
Risk-based Comprehensive Usability Evaluation of Software as a
Medical Device
Noemi Stuppia
1
, Federico Sternini
1,2 a
, Federica Miola
1
, Giorgia Picci
3
, Claudia Boarini
3
,
Federico Cabitza
4b
and Alice Ravizza
1
1
USE-ME-D srl, I3P Politecnico di Torino, Torino, Italy
2
PolitoBIOMed Lab, Politecnico di Torino, Torino, Italy
3
Dedalus spa, Firenze, Italy
4
DISCO, Università degli Studi di Milano-Bicocca, Milano, Italy
{giorgia.picci, claudia.boarini}@dedalus.eu, federico.cabitza@unimib.it
Keywords: Usability, Software as a Medical Device, User Interface.
Abstract: Introduction: Usability evaluation is a core aspect in risk assessment of medical devices, as it aims to ensure
the device interface safety, avoiding that usability problems at interface level are not related to harm.
Methods: Our research group applied our risk-based approach, international reference standards and
guidelines to the usability evaluation of a large family of SaMD. The methodology used for the evaluation is
an elaboration of regulatory prescriptions and is composed of a combination of quantitative and qualitative
methods. In particular, the usability evaluation is structured in a two-stage evaluation composed by formative
and summative evaluation. The formative stage is propaedeutic for the planning of the summative evaluation.
The final assessment included the analysis of quantitative data collected through three questionnaires and a
user test.
Results and discussion: Risk-based task analysis led to the identification of the most common use error
emerged during the user test performance. The three questionnaires led to different results: Heuristic analysis
allowed the identification of violations to the heuristic principles as perceived by the users and their severity;
SUS questionnaire provided an indicator of general device usability; the interview identified the usability
problems of each device with respect to their functionalities.
Conclusions: The study allowed the extensive assessment of the devices, the identification of usability issues,
and the classification in terms of criticality of each issue. In conclusion the study led to different proposals to
solve the issues and design changes.
1 INTRODUCTION
Patient care, two words that carry an array of diverse
practices that have a shared scope: to prioritize patient
health while limiting any unnecessary or potential
harm." To err is human", is a long-lasting thought that
in 1999 opened the conversation on the consequence
of human error in healthcare and triggered a new
approach to improve patient safety through design
(Institute of Medicine (US) Committee on Quality of
Health Care in America, 2000). The removal of all the
root causes of any hazardous situation is unfeasible,
but by factoring in the human element within the
a
https://orcid.org/0000-0002-5510-2296
b
https://orcid.org/0000-0002-4065-3415
design process, the manufacturer can mitigate risks
associated with proper use. The risk mitigation
approach is a core regulatory requirement for medical
device approval by authorities, worldwide. It is
specifically addressed in the European Medical
Device Regulation EU 2017/745. International
standards apply, and IEC 62366-1:2016 provides a
systematic approach for the manufacturer to analyze,
specify, develop and evaluate the usability of a
medical device as it relates to safety (International
Electrotechnical Commission, 2020). The standard
provides a framework that is suitable for all medical
devices. Nevertheless, no indication in the standard is
454
Stuppia, N., Sternini, F., Miola, F., Picci, G., Boarini, C., Cabitza, F. and Ravizza, A.
Risk-based Comprehensive Usability Evaluation of Software as a Medical Device.
DOI: 10.5220/0010825100003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 5: HEALTHINF, pages 454-462
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

provided regarding the selection of the most adequate
methods for each medical device and research is
progressing for the proposal of best usability
evaluation process (Kwak et al., 2021; Schmettow et
al., 2017). Continuing the work of the research group
for the identification of the most adequate strategy for
the usability evaluation for each device (D. Ravizza
et al., 2019), in this paper, we present how our team
chose the regulatory-approved methods for usability
assessment and used them for the usability
assessment of 10 medical software of different
complexity, and the result of this methodology.
2 METHODS
The international standard aims to reduce the risk of
medical errors due to poor interface design through
the definition of methods of usability evaluation of
the interface. Similarly, the standard also applies to
the documentation that accompanies a device and to
the training of the intended users. Following the
standard requirements, we defined an integrated and
comprehensive approach defining a two-step
usability evaluation phase that includes both methods
available at the state of the art and innovative methods
proposed within the context of this study and previous
studies (Sternini et al., 2021). Each phase has a
different purpose; therefore, different techniques are
used accordingly (D. Ravizza et al., 2019). In each
phase, we defined the chosen techniques and the
outcomes that each step should provide.
