
Best Practice in Multi-organisation Sensitive Health Data Sharing:
A Comparative Analysis of Ireland’s Data Governance Approach
for the Covid–19 Data Research Hub
Aleksandra Czarnik
1a
, Aoife Darragh
1b
, Maria Hurley
1
, Daniel O’Connell
1c
, Michele Quagliata
1
and Rob Brennan
2d
1
School of Computing & School of Law and Government, Dublin City University, Dublin 9, Ireland
2
ADAPT Centre, School of Computing, Dublin City University, Dublin 9, Ireland
daniel.oconnell245@mail.dcu.ie, michele.quagliata2@mail.dcu.ie, rob.brennan@dcu.ie
Keywords: Data Governance, Health Data, Data Security, Health Research, Public Administrative Bodies.
Abstract: This paper examines, from a data governance perspective, the creation and operation of the Irish Covid–19
Data Research Hub, a secure multi-institution collation and access-controlled source of sensitive Covid–19
epidemiological data from diverse sources. The Hub is assessed alongside international comparators and with
reference to a set of leading academic data governance models, including those developed by Khatri & Brown
(2010), Winter & Davidson (2019), and Abraham et al (2019). The analysis explores the requirements for
such data hubs balancing data protection, security, and health policy decision making. It examines the data
hub design from architectural, data access policy, and data governance perspectives. Whilst recognising
certain unique features of the Covid–19 Data Research Hub not replicated elsewhere, it highlights key data
governance strengths and gaps in the model used which may inform future development of similar hubs
supporting the exploitation of public sector data for health policy-related research. The interdisciplinary legal
and technical data governance assessment methodology described here is applicable to the increasing number
of data federation and aggregation projects increasingly being deployed in both public and private healthcare
settings.
1 INTRODUCTION
Health research operates in one of the most sensitive
of all data domains (General Data Protection
Regulation, 2018) and requires exemplary standards
of data stewardship and governance to comply with
data protection laws and to maintain public
confidence. The Data Administration Management
Association (DAMA, 2017) defines data governance
as “the exercise of authority, control, and shared-
decision making (planning, monitoring, and
enforcement) over the management of data assets”. In
addition, ethical health data governance, as described
by Hripcsak et al. 2014, must involve the structured
management, secure storage and controlled
a
https://orcid.org/0000-0001-6217-5701
b
https://orcid.org/0000-0003-3104-2767
c
https://orcid.org/0000-0001-8641-2409
d
https://orcid.org/0000-0001-8236-362X
disclosure of health data only to appropriate users, to
ensure knowledgeable and proper use of the data.
This challenge of providing secure researcher
access to sensitive health data is well recognised
internationally and the last decade in particular has
seen significant State led initiatives to develop
structurally, legally and ethically robust systems to
exploit the explosion of opportunities, including via
Big Data, in this sphere of public health
administrative data. Examples include initiatives in
the UK, (Winter & Davidson, 2018), France
(Goldberg & Zins, 2021) and Germany, (Cuggia &
Combes, 2019). Ireland lags in the development of
the infrastructure and services required to deliver
such an environment. Hence it is relevant to evaluate
Czarnik, A., Darragh, A., Hurley, M., O’Connell, D., Quagliata, M. and Brennan, R.
Best Practice in Multi-organisation Sensitive Health Data Sharing: A Comparative Analysis of Ireland’s Data Governance Approach for the Covid–19 Data Research Hub.
DOI: 10.5220/0010802100003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 5: HEALTHINF, pages 57-68
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
57

the Irish Covid-19 Health Research Data Hub jointly
developed by the Central Statistics Office,
Department of Health and Heath Research Board
against international best practice. Even defining the
terms of this comparison is challenging, due to the
diversity of national health data sharing projects.
Important related developments in academic
research on models of Data Governance (Khatri &
Brown, 2010; Winter and Davidson., Abrahams et al,
2019) have emerged which reflect best practice in the
design, build and operation of any large scale data
management system. Taken together, information
from the foregoing practical and theoretical systems
can be used to benchmark the Covid–19 Data
Research Hub in terms of data governance and to
identify strengths, gaps and opportunities for future
such initiatives by the Irish public administration.
In this paper, we investigate the research question
“to what extent can international health data sharing
hubs and academic data governance frameworks be
used to evaluate data governance in the Covid–19
Data Research Hub?”. We use this question to
conduct an analysis of data governance best practice
in the sphere of public administrative health data
access, analysis and exploitation and we show how to
evaluate the Irish approach based on comparison with
international approaches and academic models.
The contribution of this paper is by drawing on the
learnings from these models, we propose a series of
areas for focus both in the future development of the
Covid–19 Data Research Hub and for other public
administrative health data hubs and we discuss the
applicability of academic data governance models to
these. Using the Covid–19 Data Research Hub as its
model, this case study aims to illustrate a method to
critically assess the state of the art in the collation and
dissemination for statistical purposes of public sector
health research data, specifically focusing on data
protection, data governance and access control. It will
examine the requirement for this approach; the aims
of the model; the involvement of inter-organisational
collaboration and the legal and governance structures
used in its construction. The key strengths and
weaknesses of the Covid–19 Data Research Hub are
be outlined and options for alternative approaches
will be identified.
The rest of this paper is structured as follows:
Section 2 discussed related work, section 3 discusses
our case study consisting of a description of the
Covid–19 Data Research Hub, our evaluation
methodology and the evaluation itself, section 4
discusses our findings and section provides our
conclusions.
2 RELATED WORK
Any attempt to examine the data analysis approach of
public administrations in responding to the Covid–19
pandemic requires an understanding of both academic
and deployed models addressing intra and inter-
organisational data governance and exploitation of
administrative and Big Data in the healthcare related
sphere (Tse et al, 2018). This section examines the
existing literature in large scale data governance
generally across multiple organisations, particularly
from the health data hub perspective, seeking out
existing data hubs of similar nature, considering the
impacts of Big Data on research, and finally, the
evaluating impact of the use of such data sets on the
organisation and exploitation of state data resources
looking forward.
Due to the emergent nature of the data governance
domain, research on data governance with a multi-
organisational perspective in the area of health data is
still very limited. There is a pattern of literature
reviews considering the issue of data governance in
relation with health data hubs, but few papers address
it directly. One paper (Nielsen, 2017) directly notes
that within data governance published between 2007
and 2017, there have been only 62 papers directly
fitting under the description of ‘data governance’ i.e.,
not confusing data governance with data
management. Within those papers, only 11% consider
e-health, and only 3% consider e-government. This
highlights a gap in literature, as academic surveys
show that persons are generally positive about sharing
their data for research purposes (Nielsen, 2017), with
their top priorities revolving around secure databases,
data stewardship, and anonymisation or
pseudonymisation, as well as re-consent. Addressing
these concerns requires strong data governance.
