included ascending aorta (inlet), brachiocephalic
artery (O
1
), left common carotid artery (O
2
), left sub-
clavian artery (O
3
), and descending aorta (O
4
), as
shown in Figure 1A. The 3-D geometries then were
smoothed to reduce rough surfaces of the models. The
boundaries of the models were cut perpendicular to
the flow direction and all boundaries were extruded
50 mm to avoid backflow in the simulations.
Virtual Planning and Simulation of Coarctation Repair in Hypoplastic Aortic Arches: Is Fixing the Coarctation Alone Enough?
139

2.2 Stent Creation and Virtual Repair
of Coarctation
The virtual stents were selected patient-specifically.
Based on descending aorta size of each patient, a
commercially available stent with appropriate length
and diameter was chosen (Peters et al., 2009).
The stent geometries were created using Solidworks
(Waltham, MA, USA) software as circular cylinder
to mimic the shape when it expands. Two cuts were
made in the descending aorta of the patient to remove
CoA region from the native geometry as shown in
Figure 1B. Two planes that are parallel to and 2 mm
away from the cut surfaces of the native tissue were
created. A circle with the same diameter as the de-
scending aorta was drawn on the distal plane and a
straight solid extension was made to create the vir-
tual stent. The stent was merged to distal and prox-
imal sides of native tissue by lofting the surfaces.
Lastly, the merged regions were smoothed using Au-
todesk Meshmixer software (San Rafael, CA, USA).
The merged regions were selected separately and uni-
form triangles option was applied with a smoothing
scale set to 50 and constraint rings set to 1. Addi-
tional robust smoothing was performed on the small
rough surfaces by selecting them manually.
2.3 CFD Simulation
Tetrahedral meshes were generated for the native and
virtually repaired models using ANSYS Mesh (Mesh,
ANSYS, Canonsburg, PA, USA). The maximum and
minimum sizes were chosen as 0.5 mm to have a
uniform size of mesh elements since the results that
are obtained using this size were previously validated
(Aslan et al., 2020). An inflation layer with 5 layers,
1.2 growth rate, and 0.6 mm total thickness was cre-
ated at the aorta walls to resolve the boundary layer.
The number of total mesh elements of each model was
at least 1 million. The governing flow equations that
are given in (1) and (2), were solved using ANSYS
Fluent assuming the blood is Newtonian (with a vis-
cosity of 0.00371 Pa and density of 1060 kg/m
3
) and
the walls are rigid.
∂ρ
∂t
+ ∇(ρ
−→
u ) = 0, (1)
ρ
D
−→
u
Dt
= −∇p + ρ
−→
g + ∇τ
i j
(2)
In equations (1) and (2), ρ is density, u is velocity, τ is
stress tensor, and t is time. The time step size for the
computations was one fifth of the acquired flow time
step size of CMR.
At the inlet of the ascending aorta, the acquired
time-dependent velocity curve and at the outlets,
Windkessel boundary conditions were specified. The
Windkessel parameters were obtained, and patient-
specific turbulent flow (k-epsilon model) simulations
were performed following the steps in a previously
validated study (Aslan et al., 2020). The simulations
were run for 10 cardiac cycles. The converged results
were used to obtain hemodynamics.
2.4 Postprocessing and Comparison of
Hemodynamics
After obtaining CFD results, the hemodynamics was
calculated in the entire aorta and three regions of the
aorta:
1
ascending,
2
arch, and
3
descending as in-
dicated in Figure 1D. The arch was defined as the re-
gion between the brachiocephalic artery and left sub-
clavian artery. We reported TAWSS and PSPD, and
comparison between the native and virtually repaired
CoA models.
3 RESULTS
The pressure distribution for each case before and af-
ter virtual CoA repair are shown in Figure 2. The
repair of CoA reduced the maximum pressure in the
ascending aorta for all cases. In the descending part,
the pressure is more uniformly distributed after the
virtual treatment. The PSPD across the entire aorta of
each patient were obtained and listed in Table 2.
The virtual CoA repair decreased the PSPD for
Case 1 and Case 3 by 9.3 mmHg and 9.57 mmHg, re-
spectively. However, Case 2 did not show significant
improvement after the repair. From a clinical perspec-
tive, 20 mmHg pressure difference is an indicator of
intervention or reintervention to repair aortic disease.
The CoA repair successfully decreased PSPD below
20 mmHg in Case 1. Although the PSPD of Case 3
was reduced by 24% after the virtual repair, the pres-
sure difference remained higher than 20 mmHg. For
Case 2, the PSPD was close to the 20 mmHg limit be-
Table 2: The comparison of PSPD between native and re-
paired CoA geometries for three cases.