2.1 Formative
The first phase described in the IEC standard is the
formative evaluation, which aims to iterate the design
of the user interface to achieve the minimization of
usability-based risks.
The first activity was the definition of the software
functions and requirements, core to planning all the
further testing activities. The software primary
operating function, as already defined in the technical
file, were paired with one testable requirement. The
technical testable requirement was defined as the
capability to complete the primary operating function
with predetermined usability criteria that are
consistent with the intended use (e.g. in case of
primary operating function for patient incoming in an
emergency ward, the technical testable requirement is
the capability of the device to support triage, that is to
allow for the efficient association between the patient
and the proper colour code). The target quality level
was identified in terms of the number of times the
product would meet the testable requirement as well
as the number of bugs and unclear user interface
features, for example icons.
Subsequently, the formative evaluation was
performed following these iterative steps:
Preliminary analysis: it included as a first step
a general, "quick-and-dirty" overview of the
core product functionalities and general
interface aspect. We completed a cognitive
walkthrough (International Electrotechnical
Commission, 2016) and brainstorming, in a
team composed of usability experts, to
identify potential use errors, applicable
standards, known errors and complaints, and
relevant literature. The main goal of this step
is the definition of interface strengths and
weaknesses. The latter ones are then mapped
into a device risk analysis by using the
relevant questions listed in risk management
international standard ISO 14971
(International Organization for
Standardization, 2019) as a reference. With
this introductory knowledge we drafted a task
list, which is defined as a sequence of actions
that are necessary to achieve the task goal for
each operating function and each user profile
foreseen in the software.
Detailed analysis in a team supported by a
device expert (e.g. designers, product
specialist): this phase began with a brief
training of our usability team. The training
was conducted confirming and updating the
task list drafted in the preliminary phase and
then describing and simulating the core user
experience scenarios, enabling the product
experts to identify any interface pitfalls, bugs
or other details in the device that were not yet
addressed by the development team. The
training session provided the usability experts
with the proper knowledge to:
o Evaluate the primary operating
functions, defined as functions that are
directly related to the device safety or
that are frequently used.
o Execute the task analysis, which is a
technique aimed to understand the
process of learning of ordinary users by
observing them in real-life situations; it
describes in detail how they perform
their tasks and achieve their intended
goals.
Risk-based Comprehensive Usability Evaluation of Software as a Medical Device
455
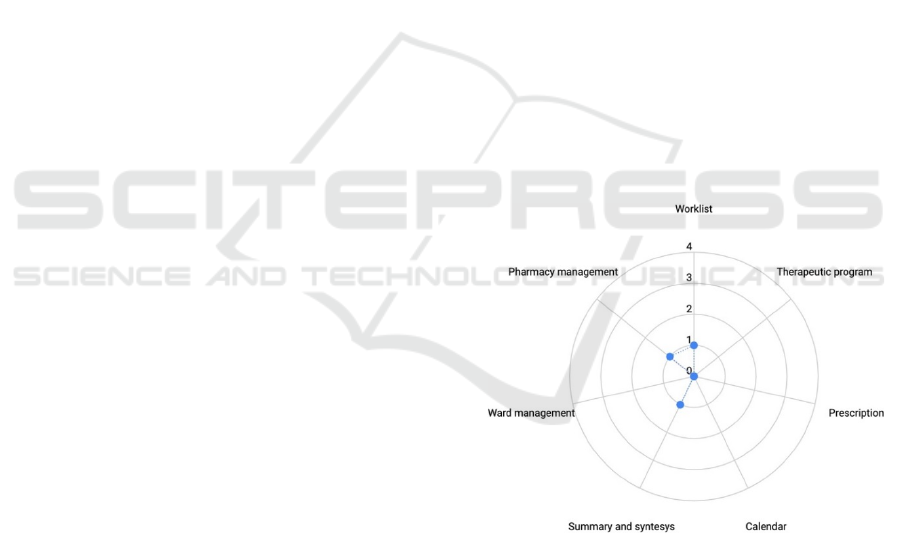
The evaluation of the primary operating function
was completed by assigning to each function a score
representative of the interface problems encountered
during the function analysis. The score ranges from 0
to 4, where 0 regards no problem, while 4 is the value
assigned to the highest risk related to the device. The
following scale was used. This scale was used for
answers both in the heuristic questionnaire and in the
primary operating function evaluation, to minimise
training of participants, ensure consistency and allow
the comparison of the scores.
