
The Investigation of the Correlation between Urine Biomarkers and
Pancreatic Ductal Adenocarcinoma
Jiahui Shen
a
Institute of Problem Solving, Beijing Normal University Hong Kong Baptist University United International College,
Zhuhai, Guangdong, China
Keywords: Pancreatic Ductal Aadenocarcinoma, Urine Biomarkers, Multinomial Logistic Regression Model, Prediction
Of Cancer.
Abstract: Pancreatic ductal adenocarcinoma (PDAC)'s low survival rate has long been a world unsolved problem. Many
past studies in recent decades proved the possibility of detecting such disease in its early stages, using a new
screening panel with several urinary biomarkers. However, limited studies truly focused on the statistical
correlation between the fluctuation of urinary biomarker concentrations and PDAC diagnosis status. Our study
sought to demonstrate a possible correlation between biomarker concentration values in urine samples and
confirmed cases of PDAC that could be used for the early diagnosis of PDAC patients. Based on the
correlation of the different biomarker measurements with our investigation, we obtained data from Kaggle
originally from an open access paper. We estimated odds ratios (ORs) and 95% CIs in a multinomial logistic
regression model. From the analysis of p-value, LYVE1, REG1B, and TFF1 are all possible biomarkers to
indicate a patient's PDAC status. Multinomial logistic regression was made to show the correlation between
selected biomarkers and diagnosis. Our study suggested that a possible real correlation exists between urinary
biomarkers' concentration and PDAC diagnosis status. Our model could be used to detect patients in their
early disease stages to some degree.
1 INTRODUCTION
Pancreatic ductal adenocarcinoma, also known as
PDAC, is the most common malignant tumor of the
pancreas. It arises from cells in the ducts or ducts of
the pancreas, hence its name. PDAC has a low
survival rate of about 9% at 5 years and is one of the
deadliest cancers in the world. Initially, the tumor
may not show any signs or symptoms. However, over
time, it may cause abdominal pain, nausea, and
vomiting, and lead to weight loss and, in most cases,
complications that eventually lead to a person's death.
In terms of today's medical research developments,
complete surgical removal of the tumor is the only
chance to cure PDAC. If the disease is detected early,
the 5-year survival rate can be increased to 70% when
the tumor is still small and resectable. However,
because pancreatic ductal adenocarcinoma is difficult
to detect at an early stage, many patients are already
at an advanced stage of cancer when diagnosed and
a
https://orcid.org/0000-0003-0924-1786
the disease is already difficult to cure. Therefore,
finding a detection method for early PDAC is an
important clinical need, which may greatly improve
the survival chances of patients.
Since the first risk prediction model for coronary
heart disease was introduced in 1976, prediction
models for various diseases, including cancer, have in
the intervening decades, several tests for Pancreatic
ductal adenocarcinoma have emerged, and the
methods can be broadly classified into two
categories. The first type is based on the use of
imaging, where patients can be distinguished from
pancreatic ductal adenocarcinoma by the radiomics
score (rad-score) using multidetector computed
tomography (MDCT), which distinguishes focal-type
autoimmune pancreatitis (fAIP) from pancreatic
ductal adenocarcinoma (MDCT). adenocarcinoma
(PDAC) (Li et al. 2021). The second category is the
use of various biomarkers to discriminate PDAC
from benign pancreatic disease and healthy
individuals. the source of most biomarkers is blood,
318
Shen, J.
The Investigation of the Correlation between Urine Biomarkers and Pancreatic Ductal Adenocarcinoma.
DOI: 10.5220/0011368200003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 318-325
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

for example, CA 19-9 and CEA in serum can be used
as detection markers (Poruk et al. 2013). Mayerle, J.
& Kalthoff, H. et al. generated metabolomic
profiles of plasma and serum samples by gas
chromatography-mass spectrometry and liquid
chromatography-tandem mass spectrometry
identification of 477 metabolites and selected nine
plasma metabolites, all of which could be identified
to distinguish pancreatic cancer from chronic
pancreatitis. Serum mRNA for LGALS9 (Galectin-9)
was detected to be highly expressed in PDAC patients
compared to the normal pancreas and could be used
as a new assay biomarker in addition to prognostic for
stage IV patients (Seifert et al. 2020). With medical
developments in the assay new blood testing
techniques with nanoparticles are added, which are
expected to distinguish PDAC patients from healthy
individuals (Caputo & Caracciolo 2020). For the
detection of the resectable phase of PDAC, the exlr
signal of PDAC can be detected by analyzing plasma
extracellular vesicle long RNA, reported (Yu et al.
2020). In addition to biomarkers in blood, there are
volatile organic compounds (VOCs) in alveolar air
(Princivalle et al., 2018) and MicroRNA (MiR) in
pancreatic fluid (Nakamura et al. 2019) both with
good sensitivity and specificity for pancreatic tumors.
One more source of biomarkers in urine, and the urine
biomarker set, including LYVE1, REG1A, and TFF1,
has been shown to be effective in the early detection
of PDAC in studies as early as 2015 (Radon et al.,
2015). In a recent study, Sahni, S. and Pandya, A. R.
et al. used a non-targeted urine metabolic panel to
identify novel metabolite biomarker profiles for
PDAC diagnosis, and six metabolites were screened
and showed very high potential in the detection and
diagnosis of PDAC in both early (stages I and II) and
late (stages III and IV) patients.
