
Incorporation of VOC-Selective Peptides in Gas Sensing Materials
Ana Rita Oliveira
1,2 a
, Efthymia Ramou
1,2 b
, Gonçalo D. G. Teixeira
1,2 c
,
Susana I. C. J. Palma
1,2 d
and Ana C. A. Roque
1,2* e
1
Associate Laboratory i4HB- Institute for Health and Bioeconomy, School of Science and Technology,
NOVA University Lisbon, 2829-516 Caparica, Portugal
2
UCIBIO – Applied Molecular Biosciences Unit, Department of Chemistry, School of Science and Technology,
NOVA University Lisbon, 2829-516 Caparica, Portugal
Keywords: Ionogels, Hybrid Gels, Peptides, Gas Sensing, Electronic Nose.
Abstract: Enhancing the selectivity of gas sensing materials towards specific volatile organic compounds (VOCs) is
challenging due to the chemical simplicity of VOCs as well as the difficulty in interfacing VOC selective
biological elements with electronic components used in the transduction process. We aimed to tune the
selectivity of gas sensing materials through the incorporation of VOC-selective peptides into gel-like gas
sensing materials. Specifically, a peptide (P1) known to discriminate single carbon deviations among benzene
and derivatives, along with two modified versions (P2 and P3), were integrated with gel compositions
containing gelatin, ionic liquid and without or with a liquid crystal component (ionogels and hybrid gels
respectively). These formulations change their electrical or optical properties upon VOC exposure, and were
tested as sensors in an in-house developed e-nose. Their ability to distinct and identify VOCs was evaluated
via a supervised machine learning classifier. Enhanced discrimination of benzene and hexane was detected
for the P1-based hybrid gel. Additionally, complementarity of the electrical and optical sensors was observed
considering that a combination of both their accuracy predictions yielded the best classification results for the
tested VOCs. This indicates that a combinatorial array in a dual-mode e-nose could provide optimal
performance and enhanced selectivity.
1 INTRODUCTION
Gas sensing is currently emerging as a critically
important technology related to a broad range of
applications such as medicine (van Hooren et al.,
2016), and early diagnosis of disease (Broza et al.,
2015; Cruz et al., 2017; Fitzgerald & Fenniri, 2017;
Krilaviciute et al., 2015; Susana I.C.J. Palma et al.,
2018; Vishinkin & Haick, 2015). Indeed, volatile
organic compounds (VOCs) are becoming
increasingly recognized as potential biomarkers
associated with disease. Artificial olfaction is the
automated simulation of the sense of smell through
the use of electronic nose devices (e-noses),
comprised by an array of chemical sensors with
a
https://orcid.org/0000-0001-8496-1746
b
https://orcid.org/0000-0003-2376-6749
c
https://orcid.org/0000-0001-7675-5926
d
https://orcid.org/0000-0002-1851-8110
e
https://orcid.org/0000-0002-4586-3024
partial selectivity coupled with signal-processing and
pattern recognition tools. The most common and
commercially available gas sensing materials include
metal oxide semiconductors (Dey, 2018) or
conducting polymers (Park et al., 2017). However,
their main drawbacks include low long-term sensor
stability, high maintenance, cumbersome and
complex instrumentation, and most importantly low
selectivity. Thus, it is of high interest to develop
better and competitive alternatives, by adapting the
components of the sensors in order to enhance their
selectivity, reliability and portability (Son et al.,
2017). In biological olfaction, odorant binding
proteins and olfactory receptors are the main tools
used to address the difficult problem of selectivity. In
artificial olfaction, these find limitations as they are
Oliveira, A., Ramou, E., Teixeira, G., Palma, S. and Roque, A.
Incorporation of VOC-Selective Peptides in Gas Sensing Materials.
DOI: 10.5220/0010797200003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 1: BIODEVICES, pages 25-34
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
25

difficult to produce, as well as to stabilise and
interface with electronic systems for signal
transduction, long-term storage and repeated use
(Barbosa et al., 2018).
Among the alternative options to tune selectivity,
peptides are one of the most attractive choices due to
their robustness, chemical diversity, compact size,
and their adaptability to extreme environments and
safely long-term storage (Cui et al., 2012; Sankaran
et al., 2012). Furthermore, they can be developed to
bind distinct targets through rational design or
discovery by panning of phage display libraries
(Kuang et al., 2010).
In this work, we investigated the incorporation of
a previously reported peptide (peptide P1), and two
designed derivatives (peptides P2 and P3) able to
discriminate single carbon deviations among benzene
and derivatives such as toluene and xylene (Ju et al.,
2015), into gel-like sensing materials yielding
electrical and optical signals in the presence of VOCs.
Two gel formulations were studied: a gelatin matrix
gelated in an ionic liquid environment
([BMIM][DCA]) (ionogels yielding an electrical
signal by monitoring the changes in ionic
conductance of the formulation) and the same matrix
with a liquid crystal component (hybrid gels yielding
an optical signal due to their birefringence). The
modified versions of P1 were obtained through the
addition of non-natural amino acids at the C-terminal
- norleucine (Nle - P2 peptide) and biphenylalanine
(Bip, - P3 peptide) - with the purpose to facilitate their
binding in the ionic liquid-liquid crystal interface, due
to their structural resemblance with 5CB (Figure 1).
These materials offer the possibility to add the
peptide selective moiety with unprecedented simple
procedures that take advantage of self-assembly and
autonomous compartmentalization, avoiding harsh
chemical reactions and peptide covalent attachment
onto surfaces.
2 MATERIALS AND METHODS
2.1 Materials and Reagents
Gelatin from bovine skin (gel strength ൎ 225 g;
Bloom, Type B), was purchased from SigmaAldrich.