0 = no problem
1 = Before using it I have to spend some time
figuring out how to do it
2 = Complicated use and makes me nervous
3 = Impossible use and/or incomprehensible
instructions
4 = Possible risk for the patient (patient
misidentification; clinical pathway interrupted)
We analysed the results of the evaluation through
a radar plot to have a glimpse of the usability risk
profile of the device. This plot, presented in Figure 1,
allows for immediate comprehension of the approval
of the design interface and whereas the device is
intuitive and requires minimal effort to complete the
main task. As the area underlying the dots increases,
the graph shows that the task is not well understood
and accepted by the evaluators.
At the end of the formative evaluation, we
designed two novel questionnaires intended to ease
the data collection during the summative phase:
Heuristic evaluation: this is a useful, efficient,
and low-cost method that we proposed to
evaluate patient safety features of medical
devices through the identification of usability
problems. Furthermore, it provides an
estimation of the severity of these problems.
The questionnaire is intended to be composed
by carefully formulated questions and closed
answers. The questions are designed by the
usability experts so that the user can assess
Zhang’s heuristics, without the need for
training regarding the underlying theory
(Zhang et al., 2003). Each relevant heuristic is
represented by at least one question
formulated to lead users to identify any
heuristic violation. The questions should have
a scope broad enough to allow the user to
answer the question on the base of its own
experience, without the bias given by the
moderator experience. Therefore, we designed
the questionnaire tackling the specific
heuristic defined by Zhang with proper terms
for the device type, but without including any
reference to specific situations (e.g., Are the
icons and interactions consistent with devices
you habitually use?). Then, the severity of the
heuristic violation is assessed through the
provision of a score; scores are presented with
meanings associated with the single user
experience and perception of risk. In this way,
the user could quickly answer without any
additional training, and the moderators could
relate the scores given by the users with the
violation severity.
Interview: The questionnaire was designed so
that each question was consistent with one
primary operating function of the device and
to be representative of the user interactions. It
is intended to provide an evaluation of the
primary operating functions as perceived by
the intended users.
For each technique, we analysed and represented
the best response, worst, mean and median for
completeness.
When all these activities are concluded, and the
results of the formative evaluation are correctly
reported, the usability assessment can proceed to the
summative evaluation.
Figure 1: Representation of an interview response radar
plot, the significance of the scores is reported in the text.
2.2 Summative
The summative evaluation aims to assess the
adequateness of the user interface by considering the
outcome in terms of the risk of potential user errors
and by providing evidence that all minimization of
known causes of use error is in place.
The core technique that our group employed was
the user test. We recruited 15 participants, a practical
HEALTHINF 2022 - 15th International Conference on Health Informatics
456

minimum number of participants for human factors
validation testing (Health, 2019). The tests were
carried out in a simulated-use environment to ensure
adequate observation. Additionally, to ensure patient
privacy, we created adequate simulated patients
profiles according to the mode principle (A. Ravizza
et al., 2020). By doing so, patient privacy was ensured
while we also allowed the test participants to interact
with realistic data. The mode principle allows
describing simulated patients using the data that are
most frequent in the patient population, which are
considered more representative than mean values
because the latter can be inconsistent with real data
(A. Ravizza et al., 2020). The test scenario was
designed by referring to the task analysis conducted
in the formative evaluation.
The task list, that is the main script for the user
tests, was implemented to test all of the primary
functions. Thus, within the same scenario, the user
might be asked to do multiple tasks per feature (e.g.,
inserting a new patient in the EHR by searching her
from a contact list or by inserting the personal
information in a search bar). By allowing the presence
of tasks sharing similar sub-steps, the test participant
had the option to understand the navigation pathway
better and conclusively give an informed opinion on
the interface characteristics based on multiple
interactions rather than a single one.