2.1 Healthcare Data and Data
Governance
Only recently has the potential of Big Data in
healthcare, particularly from a research perspective,
begun to be systematically explored and exploited. In
this regard, (Wang et al., 2018) identify five discrete
areas in which Big Data analytics can enhance
healthcare activities, these being analytical capability
for patterns of care, unstructured data analytical
capability, decision support capability, predictive
capability, and traceability. Literature also points to
the fact that there is increasing public and academic
perceptions of Big Data being of substantial value for
improving decision making processes, education,
healthcare, law, social media and artificial
HEALTHINF 2022 - 15th International Conference on Health Informatics
58

intelligence. Comparatively, there has not been as
much focus on the governance of said data though,
unless dealing with the risks of artificial intelligence
(Ethics Guidelines on Trustworthy AI, 2019).
Literature is also lacking in respect of data
governance of Big Data for research purposes,
particularly dealing with ‘sensitive’ data. It also often
conflates data management and data governance, or
in some cases calls for better techniques to handle
data, while omitting, or perhaps ignoring, data
governance. Yet this omission is problematic given
the related significance of the legal, digital trust, and
societal implications. Consideration is needed of data
governance by design, data interoperability, data
quality, data storage and operations, data security,
and data architecture.
2.2 National Data Hub Initiatives
Few national administrations have utilised health data
hubs within Healthcare (e-Estonia, 2021) and the area
remains emergent. The nature of health research is
such that in many instances the greatest value is to be
exploited where multiple data flows are combined to
bring new insights. As such, collaboration between
these entities requires complex inter-organisational
data governance (Lis & Otto, 2020).
Two interesting but different approaches are
explored from France and Germany by Cuggia and
Combes (2019), who examine the respective top-
down and bottom-up approaches to developing
publicly funded health data hubs in these countries. In
France, the Health Data Hub was designed to operate
on a hub and spoke model, with the central delivery
of sophisticated data sharing infrastructure supported
by highly expert staff in all relevant technical
domains, including IT, engineering, medicine, law
and governance. In this Top-Down model, projects
were then selected to be incorporated into the data
sharing infrastructure in two groups - one involving
only public or academic collaborators, the other
offering access also to industry partners. In Germany,
the Medical Informatics Initiative was developed
using a Bottom-Up methodology, reflecting the
federal structure of that country’s public
administration, with locally developed Data
Integration Centres and locally promoted use cases,
building on existing regional (Land) based e-health
strategies. The most successful of these use cases
were then selected to graduate to a subsequent phase
of work, during which the respective projects are to
be grown and networked. The German model focuses
on the importance of encouraging stakeholder
confidence in health data sharing, with strong
electronic consent declarations, trusted third party
technologies for identity management, clearly
defined data rules and access structures, an emphasis
on semantic interoperability, data sharing modalities
and audit criteria. The paper does not reach a
definitive conclusion as to which approach, Top
Down or Bottom Up is the most appropriate, but gives
a clear indication that in both respects the key criteria
include the drive for interoperability, data quality and
citizen involvement and trust dynamics.
Literature within the framework of public
administrative bodies collaborating to create data
hubs also shows great discontinuity though, as there
has been an increase of jointly created networks, and
data collections, in which public administrative
bodies collaborate to construct. However, the same
literature generally does not consider the
collaboration of administrative bodies for the
purposes of research-based datasets, and particularly
the impact that their collaboration may have in the
creation of them.
Estonia (McBride et al. , 2018) is widely
recognised as leader as regards the overall digitisation
of the delivery of government services and its
approach to digital state service delivery, including in
the healthcare sphere, although it does not
specifically inform the instant issue of public health
research responses in a time of pandemic. Therefore
while the technological design and data governance
protections inherent in its model were ground-
breaking and radical in the 1990s when their project
commenced, their application to the present problem
turns less on specific issues of access to health
research data and more on the Estonian State’s
approach to designing digital government on the basis
of a common national commitment to the use of Base
Registries for the collection, use and re-use of citizen
data; a very robust identity verification and
management infrastructure, underpinned by (Public
Key Infrastructure) PKI based authentication and
digital signatures and a transparent “service layer” via
which all Estonians can both access all State services
and view who in the State sector has accessed their
personal data, for what purpose with a full access
audit trail. This model offers possible indicators of a
route map to sustaining public trust in the use and re-
use of personal health information in Ireland, post
pandemic.
2.3 Data Governance Models
Managing the inter-organisational dynamics of data
governance in Big Data research is also a theme in
research by (Lis & Otto, 2020), who define the
Best Practice in Multi-organisation Sensitive Health Data Sharing: A Comparative Analysis of Ireland’s Data Governance Approach for the
Covid–19 Data Research Hub
59

characteristics of interorganisational data governance
around the themes of scope, purpose, goals, roles and
organisation, modes and governance and distinguish
between the more traditional intra-organisational data
governance tasks of assigning decision rights and
accountabilities and the more complex challenges of
inter-organisational data governance, which
frequently involves platform based technical
infrastructures.
An interesting model for approaching data
governance in the specific case of personal health
information is set out by (Winter and Davidson,
2019), who explore Helen Nissenbaum’s approach to
privacy (2009). She describes privacy not as a right to
secrecy or control but as an appropriate flow of
personal information within particular social
contexts. In the Winter and Davidson model (2018),
Data Governance in the area of Personal Health
Information (PHI) should be governed based around
five analytical dimensions – the data domain; the
stakeholders, the value or the application of the PHI,
the governance goal and the governance forum. This
paper explores the particular use case of the Royal
Free Trust and Alphabet’s DeepMind Health
initiative and highlights conflicts between the
partners in respect of key aspects in particular of the
governance goals, governance forms and the value
achieved through the initiative.
Khatri and Brown (2010) is considered the
foundational model of modern data governance and
iterates 5 key data decision domains: Data principles,
Data Quality, Meta Data, Data Access, and Data
Lifecycle. Winter and Davidson (2018) further
develop this model in their 2019 paper also
documenting 5 “dimensions” of governance for
Public Health Data, focusing in inter alia on the role
of Stakeholders (incorporating Direct, Indirect and
Public Health System related) and more specifically
calling out the Value or Application of the work,
generally encompassed Khatri and Brown’s Data
Principles (2013), while a composite synthesis of
research papers published by Abrahams et al. in
(2019) reviews 145 research and practitioner papers
in the sphere of data governance generally published
between 2001 and 2019. The latter define a pyramidal
governance structure, in which data, domain and
organisational scope are counterbalanced by
Governance Mechanisms, all framed by
organisational legal and technical “antecedents” pre
data ingestion and influenced by risk management
and performance related “consequences” post hoc.
Taken together, these three studies provide a
comprehensive governance framework via which to
evaluate research data hubs (see Table 1 below).