PSPD (mmHg)
Case 1
Native 24.72
Repaired CoA 15.42
Case 2
Native 17.45
Repaired CoA 17.26
Case 3
Native 44.27
Repaired CoA 34.70
BIOINFORMATICS 2022 - 13th International Conference on Bioinformatics Models, Methods and Algorithms
140
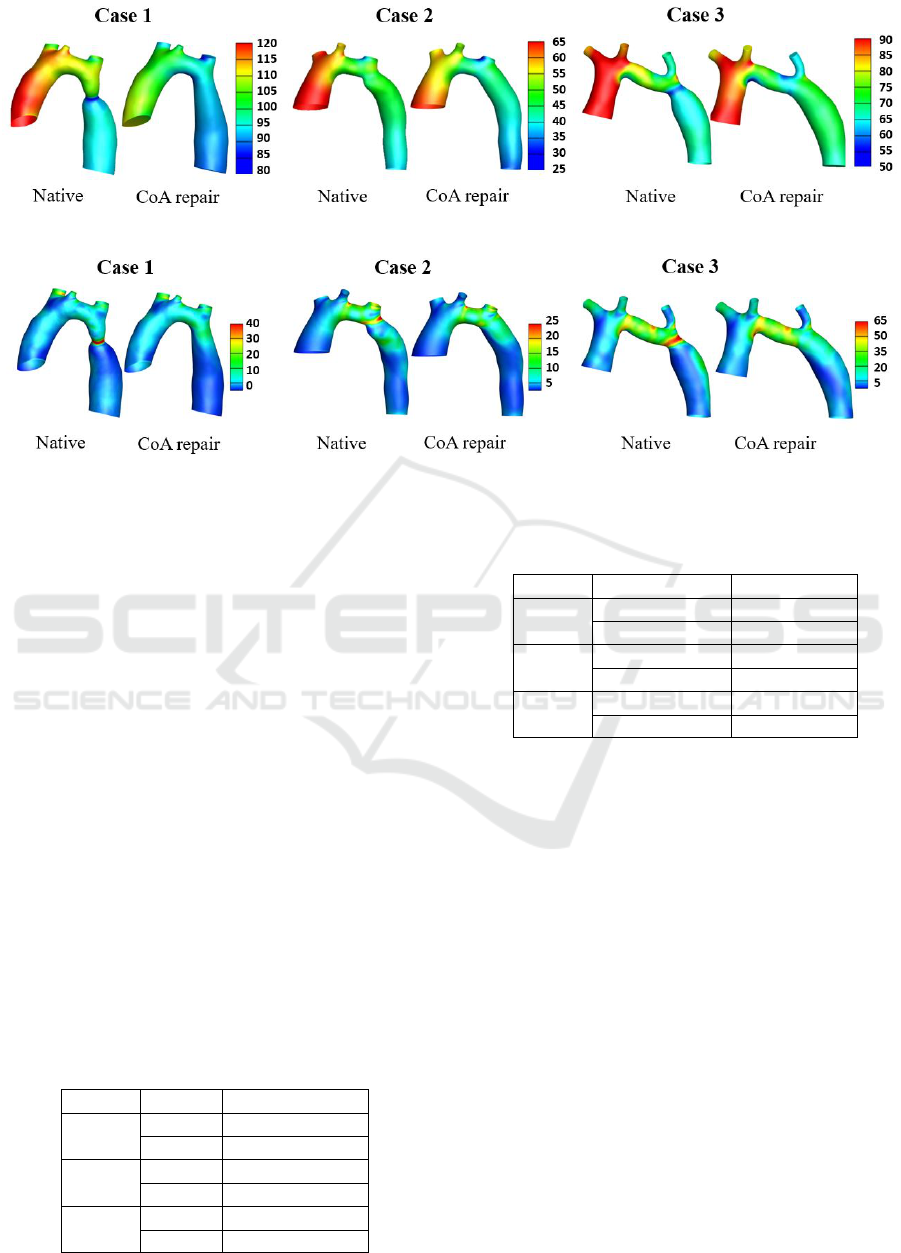
Figure 2: The pressure distribution of native and repaired CoA models of three cases. The unit of the pressure scale is mmHg.
Figure 3: The TAWSS distribution of native and repaired CoA models of three cases. The unit of the TAWSS scale is Pascal
(Pa).
fore the virtual repair and it remained the same post-
repair.