The liquid crystal 4-cyano-4’-pentylbiphenyl (5CB),
was acquired from TCI Europe, and the Ionic liquid
1-Butyl-3-methylimidazolium chloride
([BMIM][DCA], ˃98%)) was purchased from
IoLiTec. Peptides 1, 2 and 3 were purchased from
Genecust (purity >95,9%). Ethanol (purity 99.8%)
and Fluorescein isothiocyanate (FITC) Isomer I (99%
purity) were purchased from Sigma-Aldrich, while
benzene, hexane, xylene and toluene were supplied
by Fisher Scientific, and acetone (purity 99.5%) was
purchased from Honeywell.
2.2 Confirmation of Peptide P1
Binding by Multi-Parametric
Surface Plasmon Resonance
(MP-SPR)
For the MP-SPR studies, an Au-glass slide was
modified with 3-mercaptopropionic acid (3-MPA), a
linker with a hydroxyl group and a sulfhydryl group,
which binds to the Au surface thiols. For the
immobilization of the P1 peptide (10 uM in 20 mM
sodium phosphate solution, at pH 7) on the surface
EDC/NHS chemistry was used, and Ethanolamine
was used as a blocking agent for extremities where P1
was not bound. This method was used to produce the
sample placed in the peptide chamber, whereas the
control chamber contained an Au-glass slide with all
the previously described functionalization, minus the
immobilization of the peptide step. The MP-SPR
signal variation within time was measured by using
two wavelengths, 670 nm and 785 nm (mDeg), being
the average of the signal measured at the two
wavelengths defined as ∆mDeg.
2.3 Incorporation of the Peptides into
Ionogels
For the production of ionogel sensors we used the
same protocol as previously described with the
addition of peptide solutions (Abid Hussain et al.,
2017). The final formulation was pipetted onto
interdigitated golden electrodes deposited on
untreated glass slides and spread into a thin film using
a TQC film applicator (Automatic Film Applicator
Standard, TQC) with a 15 μm thickness. After
production, the peptide ionogels were left to dry on a
sealed clean petri dish, in a humidity box with
environmental control. Control ionogels (without the
incorporation of a peptide component) were also
prepared, for comparison purposes.
2.4 Incorporation of the Peptides into
Hybrid Gels
Peptide hybrid gels were formulated by mixing all
components plus the peptide solution following
protocols previously described (C. Esteves et al.,
2019; A. Hussain et al., 2017). When gelation occurs,
the gel compositions were deposited on top of
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
26

untreated glass slides and spread into thin films using
a TQC film applicator (Automatic Film Applicator
Standard, TQC) with a 30 μm thickness. After
production, the gels were stored within an
environment of controlled humidity. Control hybrid
gels (without a peptide component) were also
prepared for comparison purposes.
2.5 Hybrid Gel Characterization
The formation of ionic liquid droplets and the
morphological characteristics and differences
between the hybrid gels containing the P1, P2 and P3
peptides were assessed via a ZEISS, Observer.Z1
Polarized Optical Microscope (POM) equipped with
an Axiocam 503 color camera. Pictures were taken
with crossed polarizers and in bright field and were
processed with the ZEN 203 software.
Regarding the morphological stability of the
hybrid gels upon storage over time, POM photos with
crossed polarizers of the same region of interest were
taken for a 30-day period. A costume-made python
scrip (Python 3.6.2) was used to analyze the photos.
The scrip uses binary images to calculate the
differences between them.
2.6 VOC Sensing using Ionogels
All the prepared ionogels (peptide-based and
controls) were assessed in an in-house tailor-made
electrical e-nose device (Hussain et al., 2017). The
device detects changes in the conductance upon VOC
adsorption and desorption. The sensors were placed
in a hermetically sealed array chamber and exposed
to a sequence of six volatiles – hexane, benzene,
toluene, xylene, ethanol and acetone – similar to the
investigation of Ju et al. (Ju et al., 2015). The solvents
were kept in a bath thermostatized at 37ºC and their
respective vapors were pumped to the array chamber
(exposure period) followed with ambient air via a
second air pump (recovery period). The films were
exposed to each gas analyte for 45 consecutive cycles,
each cycle consisting of 5 sec of exposure followed
by 10 sec of recovery, in total 7 min and 30 sec of test
duration per VOC. The electrical signals of the
sensors were acquired at a sampling rate of 90 Hz, and
assays were performed in duplicates.
Processing and evaluation of the signals obtained
was performed, using methods described in our
previous publications (C. Esteves et al., 2019; S. I. C.
J. Palma et al., 2019; Rodrigues et al., 2020; Santos et
al., 2019b). Python programming tools were used to
process the signals retrieved by the e-nose device.
For the evaluation of the sensors’ performance
regarding VOC identification, machine learning
methods were applied to the individual cycles.
Briefly, twelve features representative of the signals
morphology were extracted from each cycle and used
as input variables in an automatic classifier based on
Support Vector Machines (SVM) algorithm, using a
radial basis kernel and hyperparameters C = 100 and
y = 0.1. The dataset for each sensor formulation was
composed by 30 cycles per VOC. Two thirds of this
dataset were used as training set and one third as
validation set for the automatic classifier. Accuracy
bar plots, which exhibit the percentage of the correct
predictions of the classifier were used to represent the
sensors performance.
2.7 VOC Sensing using Hybrid Gels
The optical e-nose device is designed to monitor the
light transmitted by the hybrid sensors and convert it
into voltage. The detailed setup has been described in
our previous works (Esteves et al., 2019; Hussain et
al., 2017; Palma et al., 2019; Rodrigues et al., 2020).
The sensors are placed in a hermetically sealed
detection chamber and each one is paired with a
polarizer and a corresponding analyzer. The VOC
experiments, as well as the data analysis of the signals
were conducted and processed as described in the
previous section.
3 RESULTS AND DISCUSSION
3.1 Assessing VOC Selectivity in
Ionogel: Electrical e-Nose
The affinity and selectivity of peptide P1 towards
VOCs was firstly assessed by MP-SPR. Our results
indicated a preferred binding of the peptide P1
towards VOCs following the order benzene<xylene<
toluene<ethanol<acetone<hexane, as can be seen by
the increase of the ΔmDeg values (see Table 1).