At the beginning of the user test, due to the
complex interface of the medical device, we invited
the device expert to conduct an introductory speech
and a brief training session to give proper information
about the intended use of the device and the purpose
of the user test. The speech aims to ease the
participants into the experience, by providing them
with a basic introduction on what they will later see.
More importantly, the speech helps them focus on the
crucial aspect of their contribution, which is to report
what they perceive, what they reason about, and
which action they will take accordingly. This
decomposition allowed the interviewer, during the
test, to assess the level of individual user interaction
with the specific task. Moreover, by using the PCA
approach (International Electrotechnical
Commission, 2016), the interviewer was able to
identify the main categories of use errors which
stemmed from perception, cognition and action
errors. Besides, potential use problems can be
targeted by asking the user about the consequences of
a failed task. As prescribed by the standard, we
trained the moderators to not intervene during the
user test, but to limit the intervention only if the user
could not complete the task autonomously.
The participants tested the functionalities of the
devices following the task list with the supervision of
the moderators and, for each user task use, the
moderators evaluated the user actions with the
following policy:
ok: the task was completed without error
ue (user error): the user was not able to
complete the task and requires help from the
moderator, or the user made an error that had
no impact on the patient (e.g., the input of the
password with the caps lock on), or the user
knowingly neglected to complete a task
ce (critical error): the user made an error that
has an impact on clinical risk. e.g., ignored a
notification regarding critical clinical risk (for
example, drug interaction); skipped the patient
identification.
te (technical error): task not completed due
system failure.
The purpose of analysing the task performed by
users was to evaluate the presence of ce (critical error)
and to identify which task may have caused
uncertainty or confusion. The result of the task
completion is an informative source of improvements
for technical manuals and training sessions, allowing
the designers to understand which require additional
clarity in the instructions and more examples during
training. Additionally, it can provide feedback on the
unsolved technical issues occurring during normal
use.
Additionally, during the user test, the participants
may comment on the device performance (in terms of
usability), and the moderators may propose open-
ended questions to the users, which may lead to
additional problems and uncertainty information and
further product improvement. We encourage