Based on the foregoing analysis, our study can
seek to fill gaps in the current literature, in particular
as regards connecting Big Data, the State sector,
personal freedoms, research ethics and data
governance. The lack of extensive published
information on multi-organisation health data hubs
suggests a gap where our comparative analysis could
add value. Additionally, the review uncovered that
while there are live medically oriented hubs
internationally which bear some similarities to the
Irish data hub, none of these systems could be said to
identically match the comprehensively centrally
driven model for Health Data Hub. While the German
Medical Informatics Initiative, through its focus on
clearly defined data rules and access structures,
semantic interoperability, data sharing modalities and
audit criteria appears to share the most similarities to
the Irish hub it still does not share the same function
as the Irish hub which is to ultimately provide a
statistically robust, secure and controlled
environment for the statistical analysis of relevant
data sources to inform the Government’s Covid–19
response.
3 CASE STUDY
This case study to critically assess the Covid–19 Data
Research Hub, compares it with similar
administrative data hubs in order to identify any key
strengths and weaknesses. In order to provide a
proper evaluation, given we could not rely solely on
a comparison of international prototypes we had to
look to models such as the five key decision domains
for effective data use proposed by Khatri and Brown
(2010), the conceptual framework proposed by
Winter and Davidson (2018) as well reaching out to
industry professionals who could provide us with
greater insight into the nuances of how the Covid–19
Data Research Hub was developed.
The steps which were necessary to achieve our
research objectives for this case study included the
following:
• Speaking with members of the public
administrative bodies involved in the Covid–
19 Data Research Hub so as to validate or
invalidate some of our own assumptions.
• Establishing the existence of clear data
governance structures specifically regarding
data access.
• Establishing whether international models
such as those outlined above were examined
during the course of the development of the
HEALTHINF 2022 - 15th International Conference on Health Informatics
60
Covid–19 Data Research Hub. Identifying
whether the Covid–19 Data Research Hub
may be able to incorporate features of models
abroad
• Identifying whether the Covid–19 Data
Research Hub diverges significantly from
international standards.
3.1 Covid–19 Data Research Hub
The development of the Covid–19 Data Research
Hub has been a novel undertaking in an Irish context,
precipitated by necessity. The Covid–19 Data
Research Hub is defined here as a technical
architecture which enables secure health data sharing
between Irish public administrative bodies and
approved users in a format that is controlled,
accessible and usable. The infrastructure and
underpinning governance approach were modelled on
best international practice, with a particular emphasis
on data confidentiality and strong governance. It
represents a federated governance and data sharing
initiative as following a decision by the Minister for
Health to authorise it, the Central Statistics Office
was given legal authority to process special category
health data under the control of the Department of
Health and the HSE. This was to facilitate secure,
reliable data access to approved researchers and
thereby to facilitate Covid–19 related analysis.
Looking beyond the current emergency period,
population-level data similar to that stored in the
Covid–19 Data Research Hub may also be a valuable
tool, for example, for designing medical management
algorithms and guidelines (Sharma, Borah and
Moses, 2021).
In response to the Covid–19 pandemic the CSO
began receiving research and analysis relevant data
flows from the HSE (Health Service Executive) and
other public bodies. Consequently, the Covid–19
Data Research Hub was created to make Covid–19
relevant datasets compiled by the CSO from diverse
administrative data sources securely available to
researchers via the CSO Researcher Microdata Files
(RMF) process under Section 20(c) of The Statistics
Act, 1993. The use of a RMF process was designed to
implement the possibility for statistical analysis in a
manner that protects the confidentiality of the data
and ensures that such data is only made available for
use for statistical purposes and to a restricted number
of specifically approved researchers. It was
developed after extensive consultation between the
CSO, the Health Research Board (HRB), the
Department of Health (DoH) and the HSE.
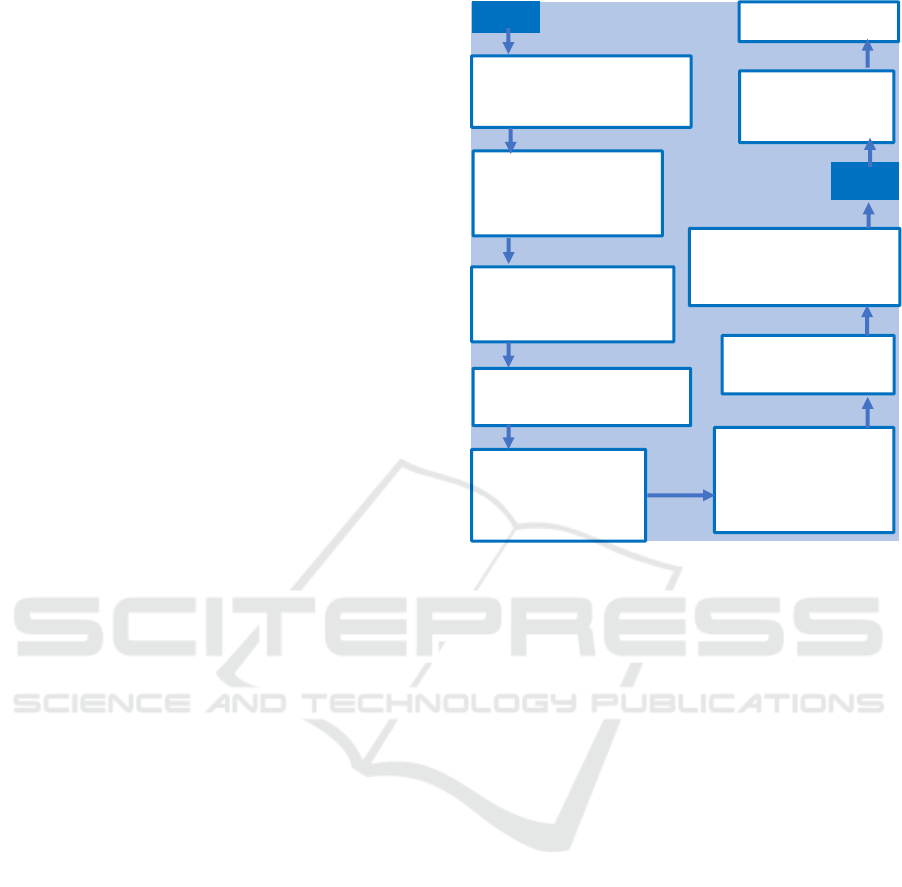
Figure 1: HSE to CSO Health data flows.
From a technical perspective, the process which
transforms data received by the CSO to data available
to the researchers is shown in Figure 1.
HSE and DoH data is transferred by Secure File
Transfer Protocol (SFTP) to a CSO remote server
with the use of encryption and secure transmission
mechanisms from the HSE. Each data flow is dealt
with individually and is stored safely in its original
format in what is called the “Migration Tier” of the
Administrative Data Centre (ADC) of the CSO.
Access to such raw data in the Migration Tier is
confined to a small number of ADC staff for
processing purposes only.
Each entire dataset is then converted from its
original format to a format compatible with the
Statistical Analysis System (SAS) and stored in what
is called the “Source Tier” of the ADC. SAS is the
primary statistical software used by the CSO to
analyse data. Access to such second Tier is limited to
ADC staff for processing and a limited number of
CSO staff with fully documented and approved
reasons which justifies the use of such confidential
data for limited internal or analysis purposes.