The three peptides were then incorporated into the
ionogel gelatin matrices and spread as thin films onto
interdigitated electrodes to be tested in an in-house
developed electrical e-nose. VOCs adsorption to the
ionogels affects the ion mobility within the materials.
Therefore, the ionogels admittance changes and an
electric response can be obtained. This is a reversible
process upon VOC desorption and the basis for
electrical VOC sensing with the electric E-nose (S. I.
C. J. Palma et al., 2019).
Incorporation of VOC-Selective Peptides in Gas Sensing Materials
27
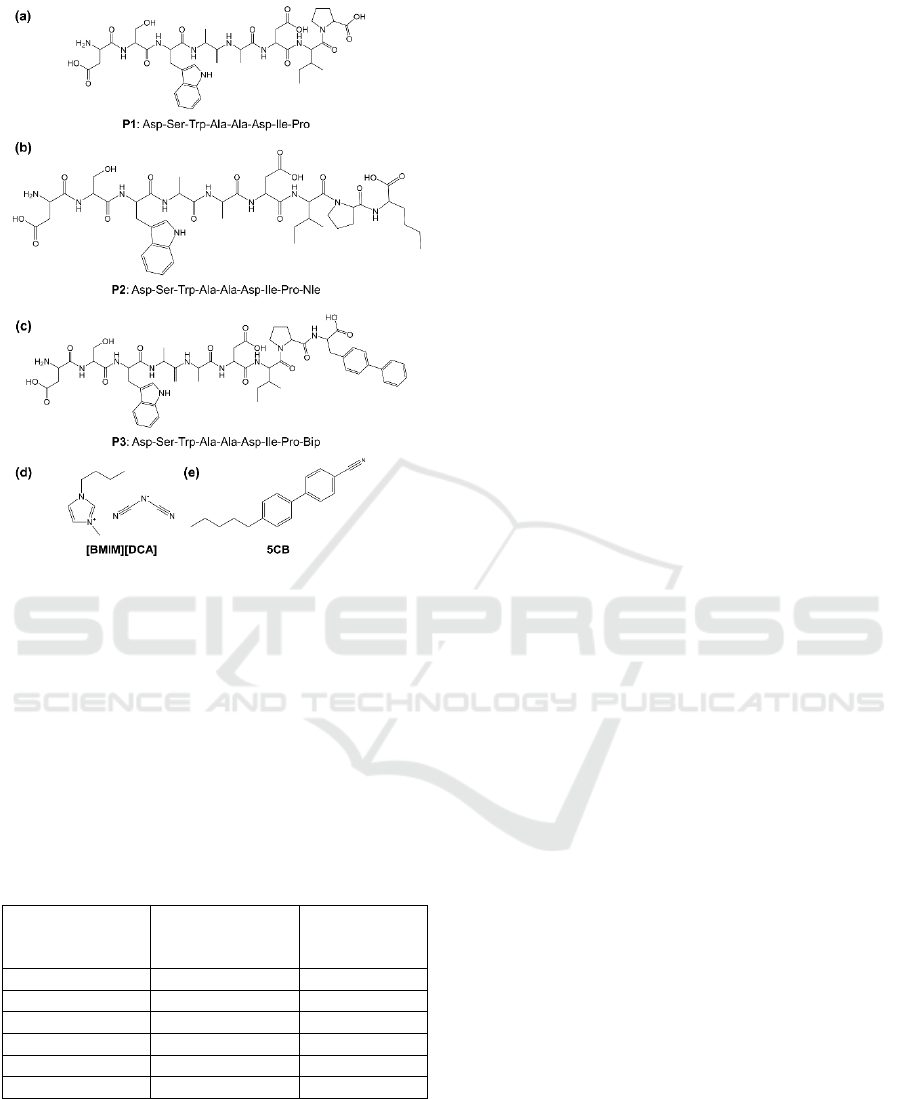
Figure 1: Chemical structure of (a) peptide 1, (b) peptide 2,
(c) peptide 3, (d) ionic liquid 1-Butyl-3-methylimidazolium
dicyanamide ([BMIM] [DCA]) and (e) liquid crystal 4-
Cyano-4’-pentylbiphenyl (5CB).
Table 1: Summary-results of the interaction between the P1
functionalized Au surface and the VOCs. ∆mDeg (peptide-
control) = ∆mDeg peptide-∆mDeg control, where ∆mDeg
peptide is the average of the signal measured at the 670 and
785 nm wavelengths in the peptide chamber, and ∆mDeg
control is the average of the signal measured at the 670 and
785 nm wavelengths in the control chamber. Relative signal
variation = the relative signal representing how much the
signal increased on the peptide chamber, when compared to
the control chamber.
VOCs
ΔmDeg
(Peptide-
Control)
Relative
signal
variation
Acetone 5.76 ± 2.16 0.21
Ethanol 2.20 ± 3.63 0.33
Benzene 32.54 ± 3.35 5.25
X
y
lene 14.69 ± 2.97 1.20
Toluene 5.61 ± 4.36 0.46
Hexane 6.19 ± 3.94 0.17
Through the sequential exposure of the peptide
ionogel formulations to the 6 tested volatiles - hexane,
benzene, toluene, xylene, ethanol and acetone -
variations in the conductance of the sensors were
monitored in real time and after signal processing (as
described in Materials and Methods section) typical
relative amplitude responses are presented in Figure
2(a).
The sensor responses exhibit a variability in their
profiles, characteristic of the corresponding gas
analyte exposure. For example, all of the sensors
during the linear 6C-alkyl chain hexane exposure
respond with a downwards curve, while all other
volatiles generate an upwards response signal. The
majority of the sensors respond rather quickly (within
seconds) upon VOCs exposure, during which most
signals never reach a plateau, with the exception of
xylene in control and P3 ionogels. Upon recovery,
almost all sensor signals return to the baseline in a
similar way.
After signal collection machine learning-based
tools were implemented to analyze the data. The
signals were divided into cycles and were then
normalized. A set of features corresponding to the
curve morphology (C. Esteves et al., 2019; S. I. C. J.