The PSPD were calculated in region
2
(arch) and
3
(descending) of the aorta to identify the contribu-
tion of TAH to the overall PSPD after the CoA repair.
The results are shown in Table 3. The CoA repair de-
creased the PSPD in region
3
by 12 mmHg and 1
mmHg for Case 1 and 3, respectively. The PSPDs in
region
2
after the repair were 7.9 mmHg for Case 1,
24.1 mmHg for Case 3. TAH contributed more to the
overall PSPD than CoA. For Case 2, the changes af-
ter the CoA repair were similar with higher PSPD in
region
2
than region
3
.
Compared to Case 1 and 3, the descending aorta of
Case 2 was the longest (61% of overall aorta length)
and the percent increase in diameter after the CoA re-
pair was the smallest (22%). Therefore, a larger PSPD
in descending part and a smaller change in overall
PSPD after the CoA repair for Case 2 is expected.
The TAWSS distributions for the three patients
are shown in Figure 3. The highest magnitudes of
Table 3: The comparison of PSPD in TAH region
2
and
CoA region
3
after CoA repair.
Region PSPD (mmHg)
Case 1
2
7.9
3
1.0
Case 2
2
7.3
3
5.6
Case 3
2
24.1
3
2.0
Table 4: The comparison of maximum TAWSS between na-
tive and repaired CoA geometries for the three patients.
Geometry TAWSS (Pa)
Case 1
Native 38
Repaired CoA 18
Case 2
Native 26
Repaired CoA 16
Case 3
Native 70
Repaired CoA 34
TAWSS were observed in Case 3. The virtual stent
repair fixed the region with high TAWSS in the CoA
area and all three cases showed reduced maximum
TAWSS after the repair. The maximum TAWSS val-
ues in the descending aorta before and after the repair
are shown in Table 4. The improvement in TAWSS
was in the repaired region but the magnitudes re-
mained higher than the rest of the overall TAWSS in
descending aorta for all cases. The TAWSS in the aor-
tic arch of Case 3 was high in the native case and since
the TAH in the arch was not repaired, the TAWSS
remained the same in that region. The higher val-
ues of TAWSS in the arch and repaired CoA region
persisted even after the CoA repair compared to the
rest of the aorta but overall magnitudes of TAWSS in
the descending aorta decreased compared to the na-
tive cases.
Virtual Planning and Simulation of Coarctation Repair in Hypoplastic Aortic Arches: Is Fixing the Coarctation Alone Enough?
141

4 DISCUSSION
This study focused on evaluating PSPD and TAWSS
of three patients with conditions of TAH and CoA.
We investigated the improvements in hemodynamics
after repairing only CoA virtually via stent placement
and leaving TAH untreated. Although the same vir-
tual treatment was applied to all three patients who
were diagnosed with the same diseases, the improve-
ment in PSPD after the CoA repair was inconsis-
tent. One of the cases showed almost no reduction in
PSPD and one other case showed 9 mmHg reduction
with the PSPD still higher than 20 mmHg, the typi-
cal threshold for intervention. Only one case success-
fully decreased PSPD from a higher value to below
20 mmHg. The pressure drops in TAH regions con-
tributed more to overall PSPD than pressure drops in
descending aorta. The results suggest that the CoA re-
pair alone in patients who also suffer from TAH does
not guarantee satisfactory PSPD in all cases. It should
be noted that the case with the PSPD higher than 20
mmHg after the repair was the youngest patient case,
a 9 months old infant. In very young patient cases,
the doctors would opt for surgical repair of isolated
coarctations, thus the virtual stenting may not neces-
sarily apply for Case 3 which may have influenced the
results.
A TAWSS that is higher than 50 dyne/cm
2
(5 Pa)
is shown to be associated with platelet aggregation in
previous studies (Kwon et al., 2014). The higher val-
ues of WSS were observed in descending aorta even
after CoA repair. But the region with the high TAWSS
was the proximal region where descending aorta and
left subclavian artery connects and it is a very small
area compared to the entire descending aorta region.
The TAWSS in the aortic arch was very high com-
pared to repaired CoA region, especially for Case 3,
reaching up to 60 Pa. Therefore, platelet formation
could likely occur in this region due to untreated TAH.