Afterwards, a pseudonymised version of each data
flow is also created in SAS and stored in what is
called the Analysis Tier. All access requests for
analysis purposes are with respect to pseudonymised
data only, therefore to this third Tier.
HSE
a21_src: HSE Coronavirus
Assessments, Test Referrals
and Facilities data
C19HospitalCases_src:
Recorded Hospital
Cases as a result of
Covid
–
19
CIDR_src: HSE
Computerised Infectious
Disease Reporting System
HIPE_src: Hospital
Inpatient Discharge Data
NOCA_src: National
Office of Clinical
Audit Intensive Care
Unit Data
SBAR_src: Situation,
Background,
Assessment,
Recommendation,
Shift
handover
data
CCT_src: Covid Care
Tracker Data
Vaccination Info Data:
Record of vaccinations
administered for Covid–19
CSO
Production of
pseudonymised
RMF files
Approval process
Best Practice in Multi-organisation Sensitive Health Data Sharing: A Comparative Analysis of Ireland’s Data Governance Approach for the
Covid–19 Data Research Hub
61
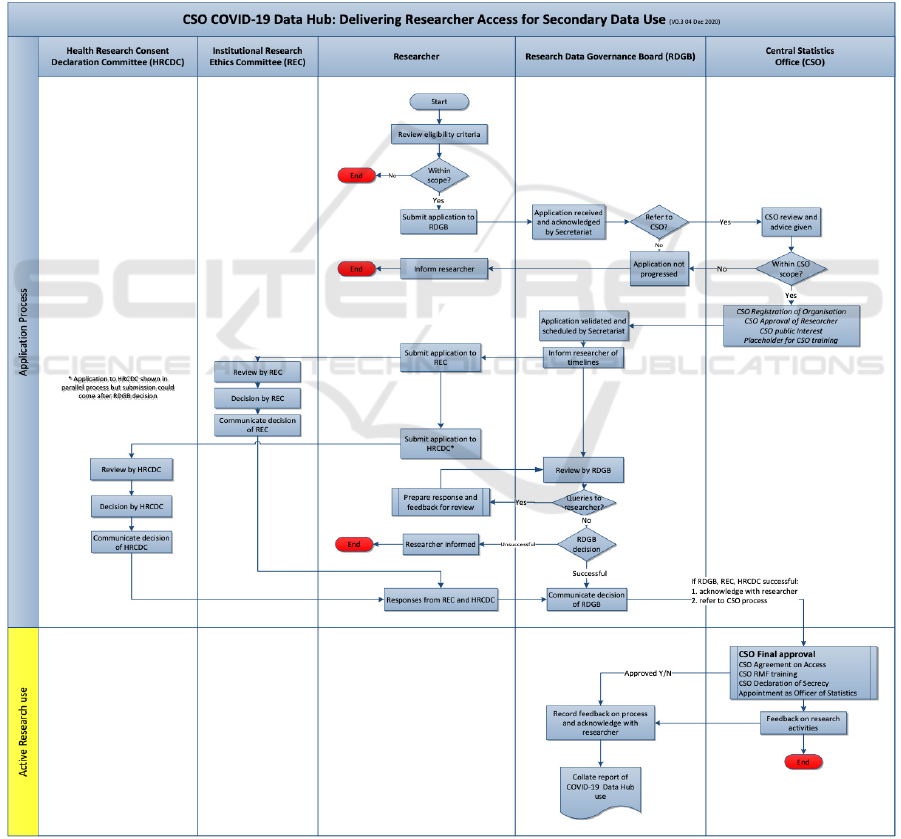
All data flows and datasets involved in the
Migration, Source and Analysis Tiers of ADC are
registered on the internal ADC Data Portal. This
online portal, which uses the CSO intranet, includes a
register of all available data stored, including
metadata and a list of registered users for each data
flow.
Once the data has undergone all the above-
explained processing and is stored in a
pseudonymised form in the Analysis Tier, researchers
may access the RDP via a Citrix connection using
unique credentials. The microdata, at all times,
remains on a CSO server as the RDP is a secure,
locked-down environment from which no data can be
extracted without permission. There is also no
internet/email access and nothing can be copied to the
local PC.
When a researcher has completed work on a file
that they wish to have exported as an output, they may
contact the data custodian in the CSO. Only such
nominated custodians have permissions set to allow
access to the researchers’ inputs and outputs folders
after checking for compliance with statistical
disclosure control.
As declared in the relevant DPIAs by the CSO, the
data will then be retained for as long as necessary to
respond to the pandemic.
Figure 2: Data Access Process Map.
HEALTHINF 2022 - 15th International Conference on Health Informatics
62

3.2 Methodology
The Irish model will be evaluated by comparison with
similar administrative data hubs in operation. The
background literature was highly informative in the
describing international comparator data hubs and
four were selected: Health Data Hub (HDH) in France
and the German Medical Informatics Initiative (MII),
the UK’s partnership between the Royal Free Trust
and Alphabet’s DeepMind Health (DMH) AI led
medical data collaboration and the design and
delivery of Estonia’s Digital Government model.
This was complemented by access to the
underpinning DPIA (Data Protection Impact
Assessment) documentation for the Irish Data
Research Hub, which provided a detailed insight into
the design and execution of that model and its
associated governance. This was complimented by an
interview with a key stakeholder (discussed below).
Each of these data hubs have been evaluated in
accordance with their adherence to the data
governance principles and domains laid out in Khatri
and Brown and refined by the 2019 review by
Abrahams et al. This gives a common basis rooted in
best practice to evaluate the current solutions and the
Irish Covid-19 Data Research Hub.
The opportunity of access to a key stakeholder in
the Irish Data Research Hub permitted a more
detailed and nuanced examination of the dynamics
and structures underpinning this initiative. A senior
manager with responsibility for Statistical System
Co-Ordination in the CSO, was interviewed using the
following as a discussion guide. The interview was
semi-structured, intended to provide reliable and
comparable data. Open-ended questions were used to
obtain answers which were not focused on what the
interviewee feels should be utilized within their
organisation, but what is. The interview lasted for an
hour and was transcribed verbatim.
The topics discussed were as follows:
1. Description of objectives of the development of the
Covid–19 Data Research Hub and the interviewee’s
role.
2. Options for project design considered by the
interviewee.
3. Whether or not international exemplars were
examined by the interviewee, and whether any
conclusions were reached if affirmatively answered.
4. The key factors which influenced the final
approach and model for the finalised approach to the
Covid–19 Data Research Hub.
5. Based on international comparators or learnings
since the Covid–19 Data Research Hub has gone live,
the assessment the interviewee would give of the
relative strengths/weaknesses in each model and in
the final Irish model
6. A description of any roadblocks or inhibitors which
forced compromises in design and delivery choices
which were ultimately taken.