Palma et al., 2019; Santos et al., 2019a) was extracted
and used as input for an SVM-based automatic
classifier. The accuracy % of correct VOC prediction
is presented in an accuracy bar plot seen in Figure
2(b), depicting that the tested ionogel compositions
exhibit distinct selectivity for the tested VOCs.
For example, P2 and P3-based sensors were able
to discriminate and identify hexane and xylene,
respectively, with an 100% accurate classification.
This could be associated with the presence of the
similar non-canonical aminoacid norleucine and
biphenylalanine moieties in P2 and P3 peptides
respectively and their similarity to the structure of the
volatiles in question, possibly enhancing the
interaction between the sensors and the volatiles. On
a similar vein, the control ionogel provides great
classification results for acetone and ethanol, with a
100% accuracy. The accuracy scores for the P1
ionogel were not as high (e.g. for toluene and xylene)
which suggests that certain signal profiles produced
by the formulation exhibited similar characteristics,
thus not allowing the distinction of the corresponding
analytes. Overall, the control ionogel formulation
achieved the best global accuracy score (91%)
followed by the P3-based ionogel (86%).
These results indicate that the incorporation of the
peptides onto the gelatin ionogels did not yield an
improvement of selectivity towards benzene and
aromatic compounds. This is mainly due to the fact
that the control ionogel already displayed excellent
discriminating behavior towards the particular VOCs
tested, as conductance and selectivity are already
driven by the ionic liquid component.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
28
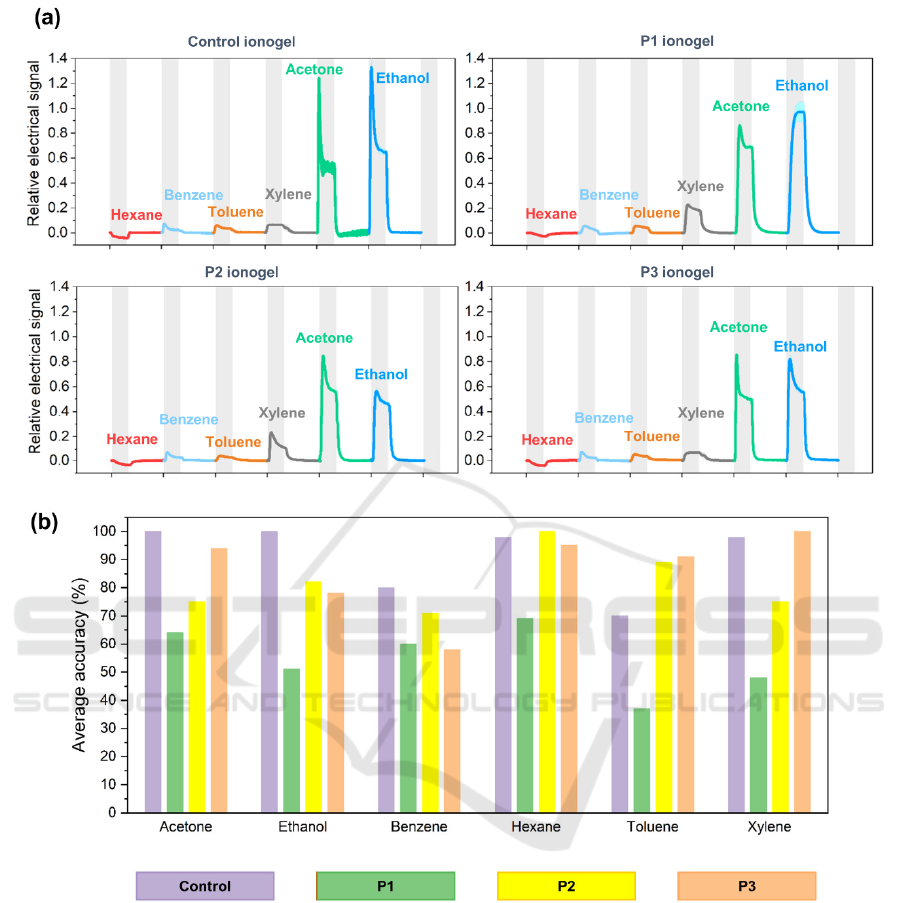
Figure 2: Electrical e-nose results for Control, P1, P2 and P3-based ionogels. (a) Typical cycle signals of all the sensors upon
exposure to different VOCs. Each curve represents the average and standard deviation of at least 19 replicate cycles from the
same sensor. VOC exposure periods (5 s) are highlighted in grey, and each cycle corresponds in total to 15 s. (b) Comparison
of the VOC prediction accuracies obtained for all the ionogels tested in the electrical e-nose.
3.2 Assessing VOC Selectivity in
Hybrid Gels: Optical e-Nose
The peptides P1, P2 and P3 were incorporated into
hybrid gel formulations by simply adding them to the
formulation and promoting autonomous self-
assembly of the different moieties into the hybrid gel
compartments. The formation of ionic liquid – liquid
crystal droplets was observed by bright field and
polarized optical microscopy (Figure 3).
All the hybrid gels (control and peptide-based)
exhibit polydisperse 5CB droplets as previously
noted (Esteves et al., 2019; Hussain et al., 2017;
Palma et al., 2019), featuring a radial configuration
which under the POM gives rise to a distinctive
Maltese cross pattern (Drzaic, 1995). The
characteristic core defect located in the center of the
droplet can be observed in the bright field pictures
(Esteves et al., 2019; Hussain et al., 2017) – seen in
Figure 3 (a), (e) and (j). The radial droplet profile
Incorporation of VOC-Selective Peptides in Gas Sensing Materials
29

Figure 3: Representative POM images of control hybrid gel (a), (e) and (i)), peptide 1 hybrid gel (b), (f) and (j)), peptide 2
hybrid gel (c), (g) and (k)) and peptide 3 hybrid gel (d), (h) and (l)).
suggests that the liquid crystal molecules adopt a
homeotropic alignment near the ionic liquid interface,
which is attributed to the interactions occurring
between the alkyl chain of the ionic liquid and 5CB
(C. Esteves et al., 2019; Carina Esteves et al., 2020;
A. Hussain et al., 2017).
We need to point out that in the case of P2-based
and P3-based hybrid compositions, apart from the
radial droplets, some irregularly shaped and randomly
oriented droplets were also observed. This finding
suggests that both the Nle (in the case of the P2
peptide) and Bip (in the case of the P3 peptide)
moieties interfere with the liquid crystal anchoring on
the ionic liquid interface.