collecting the notes from the user impression; once
vetted, they can be a valuable source for further
product improvement.
At the end of the simulated use, we let users
evaluate the devices with three different metrics:
interview, heuristic questionnaire, and System
Usability Scale (SUS). The first two are the
techniques designed during the formative evaluation,
while the SUS questionnaire provides a "quick and
dirty," reliable tool for measuring usability. It consists
of a 10-item questionnaire with five response options
for respondents; from Strongly agree to strongly
disagree (Jordan et al., 1996).
The questionnaires were briefly described by the
moderators to the participants and then filled
autonomously by the participants.
Risk-based Comprehensive Usability Evaluation of Software as a Medical Device
457
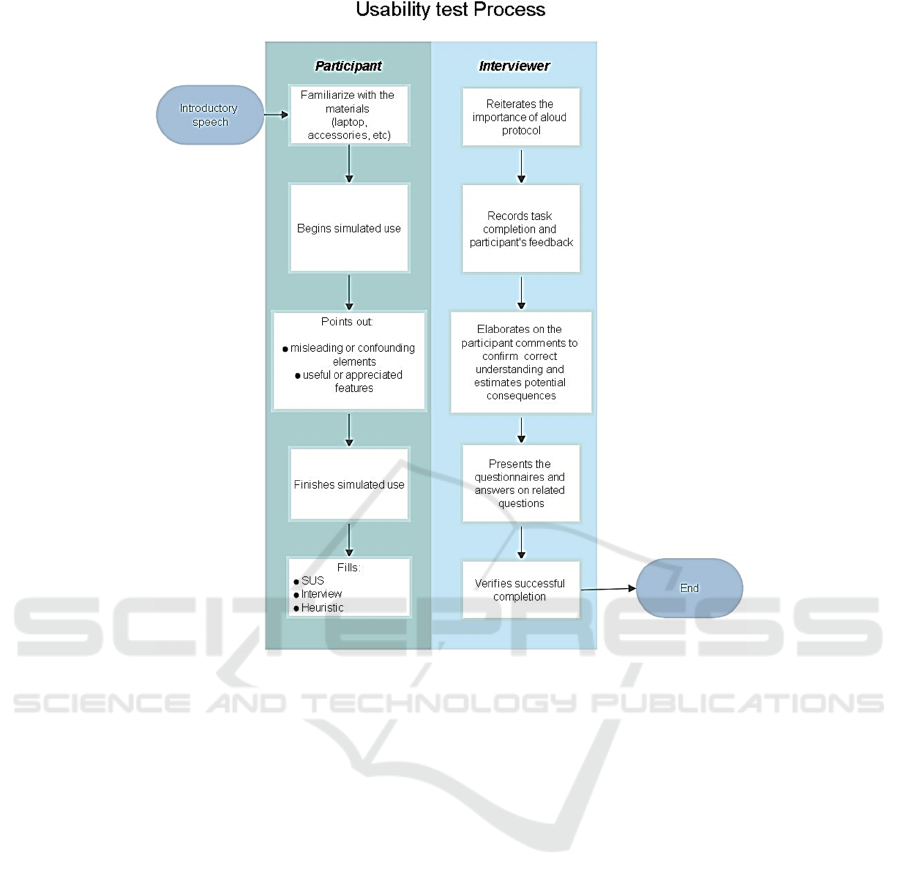
Figure 2: This caption has more than one line so it has to be set to justify.
After the testing phase, all the results are collected
and analysed. The analysis and the result report
complete the analysis of the medical device interface
and to assess its usability. A summary of the test
process is presented in Figure 2.
3 RESULTS
The methodology described above was applied to the
usability evaluation study of ten products
manufactured by Dedalus SPA. The study was aimed
at the usability evaluation of 10 different SaMDs
designed to help the management of the care path of
hospital patients, in different wards. Considering that
the work domains varied between groups, we
analysed different work domain ontologies that are
associated with different levels of intrinsic
complexities and corresponding risks. They were
grouped in families according to the intended use:
EPMA, identified by the intended use “
Electronic prescribing and medicine
administration ” . The intended users are
oncologists, nurses, nuclear medicine
physicians, radiologists, radiology technicians
and general physicians.
AID, identified by the intended use “
Operating and emergency room assistance”.
The intended users are trauma surgeons,
orthopaedics, general surgeons,
anaesthesiologists, nurses and perioperative
nurses.
ERH, identified by the intended use “
Electronic health record and screening”. The
intended users are general practitioners.
The recruitment of the user group included people
with different level of familiarity with the software.
Some of them used similar devices; some had
previously used the specific device, while others only
used paper records in their administrative and medical
operations. The difference level of experience with
different age group allowed for a complete result that
can reflect the real-use applications.
HEALTHINF 2022 - 15th International Conference on Health Informatics
458
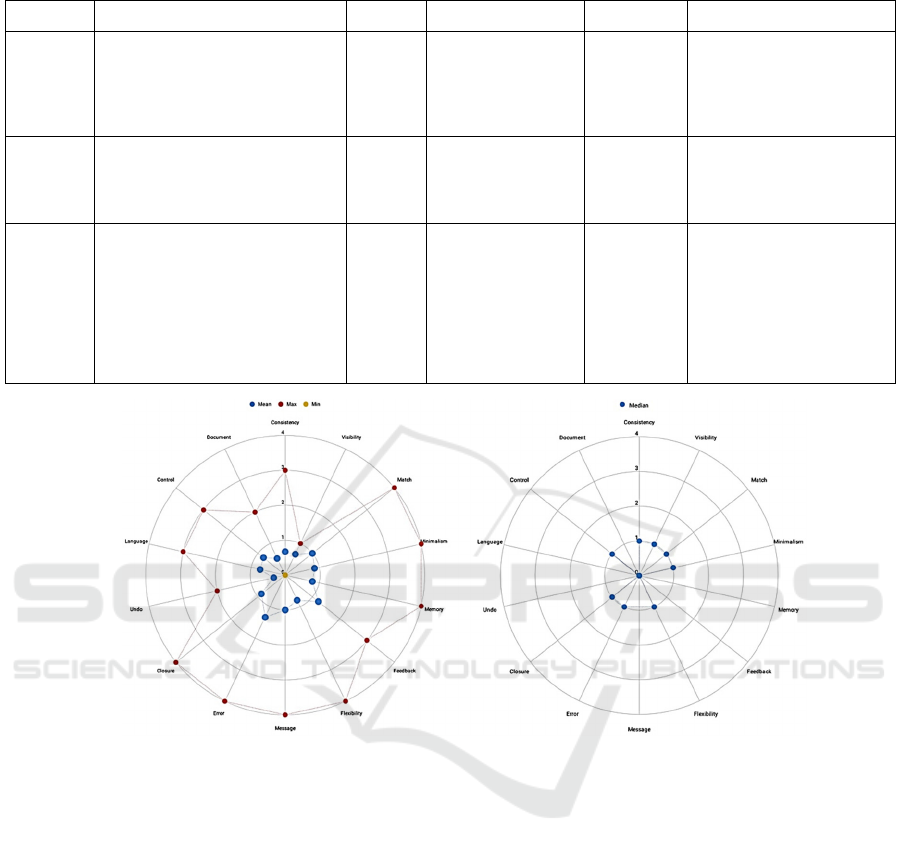
Table 1: Summary of most relevant use-errors.