7. The steps the interviewee would address in respect
of the aforementioned roadblocks for future
initiatives or learnings which would influence
alternative decision making.
8. General remarks the interviewee would wish to add
in regard to the mechanisms available in Ireland to
leverage administrative data in support of public
policy development.
Together the interview and data governance
evaluation enabled a structured, comparative analysis
of the Covid-19 Data Hub in terms of international
best practice and theoretical soundness.
3.3 Evaluation
The findings of the evaluation are synthesised in
Table 1 which provides a column for each data hub
assessed and a row for each data governance
dimension following to Khatri and Brown. First we
examine the Irish Covid -19 Data Research Hub in
isolation according to the academic principles of data
governance and then a comparative analysis is
provided with respect to the international models
examined.
The academic Data Governance models evaluated
indicate key strengths in the Irish model (see Table
1), in particular in the data governance areas of Data
Access and Data Principles, however the real value
and application of public health information depends
on the engagement, trust and sustained cooperation of
all stakeholders and there appear to be vulnerabilities
here, especially as regard metadata standards, data
lifecycle management and individual level data
transparency.
The main objective of the Covid–19 Data
Research Hub is to inform decision-making during
the national emergency based on research undertaken
by approved individuals. Pseudonymisation of the
data held on the system protects the privacy of
individuals and international comparison indicates
this to be a standard. However, weak or absent meta
data standardisation is a vulnerability from a Data
Quality and Access perspective and in a longer-term
perspective, in particular for more ordinary-time
purposes, may hinder the value of the data from a
researcher’s perspective.
Governance goals illustrate the objectives
targeted by implementing a governance method. By
robustly governing the data contained in the Covid–
Best Practice in Multi-organisation Sensitive Health Data Sharing: A Comparative Analysis of Ireland’s Data Governance Approach for the
Covid–19 Data Research Hub
63

19 Data Research Hub it is hoped to provide a secure
source for researchers to access pseudonymised data
relating to the Covid–19 pandemic and its effects and,
by extension, to demonstrate the opportunities for
further public sector policy to be informed by parallel
type research.
Governance forms indicate externalities that
impact on achieving the goals set out. These include
organisational units, practices, policies and
regulations and technologies involved in the
management of the data. The CSO ensured that all
necessary protocols under the Statistics Act, the Data
Protection Act/Health Research Regulations were
employed in the collaborative process. The
establishment of the Research Data Governance
Board (RDGB) acts as an added safeguard in
supporting governance and transparency of the
application process for approved researcher status.
Overall, from the perspective of formal or
academic governance, the Irish model presents
opportunities for improvement, on a solid and
verifiable governance base.
From the perspective of practical implementation
of other data hub models, Estonia (see Table 1) has
stolen a march in the digitisation of their public
administrative systems generally. Designed for
broader purposes than the Irish Covid–19 Data
Research Hub, their system allows residents to access
personal health data amongst a range of all the data
they share with the public administrative system,
sharing this data with doctors and healthcare
professionals whilst having full visibility of its use.
This creates ease of engagement for both parties and
removes the need for manual file transfer as it can be
carried out online. Regarding the Irish data hub, this
system operates in a more detached manner, ingesting
information specifically related to instances of
Covid–19, processing it for governance and onward
access purposes, with no option for dynamic sharing
of datasets. Only approved researchers will have
access to the data hub, following an extensive process
involving the HRB, the CSO and the RDGB.
The French Health Data Hub operates on the
principle of encouraging research, much like the Irish
data hub. A key feature of the French HDH is
Artificial Intelligence (AI), which is not yet included
in any aspect of the Irish Covid–19 data hub, although
there are clear opportunities for the deployment of
Machine Learning techniques. The HDH aims to
expand the area of digital health by including multiple
parties in the data sharing system. Similarly, the Irish
Covid–19 Data Research Hub developed with
involvement from a number of public administrative
bodies, collaborating to ensure all bases were covered
regarding the data transfer by the HSE to the CSO,
the application process managed by the HRB and the
system for approvals
Germany has developed a Medical Informatics
Initiative focusing on the promotion of training and
educating among selected healthcare actors but relies
heavily on local cooperation and is as yet unproven at
a national level. The bottom-up nature of its operation
offers assurance at a governance level whilst risking
constraints in terms of broader utility, across its
audience of data scientists, data stewards, doctors,
patients, research and universities. It is hoped that this
data hub will provide insights into medical research
and improve treatment decisions. Access to the Irish
Covid–19 Data Research Hub is limited to approved
researchers, as mentioned above, in a bid to inform
public bodies of evidence to support policy decisions.
3.3.1 Data Hub Stakeholder Interview
Summary
The interviewee noted the objectives were to
encourage research on Covid–19 across a broad group
of researchers, consistent with the Statistics Act 1993
and health research obligations. He noted that a safe
haven for research has been a necessity, which
complied with all law and recognized the status of
health data as ‘special category data’.
Regarding the “state of the art” in data hub design
and execution, it was noted that the Health Research
Board (HRB) is internationally connected and well
aware of international trends in areas relating to
process maps, research ethics, and public interest,
while the CSO is a globally active National Statistical
Institute, operating to transnational standards.
Accordingly, Irish Health Research Regulations and
CSO Statistical Data Governance Standards are
considered state of the art and heavily influenced the
governance model followed by the CSO and the HRB
in developing the data hub.
The interviewee’s principal governance and risk
factors in the Data Research Hub design & architecture
included ethics, consent, public interest, legislation,
metadata and data availability, lack of persistent
identifiers, and consideration of international trends in
data hub creation and management. The interviewee
expressed the view that the data hub was strong in the
area of research ethics and consent, with both heavily
reflected in the model, while noting that the absence of
advance data subject consent could be discounted to
some extent by the public interest imperative of Covid–
19 related responses. Nonetheless, approval to access
such data is given from an independent source, Health
Research Consent Declaration Committee.