The limitations of this study include the rigid as-
sumption of the aorta walls to simplify the computa-
tion and reduce the computation time. Since the as-
sumption was made for all models and the purpose of
this study was to demonstrate improvements in hemo-
dynamics compared to native geometries, the rigid
wall assumption does not significantly affect the com-
parison. Also, previous studies (Siogkas et al., 2011)
showed that the results of the blood flow simulations
obtained in arterial segments assuming rigid walls and
modeling deformable walls were similar. Although
the number of patient cases is small to draw a con-
clusion for answering the question proposed in the
title, we demonstrated a systematic method for vir-
tual planning and simulation of CoA repair that can be
used for more cases. In addition, the anatomy of each
aorta was distinctive which allowed observing differ-
ent improvements in results after virtual CoA repair.
The method we used in this study to predict the
PSPD was previously validated in cases with CoA by
comparing the simulation results and invasive pres-
sure measurements from the patients (Aslan et al.,
2020). We performed virtual repair of CoA to inves-
tigate the changes in PSPD. In a future study, inva-
sive PSPD measurements of the patients after the stent
placement could be used to validate the simulation re-
sults of virtual CoA repairs.
Lastly, we modeled different size stents based on
patient-specific length and diameters and used one
type of repair to increase CoA diameter. In clinical
applications, different type of stents may result in dif-
ferent hemodynamics, affecting the TAWSS in partic-
ular (Kwon et al., 2014) in the repaired CoA region.
Therefore, it may affect the virtual treatment planning
for CoA repair and should be taken into consideration.
This study used virtual repair and CFD to help
doctors determine whether the CoA treatment alone
is sufficient in patients who also suffer from TAH. In
a future study, virtual treatment could be performed
to repair both CoA and TAH to help clinicians decide
if replacing minimally invasive stent treatment with
a surgery to repair both defects in different patients
would be more advantageous.
5 CONCLUSIONS
We investigated the improvements in PSPD and
TAWSS after repairing the CoA using virtual stents
in aortas that also have TAH. We showed that after
repairing the CoA, TAWSS was improved in the de-
scending aorta and PSPD was not always satisfactory.
Patient-specific stent selection, stent placement, and
flow simulations were performed. This study is the
first to investigate whether the stent repair that caries
less risks for patients than a surgery would result in
satisfactory PSPD and TAWSS in patient who also
suffers from TAH. The results of our study could help
clinicians determine the most appropriate treatment in
patients with CoA and TAH. Combination of imaging
and patient-specific CFD simulations is an important
tool in treatment planning and could change the selec-
tion and outcomes of repairs.
ACKNOWLEDGEMENTS
This work was supported by National Institute
of Health under grants NHLBI-R01HL143468 and
R21/R33HD090671. The authors acknowledge the
BIOINFORMATICS 2022 - 13th International Conference on Bioinformatics Models, Methods and Algorithms
142

supercomputing resource at the Advanced Research
Computing at Hopkins (ARCH) that made available
for conducting the research reported in this paper.
REFERENCES
Alkashkari, W., Albugami, S., and Hijazi, Z. M. (2019).
Management of coarctation of the aorta in adult pa-
tients: state of the art. Korean circulation journal,
49(4):298–313.
Aslan, S., Mass, P., Loke, Y.-H., Warburton, L., Liu,
X., Hibino, N., Olivieri, L., and Krieger, A. (2020).
Non-invasive prediction of peak systolic pressure drop
across coarctation of aorta using computational fluid
dynamics. In 2020 42nd Annual International Confer-
ence of the IEEE Engineering in Medicine & Biology
Society (EMBC), pages 2295–2298.
Dux-Santoy, L., Guala, A., Sotelo, J., Uribe, S., Teixid
´
o-
Tur
`
a, G., Ruiz-Mu
˜
noz, A., Hurtado, D. E., Valente,
F., Galian-Gay, L., Guti
´
errez, L., et al. (2020). Low
and oscillatory wall shear stress is not related to aor-
tic dilation in patients with bicuspid aortic valve: a
time-resolved 3-dimensional phase-contrast magnetic
resonance imaging study. Arteriosclerosis, thrombo-
sis, and vascular biology, 40(1):e10–e20.
Forbes, T. J., Garekar, S., Amin, Z., Zahn, E. M., Nykanen,
D., Moore, P., Qureshi, S. A., Cheatham, J. P., Ebeid,
M. R., Hijazi, Z. M., et al. (2007). Procedural results
and acute complications in stenting native and recur-
rent coarctation of the aorta in patients over 4 years of
age: a multi-institutional study. Catheterization and
Cardiovascular Interventions, 70(2):276–285.
Goubergrits, L., Riesenkampff, E., Yevtushenko, P.,
Schaller, J., Kertzscher, U., Berger, F., and Kuehne,
T. (2015). Is mri-based cfd able to improve clinical
treatment of coarctations of aorta? Annals of biomed-
ical engineering, 43(1):168–176.