Software Use error Severity Principle violated Prevalence Recommended solution
Software
A
User failed to add a product to the
warehouse
not
critical
Giving control and
freedom to the user
about reversible
actions
25% Add another selection option
and provide a double-check
with a summative message
(pop-up windows) when the
user confirms the action
Software
B
The user was unable to correctly
find and fill the mandatory tabs to
require and exam
not
critical
Encourage
recognition rather
than memory
40% Target this scenario in the
training session and modify
the wording associated with
the task
Software
C
The user (nurse) misread the
information "reported allergies"
and read "unknown allergies".
Then she thought that the allergies
of the patient were already asked
the patient and that there is no
known allergy, while the allergy
section included several allergies.
critical
Provide a simple and
natural language:
any data that the user
has to insert must
Be presented in a
completely similar
way to the paper
format.
20%
The information about the
presence of this section and
the meaning of the keywords
used in summary included in
the dashboard must be for
future installations at
customer sites
Figure 3: Example of the heuristic response radar plot. Score 0, No problem, 1 The use is a bit complicated, the user needs
time to get used to the device use, 2 The use is complicated, the user could get nervous during the device use, 3 The use is
impossible due to design issue or non-understandable instructions, 4 Possible patient risk.
3.1 Task Completion
During the analysis of the task completion, it is
possible to quantitatively estimate the percentage of
the correctly executed tasks, technical errors and
evaluate the severity of the use error. The evaluation
of the severity of a use-error is typically not uniquely
defined and strongly relies on the interviewer's
judgement. It may lead to a modification of the risk-
analysis or just to a suggestion for further product
improvement. In this study, the moderators were
trained in advance, to minimise bias, to assign the
“critical error” class to actions that could expose the
patient to serious risk. Excluding the technical
failures, all other errors are then classified as user
errors. We summarised the most significant examples
of user errors and their classification in Table 1.
3.2 Heuristics
As cited in the methods section, the results were
evaluated according to their median, average, best
and worst values. When the mean score is lower than
one, it represents a consensus opinion related to the
specific heuristic that is partially favourable for the
product. The score equal to one is considered as a
threshold to identify efficacy problems related to the
specific heuristic. Any score equal to or higher than
two should require further analysis since it may be a
source of patient risk. In the reported example, related
to the software for emergency wards management, by
observing the worst-case evaluation, we detect
multiple at-risk categories. To better understand the
meaning of the heuristic tag, we reported the
Risk-based Comprehensive Usability Evaluation of Software as a Medical Device
459
Table 2: Example of primary operating function and testable requirement.
Primary
operating
function
Physician Nurse Interface testable technical requirements
Patient
incoming
no yes The patient incoming function shall allow assigning a color coding
according to predefined clinical criteria in an efficient manner
List yes yes The list function shall allow the monitoring of the whole set of
activities of the ER ward in an efficient manner
ER
ambulatory
patient
management
yes yes The function of patient management shall allow all the clinical
personnel, according to their privileges, to update the clinical
record in the ER ward in an efficient manner
graphical representation of the results in figure 3 and
the related comment:
Icon and colors: Widely differ from the typical
representations both in graphics and in colour
Minimalism: The user interface has
unnecessary and redundant information
Memory: The user is required to remember
much information about the patient and his
therapeutic path while using the software
Flexibility: The software does not
accommodate user desired variation
Message: The error message is not clear or
helpful
Closure: The user is often unsure if an
operation was completed or not.