HEALTHINF 2022 - 15th International Conference on Health Informatics
64
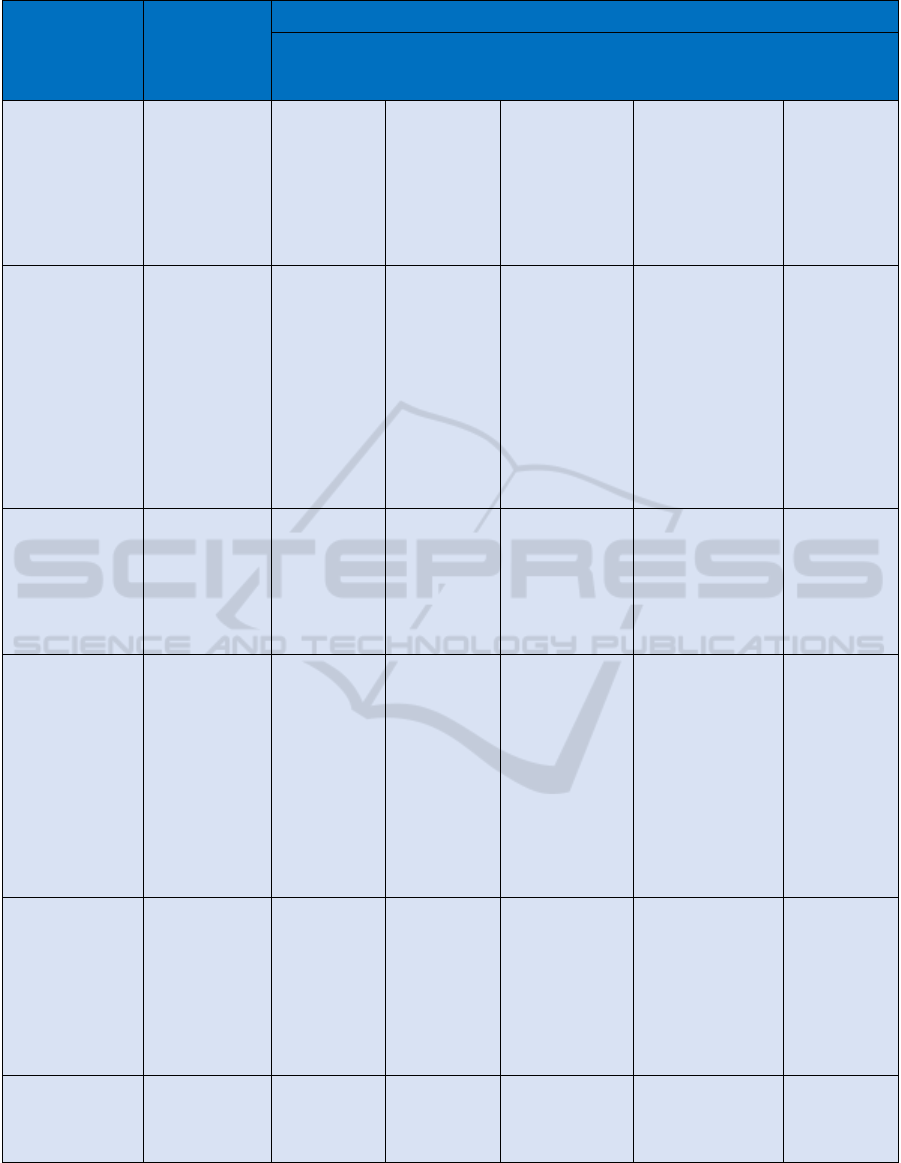
Table 1: Data Hub Evaluation based on Data Governance Domains and Antecedents.
Data Governance Data Hubs
Decision Domain Scope French HDH German MII
Covid–19
Research Data
Hub
DeepMind Health
(UK)
Estonia
Antecedents External (legal,
regulatory,
market
envt…)/Internal
(Business
Strategy; IT
architecture;
Culture envt)
Central rule
setting. Strict
national
regulatory
framework,
Hub and
Spoke
architecture
Bottom-Up
approach
Urgent national
response
Local (Health Trust
to private
contractor)
contracting
arrangement with
weekly specified
contracting
parameters
Clean Sheet –
common
baseline –
Register
based
approach
Data Principles Acceptable
uses? Desirable
behaviours?
Use & re-use
protocols?
Regulatory
Engagement
18 projects
sanctioned,
subject to
independent
oversight.
Complex
access/match
ing processes
slowing
progress.
Moving to
harmonise
Strong
commitment
to
interoperabili
ty and data
sharing
Tightly defined,
purpose
dependent
access. no open
sharing
protocols.
rigorous output
checking. close
DPC
engagement
Weakly defined.
Large data dumps
with poorly
specified outputs.
Data principles
severely criticised
according to an
Independent
Review Panel in
2017
Collect once,
use often is
the guiding
principle with
strict national
governance re
access and
use.
Maximum
transparency
Data Quality/
Domain Scope
Accuracy,
Timeliness,
Completeness,
Credibility
No evidence
of validation
No evidence
of validation
Acknowledge
unvalidated
No evidence of
validation
100%
transparent,
data viewable
by data
subject,
editable/verifi
able
Metadata/
Domain Scope
Semantic
Dictionary
Metadata
Maintenance
Implied
strong
governance,
given Hub &
Spoke Design
Due to
Bottom-Up
design,
presumed
lagging if
present at all.
Poorly
documented
in research
papers
Confirmed as a
gap. Purpose
specific
approach to
individual data
flows. urgently
requires
attention
No evidence. AI
data
mining/processing
techniques
deployed on a
“black box” basis
Register
based,
legislatively
driven
approach
ensures
commonality
and consistent
with external
parties (banks
for eID
infrastructure)
Data Access/
Governance
Form/Governance
Mechanism
Access Risk
Assessment
Access
Protocols
Access
Logging/Control
Compliance &
Security
Varied,
complex and
diverse rules
Stringent
governance,
centrally
overseen
Stringent data
provenance,
sharing,
processing and
access controls;
Ethical, Research
and governance
sign off required
No central
oversight but
confirmed patient
opt-out
Strict
legislatively
defined
governance
Data Lifecycle/
Domain Scope
Data definition,
production,
retention,
retirement
Unspecified
in literature
Unspecified in
literature
Unspecified in
literature
Unspecified in
literature
Unspecified in
literature
Best Practice in Multi-organisation Sensitive Health Data Sharing: A Comparative Analysis of Ireland’s Data Governance Approach for the
Covid–19 Data Research Hub
65

He further noted the architecture of the Data Research
Hub establishes a foundation for potential future
expansion to the education and labour market, for
which there is demand for research purposes.
The interviewee identified that a lack of metadata
for data sets is an issue, as the CSO receives data from
the HSE directly from diverse systems, each of which
was designed independently, for diverse purposes.
Researchers rely on data sets that display consistency,
are complete and ready for research purposes.
Bridging this gap is a considerable challenge. In
particular, data access, metadata availability, and the
lack of a persistent identifier created issues. It was
also identified that reluctance to embrace standard
identifiers across the public sector is problematic. In
noting that the resistance existed even prior to GDPR
and the DPC, he also noted that ‘bravery’ is now
necessary, to galvanise the effort to mobilise
administrative data for public policy development.
As a general remark, it was noted that investment
in data which is based on sensors and IoT must be
considered. However, this area raises the challenge of
the sheer size of the data flows implying a need to
engage with partners, including potential outsourced
providers, which may imply cloud solutions for data
that is not sensitive. This would represent a
considerable departure for the public sector.
3.4 Discussion
From the above, we can draw a number of
conclusions: the stakeholder confirmed the growing
trend in Europe to make data available for research
purposes has reached Ireland, but noted the
difficulties that come alongside this in respect of
legislation that limits the use of health data for
research purposes. While these difficulties have been
discussed within scholarship and in papers outlining
other European health hub systems, (Winter and
Davidson , 2018) the author made it clear that these
were not considered for direct implementation.