Kenny, D., Polson, J. W., Martin, R. P., Paton, J. F., and
Wolf, A. R. (2011). Hypertension and coarctation of
the aorta: an inevitable consequence of developmental
pathophysiology. Hypertension Research, 34(5):543–
547.
Kwon, S., Feinstein, J. A., Dholakia, R. J., and LaDisa, J. F.
(2014). Quantification of local hemodynamic alter-
ations caused by virtual implantation of three com-
mercially available stents for the treatment of aortic
coarctation. Pediatric cardiology, 35(4):732–740.
Liu, X., Aslan, S., Hess, R., Mass, P., Olivieri, L., Loke, Y.,
Hibino, N., Fuge, M., and Krieger, A. (2020). Auto-
matic shape optimization of patient-specific tissue en-
gineered vascular grafts for aortic coarctation. In 2020
42nd Annual International Conference of the IEEE
Engineering in Medicine & Biology Society (EMBC),
pages 2319–2323.
Lopez, L., Colan, S., Stylianou, M., Granger, S., Tracht-
enberg, F., Frommelt, P., Pearson, G., Camarda, J.,
Cnota, J., Cohen, M., et al. (2017). Relationship of
echocardiographic z scores adjusted for body surface
area to age, sex, race, and ethnicity: the pediatric heart
network normal echocardiogram database. Circula-
tion: Cardiovascular Imaging, 10(11):e006979.
Ma, Z.-L., Yan, J., Li, S.-J., Hua, Z.-D., Yan, F.-X., Wang,
X., and Wang, Q. (2017). Coarctation of the aorta with
aortic arch hypoplasia: midterm outcomes of aortic
arch reconstruction with autologous pulmonary artery
patch. Chinese medical journal, 130(23):2802.
Mai, C. T., Isenburg, J. L., Canfield, M. A., Meyer,
R. E., Correa, A., Alverson, C. J., Lupo, P. J.,
Riehle-Colarusso, T., Cho, S. J., Aggarwal, D., et al.
(2019). National population-based estimates for ma-
jor birth defects, 2010–2014. Birth defects research,
111(18):1420–1435.
Peters, B., Ewert, P., and Berger, F. (2009). The role of
stents in the treatment of congenital heart disease:
Current status and future perspectives. Annals of pe-
diatric cardiology, 2(1):3.
Quennelle, S., Powell, A. J., Geva, T., and Prakash, A.
(2015). Persistent aortic arch hypoplasia after coarcta-
tion treatment is associated with late systemic hyper-
tension. Journal of the American Heart Association,
4(7):e001978.
Rao, P. S. (2020). Neonatal (and infant) coarctation of the
aorta: management challenges. Research and Reports
in Neonatology, 10:11.
Schubert, C., Br
¨
uning, J., Goubergrits, L., Hennemuth, A.,
Berger, F., K
¨
uhne, T., and Kelm, M. (2020). Assess-
ment of hemodynamic responses to exercise in aor-
tic coarctation using mri-ergometry in combination
with computational fluid dynamics. Scientific Reports,
10(1):1–12.
Siewers, R. D., Ettedgui, J., Pahl, E., Tallman, T., and Pe-
dro, J. (1991). Coarctation and hypoplasia of the aor-
tic arch: will the arch grow? The Annals of thoracic
surgery, 52(3):608–613.
Siogkas, P., Sakellarios, A., Exarchos, T., Stefanou, K.,
Fotiadis, D., Naka, K., Michalis, L., Filipovic, N.,
and Parodi, O. (2011). Blood flow in arterial seg-
ments: rigid vs. deformable walls simulations. Jour-
nal of the Serbian Society for Computational Mechan-
ics, 5(1):69–77.
Su
´
arez de Lezo, J., Romero, M., Pan, M., Su
´
arez de Lezo,
J., Segura, J., Ojeda, S., Pavlovic, D., Mazuelos, F.,
L
´
opez Aguilera, J., and Espejo Perez, S. (2015). Stent
repair for complex coarctation of aorta. Cardiovascu-
lar Interventions, 8(10):1368–1379.
Torok, R. D., Campbell, M. J., Fleming, G. A., and Hill,
K. D. (2015). Coarctation of the aorta: management
from infancy to adulthood. World journal of cardiol-
ogy, 7(11):765.
Virtual Planning and Simulation of Coarctation Repair in Hypoplastic Aortic Arches: Is Fixing the Coarctation Alone Enough?
143