The peptide-based hybrid gels were tested in the
optical e-nose. Exposure to the tested gas analytes
results in a disorganization of the liquid crystal
component, triggering a phase transition to the
isotropic state. The signal responses are the
collaborative responses of the individual
compartments of the gel formulations to the
corresponding analyte. For example, hydrophobic
VOCs, such as hexane and the aromatic benzene,
xylene and toluene, are more likely to interact mainly
with the oil phase formed by the liquid crystal
molecules inside the droplets. Protic VOCs, and those
forming hydrogen bonds (e.g. ethanol), tend to
interact not only with the LC droplets but also with
the gelatin matrix itself, as previously reported
(Esteves et al., 2019; Hussain et al., 2017). The sensor
responses were repeatable and exhibited features
(such as signal profile, response/recovery profile)
characteristic of the tested volatiles.
Signal processing, analysis and presentation were
conducted using the same tools as in the electrical e-
nose results, described in the previous section. In
Figure 4(a) relative amplitude responses for each
tested hybrid peptide gel compositions and for all the
studied gas analytes is shown. The baseline on each
individual curve represents the initial light state of a
sensor, due to the presence of the liquid crystal
droplets. Upon VOC exposure the liquid crystal
component becomes isotropic, thus it cannot alter the
polarization of the transmitted light (which
subsequently cannot pass through the analyzer). This
generates an upwards response curve to a dark(er)
state for the sensor.
It is possible to observe that each sensor holds a very
characteristic amplitude signature signal, related to
the different volatiles. For example, a delayed
response upon analyte exposure is observed (e.g.
hexane for control and P1-based hybrid gels), or the
cases where a plateau in the response is reached (e.g.
the control and P3-based sensors for all volatiles, with
the exception of hexane and ethanol), and a very
distinctive flat response from P1-based sensor
towards ethanol.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
30
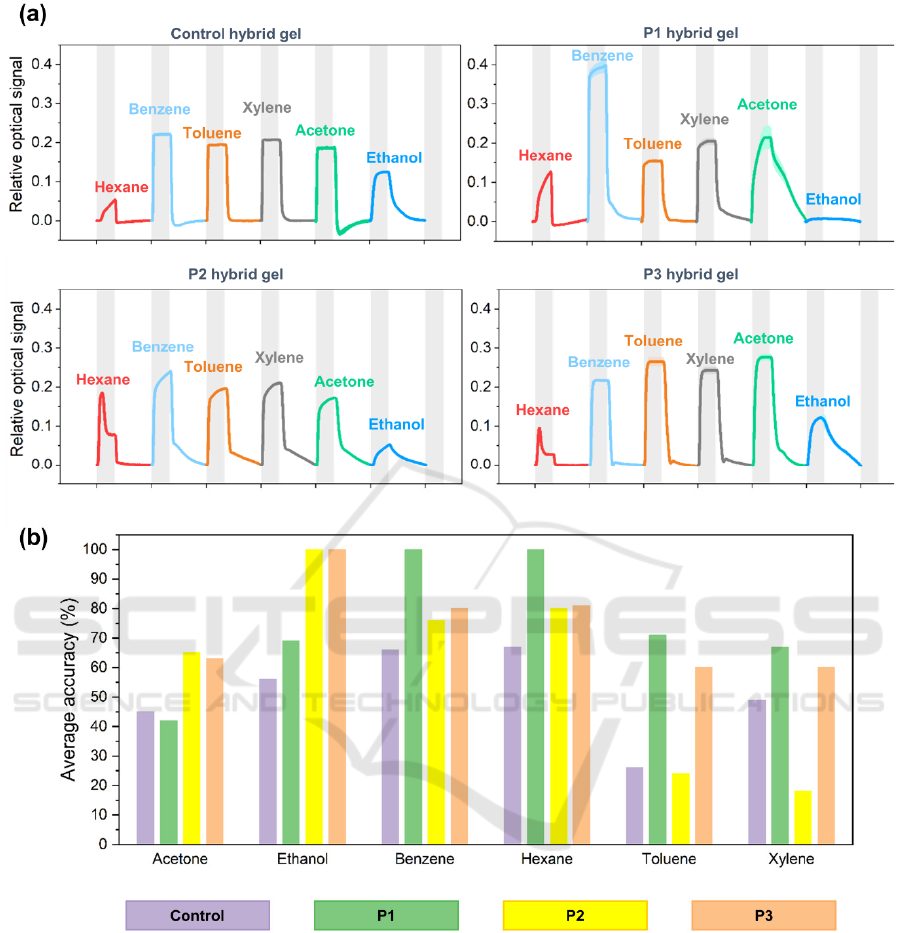
Figure 4: Optical e-nose results for the for Control, P1, P2 and P3-based hybrid gels. (a) Typical cycle signals of all the
sensors upon exposure to different VOCs. Each curve represents the average and standard deviation of at least 19 replicate
cycles from the same sensor. VOC exposure periods (5 s) are highlighted in grey, and each cycle corresponds in total to 15 s.
(b) Comparison of the VOC prediction accuracies obtained for all the sensors tested in the optical e-nose.
Another interesting observation is that although
the 10 s recovery period seems to be sufficient for the
liquid crystal to completely recuperate from
isotropisation in the control hybrid formulation
(allowing the sensors signal to return to the initial
baseline levels), the majority of the peptide-based
sensors appear to take longer to completely recover
within the 10 s period. This is evident in the case of
P1 sensors with benzene, xylene and acetone, and
both P2 and P3 sensors with all volatiles – with the
exception of hexane. It should be noted that this
recovery pattern was not detected for the ionogel
sensors previously analyzed.