Nonetheless, international trends were observed, as
noted in topic 2. Some of the international trends
observed, such as metadata (which also implies data
quality), data availability, legislation and consent
have been parts of data governance state-of-the-art
scholarship (K. C. O’Doherty et al, 2021), (Prainsack
& Buyx, 2013) (McMahon, Buyx & Prainsack, 2020),
(Cuggia & Combes, 2019). Despite the fact the
interviewee has not mentioned data governance
specifically as an influencing factor, this does not
imply that it cannot exist de facto. It should be further
noted that while data governance has been confirmed
to be an influencing success factor in prior
scholarship, (Panian, 2010) it is nonetheless not in the
mainstream yet. This is further exacerbated by the
fact that scholarship is only recently treading the
waters of data governance in international health
hubs. The interviewee, in topic 5, discussed the lack
of explicit consent for data as research assets.
Nonetheless, the interviewee interestingly mentions
the overriding public interest. Article 6(e) of the
GDPR does allow processing for the purposes of
performance of a task carried out in the public interest
(GDPR, 2018). The German Data Protection
Commission has recently approved a set of (updated)
forms used to ensure a provision for patient data for
medical research purposes (Virtuelles
Datenschutzbüro, 2021). They will be approved for
use by the Medical Informatics Initiative, which is
essentially, a data hub much like the Covid–19 Data
Research Hub developed by the HSE and CSO, with
the two diverging factors being that the German data
hub encompasses all medical data, as opposed to
Covid–19 related data, (MII Germany, 2021) and the
‘bottom-up’ approach taken by Germany, wherein a
consortia of hospitals, universities, and private
partners exists (Cuggia & Combes, 2019). The French
Health Data Hub, known as the ‘Plateforme nationale
des données de santé’, or HDH, is more similar to the
Irish hub, with the objective of promoting research.
Much like the Irish system, the French system was
also tested via pilot projects (“Plateforme des données
de santé, Direction de la recherche, des études, de
l’évaluation et des statistiques”, 2021). Furthermore,
the Irish system also features the employment of data
producers, as a joint venture by the HSE and CSO.
While the full comparisons between the data
governance of the French, Estonian, German, and
Irish data hubs would be extensive, our initial
research has nonetheless shown that the hubs differ
greatly. International comparisons do not play a role
in de facto application of development of health data
hubs, and this is mostly arising out of the factors
which necessitate the hub in the first place.
Conclusively, there seems to be general international
practice that simply occurs on the basis of best
practice reasoning. While international hubs were not
considered in respect of applicable features,
nonetheless, there is general international practice
used that can be found across all hubs.
4 CONCLUSION
While international Health Data Hubs exist or are in
development, they diverge as much as they intersect
as regards purpose, governance, and implementation.
HEALTHINF 2022 - 15th International Conference on Health Informatics
66

Key areas of data governance development focus
should be made a priority, in particular in order to
preserve public confidence and to support future
interoperability and long-term utility from data
sources. In particular, attention should be paid to
consent and metadata management and to data subject
transparency.
The Covid–19 Data Research Hub is
distinguished in particular by the fact that it focuses
exclusively on public sector data being made
available to academic researchers for emergency
response purposes. International standard ethics
approval is required for research projects, consistent
with comparator models in the UK, France and
Germany. Due to the retrofitting of the data access
model to diverse available sources, preliminary
consent has had to be dispensed with, although a
robust retrospective process for consent management
is in place. Rigorous researcher access protocols are
applied, and the purpose of the research is firmly
focused on public good outcomes, thus in this respect
it appears to offer a particularly high level of
assurance to data subjects individually and
collectively.
All evidence suggests the CSO’s ingestion,
collation and preparation of data for research access,
via Research Micro-Data File access, complies with
rigorous data governance standards, protecting the
privacy of data subjects and limiting access strictly to
that which is necessary. There are no “black box”
processes and Data Subjects can access full
transparency details in respect of the processing
principles applied to their data. Outputs are rigorously
checked for Statistical Disclosure. No cloud
technology is used, and data is securely held on
premise at all times.
While transparency is well documented in
general, however, the Data Subject enjoys very
limited transparency at the individual level. This
aspect cannot easily be retrofitted to a system
developed reactively and drawing on disparate
sources, not designed for this purpose. This stands in
stark contrast, for example, to the Estonian Digital
Government model where a discrete Service Layer
(Winter and Davidson (2019) ensures Data Subjects
have real time visibility on the use of their data and
the X-Road based Data Registers model ensures that
any given variable has a single consistent, auditable
source. In order to preserve public confidence,
progress in this area is desirable.
At the statistical level, the absence of strong
semantic compatibility and inter-operability/meta-
data standardisation hampers data processing, making
the role of the CSO particularly challenging. Unique
identifiers would assist considerably, as would
common meta-data standards.
This research did not reveal ideal international
comparators against which to benchmark the Covid–
19 Data Research Hub, but general learnings were
nonetheless instructive in particular as regards
general pitfalls for large scale data sharing and
analysis. The lessons learned from Estonia offer a
particularly illuminating view of the possibility for
the safe, trusted and transparent use of public
administrative data “as a public asset” and these
should be studied in particular detail in the
perspective of future investment in Irish public sector
research capability. Benchmarking against academic
data governance models reveals key weaknesses, in
particular in respect of meta data and data lifecycle
management, while issues of Data Quality validations
are also ripe for further examination.
ACKNOWLEDGMENTS
We would like to express a special thanks to Mr Paul
Morrin of the Central Statistics Office for his
assistance as part of this project. This research has
received funding from the ADAPT Centre for Digital
Content Technology, funded under the SFI Research
Centres Programme (Grant 13/RC/2106\_P2), co-
funded by the European Regional Development Fund.
For the purpose of Open Access, the author has
applied a CC BY public copyright licence to any
Author Accepted Manuscript version arising from
this submission.
REFERENCES
“Art. 6 GDPR – Lawfulness of processing,” General Data
Protection Regulation (GDPR). https://gdpr-
info.eu/art-6-gdpr/ (accessed Apr. 16, 2021).
“Art. 9 GDPR – Processing of special categories of personal
data” General Data Protection Regulation (GDPR)
https://gdpr.eu/article-9-processing-special-categories-
of-personal-data-prohibited/
Abraham R., Brockeand J. V. & Schneider J., (2019) “Data
Governance: A Conceptual Framework, Structured
Review and Research Agenda” International Journal of
Information Management vol 49 pp424-438, Dec. 2019
doi: https://doi.org/10.1016/j.ijinfomgt.2019.07.008
Cuggia M. & Combes S., ‘The French Data Hub and The
German Medical Informatics Initiatives: Two National
Projects to promote Data Sharing in Health Care’,
Yearb of Med Inform, vol 28, pp 195-202, Aug. 2019
DOI: 10.1055/s-0039-1677917.