Error: The system may be misleading
It should be noted that at least half of the user
population choose values above the "low efficacy"
score one.
3.3 SUS
The ten questions of the SUS questionnaire result in
the System Usability score
. The average value is 68.
Generally, any score higher than 81 is an optimal
response, while a score lower than 51 is critical and
unacceptable. In this study, any of the analysed
devices obtained a critical ad unacceptable score, but
the result showed that there is room for improvement.
3.4 Interview
As previously explained in the method section, we
associate a testable requirement for each primary
operating function that directly relates to safety. This
is aimed to assess whether the software successfully
provides a testable solution in the user-interface. This
is generally done by implementing alarms, color
coding or dedicated icon. An example is provided in
table 2.
Comprehensively, most of the primary operating
functions were evaluated as acceptable by the
participants, with most frequent scores equal to 0 or 1
(no risk area). However, any response equal to or and
higher than two belongs to the risk-area and needs to
be addressed. We report here a few examples of the
problems identified through this tool:
One usability problem was detected by two
different participants (physicians) in the same
task; the physician pointed out that the clinical
report at discharge was missing information
regarding the drug therapies. However, the
issue was already identified and resolved by
the manufacturer but not available in the
customization of the software designed for the
test set. The contents of the clinical report at
discharge are provided in a complete list by
the medical device software, and the actual
inclusion of one or more of the therapies (for
example administered, planned, required at
discharge, home therapy) is a customisation
choice. Consequently, to avoid uncertainty for
the users, we encouraged to highlight what is
customizable and what not during the training
session.
Both our team and expert users detected a
usability problem related to the drug
administration task. The hazardous element
was the lack of a time reference for the
administration in the primary therapy window.
The software already allowed access to that
information, but in a different window. The
results of the interview completed by the user
confirmed the usability risk identified by the
experts in the formative phase. The use of the
same metrics and plots for the formative
evaluation and interview analysis allowed us to
identify the consistencies and the gaps of the
formative analysis when compared to real use.
HEALTHINF 2022 - 15th International Conference on Health Informatics
460

4 DISCUSSION
By implementing multiple evaluation methods for the
usability evaluation, we aim to collect as many
information as possible. While a questionnaire or
task-completion can provide a numeric result, it
cannot identify specific design problems that may
need to be addressed. On the other hand, when paired
with multiple qualitative methods, such as moderator
observations, PCA techniques and open-ended
questions, these techniques allow for valid
quantification of the criticality of each issue, while
qualitative methods allow the comprehension of all
design flaws encountered during the usability testing.
Each one of the methods used during the study
covered different aspects of the usability evaluation,
and participated to the completion of the usability
assessment of the different devices, providing
different observations regarding the device safety and
interface design. Strengths and weaknesses of each
one of the results obtained are detailed in the
following paragraphs.
The task performance evaluation highlighted the
current weaknesses in terms of actions and part of the
usability process identifying the steps and the tasks
that are the most critical for the management of the
medical device safety but does not provide additional
insights related to the reasons and the semantic error
that led to the usability pitfall. Nevertheless, with the
proper integration of questionnaires and interviews,
the causes related to the pitfalls can be investigated
and understood.
The SUS questionnaire confirmed that is a
technique that does not provide any information
regarding the medical device safety, but can be used
for the evaluation and quantification of the ease of use
and user interface pleasureability and provides the
possibility for comparison with analogous devices.
The heuristic evaluation phase, even if cannot be
used directly to observe hazardous situations and
potential usability pitfalls demonstrated to be an
excellent tool for the identification of the weaknesses
of the tested user interface and to understand how to
plan and focus the improvements of the user interface.
Finally the interview questionnaire evidenced its
potential for the identification of the most critical
functionalities of the device. With respect to the
heuristic evaluation, which identifies the critical
qualities of the user interface, this tool is extremely
useful for the evaluation of the functionalities, how
they are designed and perceived by the users.