When looking at the VOC prediction accuracy bar
plot (seen in Figure 4(b)) it is noticeable that
selectivity towards benzene and hexane from the P1-
Incorporation of VOC-Selective Peptides in Gas Sensing Materials
31

based sensor was achieved. Overall, the P1-based
hybrid gel was able to distinguish and classify all the
tested volatiles presenting a 74% score of global
accuracy.
4 CONCLUSIONS
In this work we studied the incorporation of peptides
in two distinct groups of gas sensitive materials.
Ionogels, comprised of gelatin and ionic liquid,
tailored for electrical gas sensing and hybrid gels,
containing gelatin, ionic liquid and liquid crystal,
designed for optical gas sensing. A peptide (P1)
known to discriminate single carbon deviations
among benzene and derivatives was used as a model,
along with two modified versions (P2 and P3). The
set of ionogels were tested in an electrical e-nose,
designed to monitor changes in the conductance of
the sensors, and selectivity towards hexane and
xylene was observed in the case of P2 and P3 ionogel
sensors, respectively. Regarding the hybrid gels, the
incorporation of peptide P1 did not disrupt the self-
assembly of the ionic liquid crystal droplets
(suggesting that peptide P1 is mainly distributed in
the matrix), while the incorporation of peptides P2
and P3 disrupt some droplets, although the majority
exhibit a radial configuration. The set of hybrid gels
were tested in an optical e-nose, designed to monitor
the light transmitted from the materials. An enhanced
discrimination of benzene was observed for the P1-
based gel and for ethanol in the case of both P2 and
P3 hybrid gels. As a final note, we would like to
highlight the complementarity of the two distinct gel
formulations responses, since the combination of both
optical and electrical prediction accuracies could
provide the best classification accuracies for the
tested VOCs. For example, the P2 ionogel was more
Table 2: Relative accuracy scores of VOC prediction from
every ionogel and hybrid gel peptide sensors’ tested. The
relative accuracy score is defined as the difference between
the peptide-based accuracy score and the control accuracy
score.
Relative accuracy
ionogels (%)
Relative accuracy
hybrid gels (%)
VOCs P1 P2 P3 P1 P2 P3
Acetone -3 20 18 -36 -25 -6
Ethanol 13 44 44 -49 -18 -22
Benzene 34 10 14 -20 -9 -22
X
y
lene 33 13 14 -29 2 -3
Toluene 45 -2 34 -33 19 21
Hexane 18 -31 11 -50 -23 2
selective towards hexane and toluene whereas the P2
hybrid gel was more selective towards ethanol,
suggesting that additional information and enhanced
selectivity can be obtained regarding discrimination
of volatiles, by using a combinatorial sensors array in
a dual-mode e-nose. An overall view of the relative
accuracy results from all sensors, regarding
discrimination of each VOC, can be seen in Table 2.
ACKNOWLEDGEMENTS
This project has received funding from the European
Research Council (ERC) under the EU Horizon 2020
research and innovation programme [grant reference
SCENT-ERC-2014-STG-639123, (2015-2022)] and
by national funds from FCT - Fundação para a
Ciência e a Tecnologia, I.P., in the scope of the
project UIDP/04378/2020 and UIDB/04378/2020 of
the Research Unit on Applied Molecular Biosciences
– UCIBIO and the project LA/P/0140/2020 of the
Associate Laboratory Institute for Health and
Bioeconomy - i4HB, which is financed by national
funds from financed by FCT/MEC
(UID/Multi/04378/2019). The authors thank
FCT/MCTES for the PhD grants
SFRH/BD/128687/2017 and PD/BD/139800/2018.
REFERENCES
Barbosa, A. J. M., Oliveira, A. R., & Roque, A. C. A.
(2018). Protein- and Peptide-Based Biosensors in
Artificial Olfaction. Trends in Biotechnology, 36(12),
1244–1258. https://doi.org/10.1016/j.tibtech.2018.07.0
04
Broza, Y. Y., Mochalski, P., Ruzsanyi, V., Amann, A., &
Haick, H. (2015). Hybrid Volatolomics and Disease
Detection. Angewandte Chemie - International Edition,
54(38), 11036–11048. https://doi.org/10.1002/
anie.201500153
Cruz, H., Califórnia, A., Pinto, A., Fonseca, J., Palmeira-
De-Oliveira, J. G. A., Martinez-De-Oliveira, J., &
Pereira, L. (2017). Development of e-nose biosensors
based on organic semiconductors towards low-cost
health care diagnosis in gynecological diseases.
Materials Today: Proceedings, 4(11), 11544–11553.
https://doi.org/10.1016/j.matpr.2017.09.065
Cui, Y., Kim, S. N., Naik, R. R., & McAlpine, M. C. (2012).
Biomimetic peptide nanosensors. Accounts of Chemical
Research, 45(5), 696–704. https://doi.org/10.1021/
ar2002057
Dey, A. (2018). Semiconductor metal oxide gas sensors: A
review. Materials Science and Engineering B, 229,
206–217. https://doi.org/10.1016/j.mseb.2017.12.036
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
32

Drzaic, P. S. (1995). Nematic Configurations Within
Droplets. In Liquid Crystal Dispersions (pp. 99–181).
World Scientific.
Esteves, C., Santos, G. M. C., Alves, C., Palma, S. I. C. J.,
Porteira, A. R., Filho, J., Costa, H. M. A., Alves, V. D.,
Morais, B. M., Ferreira, I., Gamboa, H., & Roque, A.