Best Practice in Multi-organisation Sensitive Health Data Sharing: A Comparative Analysis of Ireland’s Data Governance Approach for the
Covid–19 Data Research Hub
67

DAMA International (2017). DAMA-DMBOK: Data
Management Body of Knowledge, Second Edition.
Bradley Beach, N.J.: Technics Publications, 2017.
De Prieëlle F., De Reuver M. & Rezaei J., (2020) “The
Role of Ecosystem Data Governance in Adoption of
Data Platforms by Internet-of-Things Data Providers:
Case of Dutch Horticulture Industry,” IEEE
Transactions on Engineering Management, vol 1, pp.
1–11, Jan. 2020, doi: https://doi.org/10.1109/
TEM.2020.2966024
e-Estonia.com ‘E-Health Records’, <https://e-estonia.com/
solutions/healthcare/e-health-record/> (accessed 13
March 2021); ‘Page d’accueil’, Health Data Hub.fr,
<https://www.health-data-hub.fr/> (accessed 13 March
2021); ‘Digital Medicine - BIH’, Berlin Institute of
Health.org, <https://www.bihealth.org/en/research/
translation-hubs/digital-medicine> (accessed 13 March
2021); ‘Our Hubs’, HDR UK.ac.uk <
https://www.hdruk.ac.uk/helping-with-health-data/our-
hubs-across-the-uk/> (accessed 13 March 2021.)
Ethics Guidelines for Trustworthy AI, High-Level Expert
Group on AI. This followed he publication of the
guidelines’ first draft in Dec 2018. https://digital-
strategy.ec.europa.eu/en/library/ethics-guidelines-
trustworthy-ai
Golberg M. & Zins M., (2021) Le Health Data Hub (suite),
Med Sci (Paris) vol. 37, no. 3, pp. 271-276, Mar. 2021,
doi: https://doi.org/10.1051/medsci/2021016.
Hripcsak G. et al, (2014) “Health data use, stewardship, and
governance: ongoing gaps and challenges: a report from
AMIA’s 2012 Health Policy Meeting.” Journal of the
American Medical Informatics Association, vol.21,
pp.204-211, Mar. 2014, doi: https://dx.doi.org/10.11
36%2Famiajnl-2013-002117
Khatri V. & Brown C. V., (2010) ‘Designing Data
Governance’ Communications of the ACM vol 53, pp
148 - 152, Jan. 2010
Lis D. & Otto B., (2020) "Data Governance in Data
Ecosystems – Insights from Organizations" presented at
Americas Conference on Information Systems, 2020,
pp. 1-10, doi: https://www.researchgate.net/
publication/343215188_Data_Governance_in_Data_E
cosystems_-_Insights_from_Organizations
Margetts H. & Naumann A., 'Government as a platform:
What can Estonia show the world?’ Oxford Politics
Research Paper, 2017 <https://www.politics.ox.ac.uk/
publications/government-as-a-platform-what-can-
estonia-show-the-world.html
McBride K., Toots M., Kalvet T. & Krimmer R., (2018)
‘Leader in e-Government, Laggard in Open Data:
Exploring the Case of Estonia’, Reuve francaise
d’administration publique vol. 167 no 3 pp 613-625
https://www.cairn.info/revue-francaise-d-
administration-publique-2018-3-page-613.htm
McMahon A., Buyx A., & Prainsack B., (2020) “Big Data
Governance Needs More Collective Responsibility:
The Role of Harm Mitigation in the Governance of Data
Use in Medicine and Beyond,” Med. Law Rev., vol. 28,
no. 1, pp. 155–182, Feb. 2020, doi: 10.1093/
medlaw/fwz016
MII Germany, “About the initiative | Medical Informatics
Initiative.” https://www.medizininformatik-initiative.
de/en/about-initiative (accessed Apr. 16, 2021).
Nielsen, O.B., (2017) A Comprehensive Review of the Data
Governance Literature, Selected Papers of the IRIS no
8 https://core.ac.uk/download/pdf/301373908.pdf
Nissenbaum H., (2009) ‘Privacy in Context: Technology,
Policy and the Integrity of Social Life’, Stanford, CA,
USA Stanford University Press, 2009
O’Doherty K. C. et al (2021) “Toward better governance of
human genomic data,” Nat. Genet., vol. 53, no. 1, Art.
no. 1, Jan. 2021, doi: 10.1038/s41588-020-00742-6.
Panian Z., (2010) Some Practical Experiences in Data
Governance in World Academy of Science, Engineering
and Technology, doi: https://citeseerx.ist.psu.edu/
viewdoc/download?doi=10.1.1.190.6948&rep=rep1&t
ype=pdf
Prainsack B. & Buyx A., (2013) “A Solidarity-Based
Approach to the Governance of Research Biobanks,”
Med. Law Rev., vol. 21, no. 1, pp. 71–91, Mar. 2013,
doi: 10.1093/medlaw/fws040.
République Française, “Plateforme des données de santé |
Direction de la recherche, des études, de l’évaluation et
des statistiques.” https://drees.solidarites-sante.gouv.fr/
article/plateforme-des-donnees-de-sante (accessed
Apr. 16, 2021).
Sharma A., Borah S. B. & Moses A. C., (2021) ‘Responses
to Covid–19: The Role of Governance, Healthcare
Infrastructure, and Learning from Past Pandemics’
Journal of Business Research vol.122, pp. 597-607, Jan
2021, doi: https://doi.org/10.1016/j.jbusres.2020.09.0
11.
Tse D., Chow C., Ly T., Tong C. & Tam K., (2018) ‘The
Challenges of Big Data Governance in Healthcare’, in
17th IEEE International Conference On Trust, Security
And Privacy In Computing And Communications/ 12th
IEEE International Conference On Big Data Science
And Engineering, 2018, pp.1632-1636, doi:
https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnu
mber=8456108.
Virtuelles Datenschutzbüro, “Datenschutzbehörden des
Bundes und der Länder akzeptieren die Einwilligungs-
dokumente der Medizininformatik-Initiative.”
https://www.datenschutz.de/datenschutzbehoerden-
des-bundes-und-der-laender-akzeptieren-die-
einwilligungsdokumente-der-medizininformatik-
initiative/ (accessed Apr. 16, 2021).
Wang Y., Kung L. & Byrd T., (2018) ‘Big Data Analytics
– Understanding Capabilities and Potential Benefits for
Healthcare Organisations’, Technology Forecasting
and Social Change vol 126, pp. 3-13, Jan. 2018 , doi:
https://doi.org/10.1016/j.techfore.2015.12.019
Winter J. S. & Davidson E., (2018) ‘Big Data Governance
of Personal Health Information and Challenges to
Contextual Integrity’ The Information Society vol 35,
pp 36 - 51, Dec. 2018, doi: https://doi.org/
10.1080/01972243.2018.1542648
HEALTHINF 2022 - 15th International Conference on Health Informatics
68