5 CONCLUSIONS
As part of the validation activities of the usability
aspects, our group acted as an external reviewer of the
compliance of a series of software as medical devices
with respect to the current applicable standards and
Medical Device Regulation in Europe. To complete
the evaluation of these devices, we used an approach
derived from the applicable standards and other
pertinent sources. We explicitly tailored them to
ensure a complete overview of the device usability
composed by structured methods. We applied this
approach in this review, during the formative and
summative phases of design. The proposed
methodology for the activities is highly informative,
repeatable, it allows for comparisons between
different devices and complies with the current
applicable standards. Our approach allowed a clear
presentation of the results both to the developers and
to the regulatory authorities. In future studies, we will
analyse, improve, and standardise this methodology
in order to obtain a structured workflow and a
framework of techniques for the usability evaluation
of SaMDs.
REFERENCES
Health, C. for D. and R. (2019, September 2). Applying
Human Factors and Usability Engineering to Medical
Devices. U.S. Food and Drug Administration; FDA.
https://www.fda.gov/regulatory-information/search-
fda-guidance-documents/applying-human-factors-and-
usability-engineering-medical-devices
Institute of Medicine (US) Committee on Quality of Health
Care in America. (2000). To Err is Human: Building a
Safer Health System (L. T. Kohn, J. M. Corrigan, & M.
S. Donaldson, Eds.). National Academies Press (US).
http://www.ncbi.nlm.nih.gov/books/NBK225182/
International Electrotechnical Commission. (2016).
IEC/TR 62366-2:2016 (1st ed.). IEC.
https://www.iso.org/cms/render/live/en/sites/isoorg/co
ntents/data/standard/06/91/69126.html
International Electrotechnical Commission. (2020). IEC
62366-1:2015+AMD1:2020 (1.1). IEC. https://
webstore.iec.ch/publication/67220#additionalinfo
International Organization for Standardization. (2019). ISO
14971:2019 (3rd ed.). ISO. https://www.iso.org/cms/
render/live/en/sites/isoorg/contents/data/standard/07/2
7/72704.html
Jordan, P. W., Thomas, B., McClelland, I. L., &
Weerdmeester, B. (1996). Usability Evaluation In
Industry. CRC Press.
Kwak, H., Oh, H., Cha, B., & Kim, J. M. (2021). The
assessment of usability of pain medical device by
physiatrists and physiotherapists. Medicine, 100(38),
Risk-based Comprehensive Usability Evaluation of Software as a Medical Device
461

e27245. https://doi.org/10.1097/MD.0000000000027
245
Ravizza, A., Sternini, F., Giannini, A., & Molinari, F.
(2020). Methods for Preclinical Validation of Software
as a Medical Device: Proceedings of the 13th
International Joint Conference on Biomedical
Engineering Systems and Technologies, 648–655.
https://doi.org/10.5220/0009155406480655
Ravizza, D., Sternini, F., Lantada, A., Sánchez, L., Sternini,
F., Ravizza, D., & Bignardi, C. (2019). Techniques for
Usability Risk Assessment during Medical Device
Design: Proceedings of the 12th International Joint
Conference on Biomedical Engineering Systems and
Technologies, 207–214. https://doi.org/10.5220/
0007483102070214
Schmettow, M., Schnittker, R., & Schraagen, J. M. (2017).
An extended protocol for usability validation of
medical devices: Research design and reference model.
Journal of Biomedical Informatics, 69, 99–114.
https://doi.org/10.1016/j.jbi.2017.03.010
Sternini, F., Isu, G., Iannizzi, G., Manfrin, D., Stuppia, N.,
Rusinà, F., & Ravizza, A. (2021). Usability Assessment
of an Intraoperative Planning Software: Proceedings of
the 14th International Joint Conference on Biomedical
Engineering Systems and Technologies, 483–492.
https://doi.org/10.5220/0010252904830492
Zhang, J., Johnson, T. R., Patel, V. L., Paige, D. L., &
Kubose, T. (2003). Using usability heuristics to
evaluate patient safety of medical devices. Journal of
Biomedical Informatics, 36(1–2), 23–30.
https://doi.org/10.1016/S1532-0464(03)00060-1
HEALTHINF 2022 - 15th International Conference on Health Informatics
462