C. A. (2019). Effect of film thickness in gelatin hybrid
gels for artificial olfaction. Materials Today Bio,
1(December 2018), accepted manuscript.
https://doi.org/10.1016/j.mtbio.2019.100002
Esteves, Carina, Ramou, E., Porteira, A. R. P., Moura
Barbosa, A. J., & Roque, A. C. A. (2020). Seeing the
Unseen: The Role of Liquid Crystals in Gas-Sensing
Technologies. Advanced Optical Materials, 8(11).
https://doi.org/10.1002/adom.201902117
Fitzgerald, J., & Fenniri, H. (2017). Cutting edge methods
for non-invasive disease diagnosis using e-tongue and
e-nose devices. Biosensors, 7(4). https://doi.org/
10.3390/bios7040059
Hussain, Abid, Semeano, A. T. S., Palma, S. I. C. J., Pina,
A. S., Almeida, J., Medrado, B. F., Pádua, A. C. C. S.,
Carvalho, A. L., Dionísio, M., Li, R. W. C., Gamboa,
H., Ulijn, R. V., Gruber, J., & Roque, A. C. A. (2017).
Tunable Gas Sensing Gels by Cooperative Assembly.
Advanced Functional Materials, 27(27), 1–9.
https://doi.org/10.1002/adfm.201700803
Ju, S., Lee, K. Y., Min, S. J., Yoo, Y. K., Hwang, K. S.,
Kim, S. K., & Yi, H. (2015). Single-carbon
discrimination by selected peptides for individual
detection of volatile organic compounds. Scientific
Reports, 5, 9196. https://doi.org/10.1038/srep09196
Krilaviciute, A., Heiss, J. A., Leja, M., Kupcinskas, J.,
Haick, H., & Brenner, H. (2015). Detection of cancer
through exhaled breath: A systematic review.
Oncotarget, 6(36), 38643–38657. https://doi.org/
10.18632/oncotarget.5938
Kuang, Z., Kim, S. N., Crookes-Goodson, W. J., Farmer, B.
L., & Naik, R. R. (2010). Biomimetic chemosensor:
Designing peptide recognition elements for surface
functionalization of carbon nanotube field effect
transistors. ACS Nano, 4(1), 452–458. https://doi.org/
10.1021/nn901365g
Palma, S. I. C. J., Esteves, C., Padua, A. C. C. S., Alves, C.
M., Santos, G. M. C., Costa, H. M. A., Dionisio, M.,
Gamboa, H., Gruber, J., & Roque, A. C. A. (2019).
Enhanced gas sensing with soft functional materials.
2019 IEEE International Symposium on Olfaction and
Electronic Nose (ISOEN), 1–3. https://doi.org/
10.1109/ISOEN.2019.8823178
Palma, Susana I.C.J., Traguedo, A. P., Porteira, A. R., Frias,
M. J., Gamboa, H., & Roque, A. C. A. (2018). Machine
learning for the meta-analyses of microbial pathogens’
volatile signatures. Scientific Reports, 8(1), 1–15.
https://doi.org/10.1038/s41598-018-21544-1
Park, S. J., Park, C. S., & Yoon, H. (2017). Chemo-
electrical gas sensors based on conducting polymer
hybrids. Polymers, 9(5), 155. https://doi.org/10.3390/
polym9050155
Rodrigues, R., Palma, S. I. C. J., G. Correia, V., Padrão, I.,
Pais, J., Banza, M., Alves, C., Deuermeier, J., Martins,
C., Costa, H. M. A., Ramou, E., Silva Pereira, C., &
Roque, A. C. A. (2020). Sustainable plant polyesters as
substrates for optical gas sensors. Materials Today Bio,
8(August). https://doi.org/10.1016/j.mtbio.2020.100
083
Sankaran, S., Khot, L. R., & Panigrahi, S. (2012). Biology
and applications of olfactory sensing system : A review.
Sensors and Actuators: B. Chemical, 171–172, 1–17.
https://doi.org/10.1016/j.snb.2012.03.029
Santos, G., Alves, C., Pádua, A. C., Palma, S., Gamboa, H.,
& Roque, A. C. (2019a). An optimized e-nose for
efficient volatile sensing and discrimination.
BIODEVICES 2019 - 12th International Conference on
Biomedical Electronics and Devices, Proceedings;
Part of 12th International Joint Conference on
Biomedical Engineering Systems and Technologies,
BIOSTEC 2019, 36–46. https://doi.org/10.5220/000
7390700360046
Santos, G., Alves, C., Pádua, A. C., Palma, S., Gamboa, H.,
& Roque, A. C. (2019b). An optimized e-nose for
efficient volatile sensing and discrimination. In A.
Roque, A. Fred, & H. Gamboa (Eds.), BIODEVICES
2019 - 12th International Conference on Biomedical
Electronics and Devices, Proceedings; Part of 12th
International Joint Conference on Biomedical
Engineering Systems and Technologies, BIOSTEC
2019 (pp. 36–46). SCITEPRESS. https://doi.org/
10.5220/0007390700360046
Son, M., Lee, J. Y., Ko, H. J., & Park, T. H. (2017).
Bioelectronic Nose : An Emerging Tool for Odor
Standardization. Trends in Biotechnology, 35(4), 301–
307. https://doi.org/10.1016/j.tibtech.2016.12.007
van Hooren, M. R. A., Leunis, N., Brandsma, D. S.,
Dingemans, A. M. C., Kremer, B., & Kross, K. W.
(2016). Differentiating head and neck carcinoma from
lung carcinoma with an electronic nose: a proof of
concept study. European Archives of Oto-Rhino-
Laryngology, 273(11), 3897–3903. https://doi.org/
10.1007/s00405-016-4038-x
Vishinkin, R., & Haick, H. (2015). Nanoscale Sensor
Technologies for Disease Detection via Volatolomics.
Small, 11(46), 6142–6164. https://doi.org/10.1002/
smll.201501904
Capelli, L., Sironi, S., & Del Rosso, R. (2014). Electronic
noses for environmental monitoring applications.
Sensors, 14(11), 19979–20007. https://doi.org/10.3390/
s141119979
Chehri, A., Farjow, W., Mouftah, H. T., & Fernando, X.
(2011). Design of wireless sensor network for mine
safety monitoring. 2011 24th Canadian Conference on
Electrical and Computer Engineering (CCECE),
Niagara Falls, ONT, 001532–001535.
https://doi.org/10.1109/CCECE.2011.6030722
Cui, Y., Kim, S. N., Naik, R. R., & McAlpine, M. C. (2012).
Biomimetic peptide nanosensors. Accounts of Chemical
Research, 45(5), 696–704. https://doi.org/10.1021/
ar2002057
Dey, A. (2018). Semiconductor metal oxide gas sensors: A
review. Materials Science and Engineering B, 229,
206–217. https://doi.org/10.1016/j.mseb.2017.12.036
Incorporation of VOC-Selective Peptides in Gas Sensing Materials
33

Drzaic, P. S. (1995). Nematic Configurations Within
Droplets. In Liquid Crystal Dispersions (pp. 99–181).
World Scientific.
Esteves, C., Santos, G. M. C., Alves, C., Palma, S. I. C. J.,
Porteira, A. R., Filho, J., Costa, H. M. A., Alves, V. D.,
Morais, B. M., Ferreira, I., Gamboa, H., & Roque, A.
C. A. (2019). Effect of film thickness in gelatin hybrid
gels for artificial olfaction. Materials Today Bio,
1(December 2018), accepted manuscript.
https://doi.org/10.1016/j.mtbio.2019.100002
Hussain, A., Semeano, A. T. S., Palma, S. I. C. J., Pina, A.
S. P., Almeida, J., Medrado, B. F. F., Padua, A. C. C.
S., Carvalho, A. L., Dionisio, M., Li, R. W. C.,
Gamboa, H., Ulijn, R. V., Gruber, J., & Roque, A. C.
A. (2017). Tunable Gas Sensing Gels by Cooperative
Assembly. Advanced Functional Materials, 27,
1700803. https://doi.org/10.1002/adfm.201700803
Ju, S., Lee, K. Y., Min, S. J., Yoo, Y. K., Hwang, K. S.,
Kim, S. K., & Yi, H. (2015). Single-carbon
discrimination by selected peptides for individual
detection of volatile organic compounds. Scientific
Reports, 5, 9196. https://doi.org/10.1038/srep09196
Kuang, Z., Kim, S. N., Crookes-Goodson, W. J., Farmer, B.
L., & Naik, R. R. (2010). Biomimetic chemosensor:
Designing peptide recognition elements for surface
functionalization of carbon nanotube field effect
transistors. ACS Nano, 4(1), 452–458.
https://doi.org/10.1021/nn901365g
Lefferts, M. J., & Castell, M. R. (2015). Vapour sensing of
explosive materials. Analytical Methods, 7(21), 9005–
9017. https://doi.org/10.1039/c5ay02262b
Palma, S. I. C. J., Esteves, C., Padua, A. C. C. S., Alves, C.
M., Santos, G. M. C., Costa, H. M. A., Dionisio, M.,
Gamboa, H., Gruber, J., & Roque, A. C. A. (2019).
Enhanced gas sensing with soft functional materials.
2019 IEEE International Symposium on Olfaction and
Electronic Nose (ISOEN), 1–3. https://doi.org/10.1109/
ISOEN.2019.8823178
Park, S. J., Park, C. S., & Yoon, H. (2017). Chemo-
electrical gas sensors based on conducting polymer
hybrids. Polymers, 9(5), 155. https://doi.org/10.3390/
polym9050155
Rodrigues, R., Palma, S. I. C. J., G. Correia, V., Padrão, I.,
Pais, J., Banza, M., Alves, C., Deuermeier, J., Martins,
C., Costa, H. M. A., Ramou, E., Silva Pereira, C., &
Roque, A. C. A. (2020). Sustainable plant polyesters as
substrates for optical gas sensors. Materials Today Bio,
8(August). https://doi.org/10.1016/j.mtbio.2020.100
083
Sankaran, S., Khot, L. R., & Panigrahi, S. (2012). Biology
and applications of olfactory sensing system: A review.
Sensors and Actuators: B. Chemical, 171–172, 1–17.
https://doi.org/10.1016/j.snb.2012.03.029
Santos, G., Alves, C., Pádua, A. C., Palma, S., Gamboa, H.,
& Roque, A. C. (2019a). An optimized e-nose for
efficient volatile sensing and discrimination.
BIODEVICES 2019 - 12th International Conference on
Biomedical Electronics and Devices, Proceedings;
Part of 12th International Joint Conference on
Biomedical Engineering Systems and Technologies,
BIOSTEC 2019, 36–46. https://doi.org/10.5220/000
7390700360046
Santos, G., Alves, C., Pádua, A. C., Palma, S., Gamboa, H.,
& Roque, A. C. (2019b). An optimized e-nose for
efficient volatile sensing and discrimination. In A.
Roque, A. Fred, & H. Gamboa (Eds.), BIODEVICES
2019 - 12th International Conference on Biomedical
Electronics and Devices, Proceedings; Part of 12th
International Joint Conference on Biomedical
Engineering Systems and Technologies, BIOSTEC
2019 (pp. 36–46). SCITEPRESS. https://doi.org/
10.5220/0007390700360046
Son, M., Lee, J. Y., Ko, H. J., & Park, T. H. (2017).
Bioelectronic Nose: An Emerging Tool for Odor
Standardization. Trends in Biotechnology, 35(4), 301–
307. https://doi.org/10.1016/j.tibtech.2016.12.007
van Hooren, M. R. A., Leunis, N., Brandsma, D. S.,
Dingemans, A. M. C., Kremer, B., & Kross, K. W.
(2016). Differentiating head and neck carcinoma from
lung carcinoma with an electronic nose: a proof of
concept study. European Archives of Oto-Rhino-
Laryngology, 273(11), 3897–3903. https://doi.org/
10.1007/s00405-016-4038-x
Zampolli, S., Elmi, I., Ahmed, F., Passini, M., Cardinali, G.
C., Nicoletti, S., & Dori, L. (2004). An electronic nose
based on solid state sensor arrays for low-cost indoor
air quality monitoring applications. Sensors and
Actuators, B: Chemical, 101(1–2), 39–46.
https://doi.org/10.1016/j.snb.2004.02.024
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
34