In contrast to previously published articles on the
detection of Pancreatic ductal adenocarcinoma, this
study changes the detection of PDAC from traditional
imaging methods (e.g., CT) or blood markers to urine
biomarkers. While imaging methods are expensive
and require training of dedicated personnel for
testing, while many biomarkers in the blood (e.g.,
CA19-9 serum test) can be used to diagnose PDAC,
urine instead of blood allows for completely non-
invasive sampling, high volume collection, and easily
repeatable measurements, with a smaller dynamic
range and less complex proteome than blood. In
addition, continuous ultrafiltration of blood is
expected to result in the accumulation of at least some
biomarkers in the urine, leading to higher
concentrations. Therefore, sensitivity, specificity,
positive and negative predictive values are superior to
conventional methods. Second, previous studies have
used urine biomarkers to detect PDAC, and they used
REG1A in urine as one of the biomarkers. expression
of REG1A increased with the progression of PanINs
to cancer, but REG1B was highly expressed in the
earliest PanINs, showing a better difference. Despite
their similar performance, this study confirmed that
REG1B was superior to REG1a in comparisons
between control samples and stage I-IIA PDAC
samples. therefore, all subsequent experiments in this
study used REG1B as a component of the biomarker
set.
In this paper, A secondary data analysis is
conducted to study the statistical correlation between
four urine bio-markers and Pancreatic ductal
adenocarcinoma, and to predict whether this patient
has pancreatic problems and determine whether he
has pancreatic cancer at an early stage. First, the type
of variable was determined after obtaining the data
and corresponding to obtaining the p-value.
Continuous variables were assessed using ANOVA,
while categorical variables were tested with the χ
2
-
test. Then a polynomial logistic regression model was
built to come and correlations were assessed by the
above.
2 DATA SOURCE
The data was obtained from Kaggle. It is a
community that allows users to find and publish
datasets, explore and build models in a web-based
data science environment. It is also a community
where one can collaborate with other data scientists
and machine learning engineers as well as participate
in competitions to solve data science challenges. The
data was uploaded to Kaggle by John Davis and the
data was initially derived from an open-access paper
by Silvana Debernardi and a colleague in PLoS
medicine published on December 10, 2020.We
selected this secondary data based on the relevance of
the different biomarker measurements to our study
and the number of reported NAs. These clinical
specimens come from multiple centers, such as Barts
Pancreas Tissue Bank(BPTB), University College
London (UCL), University of Liverpool (LIV),
Spanish National Cancer Research Center (ESP), the
University of Cambridge Hospital, and the University
of Belgrade. All samples were collected prior to
surgery or chemotherapy treatment and were
potentially age and sex matched. The data including
a total of 590 biomarker panels tested on urine
samples, 332 of which were collected in 2013 by
Vanessa W and colleagues to study the association
The Investigation of the Correlation between Urine Biomarkers and Pancreatic Ductal Adenocarcinoma
319

between pancreatic ductal adenocarcinoma and
urinary metabolic features, and the latter 258 samples
were collected by Debernardi and colleagues
collected additional samples at the time of the study.
The majority of samples were from BPTB and LIV
with 409 and 132 samples, respectively, with the
remaining 8% of samples originating from other
centers. The first category was 183 control samples,
with no known pancreatic disease or malignancy
confirmed. The second category was benign disease
samples, which included 119 cases of chronic
pancreatitis, 54 cases of gallbladder disease, 20 cystic
lesions of the pancreas, and 15 cases with abdominal
pain and gastrointestinal symptoms, for a total of 208
samples. The remaining 199 were all PDAC patients.
The male to female ratio remained essentially 1:1 at
291 and 299 respectively, and the mean age of the
sample is 59.1 years old.
3 RESEARCH VARIABLES
The pancreas plays a vital role in exocrine function,
helping digest protein, cholesterol, and fat. Pancreatic
cancer can severely impair the normal function of the
pancreas. Therefore, we selected four biomarkers
from urine biomarkers that are closely related to
pancreas and used their values as independent
variables in this study. The following are the main
characteristics of the four urine biomarkers and they
are all continuous variables. Variable,creatinine, as
a urinary biomarker of kidney function, is a protein
often used as an indicator of kidney function. In
patients with PDAC, decreased protein digestion may
lead to increased urinary creatinine production.
Variable, lymphatic vessel endothelial hyaluronan
receptor 1 (LYVE1) is a protein that may play a role
in tumor metastasis, growing tumors may produce
large amounts of YVLE1 for cell metastasis.
Variable, REG1B stand for regenerating family
member 1 beta is a Protein Coding gene. It may be
associated with pancreas regeneration; damaged
pancreas tissue may release large amounts of REG1B
during regeneration. TFF1 is trefoil factor 1, serves
as a variable that may be associated with regeneration
and repair of the urinary tract. Increasing TFF1 in the
gastrointestinal mucosa can help repair the damaged
digestive tract. In addition, age and gender were also
independent variables in this study. Age was a
continuous variable and gender was used as a
categorical variable, with M for male and F for
female.
The dependent variable in the study is diagnosis,
which is a categorical variable. There are three
diagnostic classifications, 1 represents control (no
pancreatic disease), 2 stands for benign hepatobiliary
disease (119 of which are chronic pancreatitis), and 3
for pancreatic ductal adenocarcinoma, i.e., pancreatic
cancer. Through the correlation between these four
urine biomarkers and the patient's diagnosis and data
analysis, the early diagnosis of PDAC will be more
accurate.
Table 1: Summary of Data Collected, including the type of independent variable, the number of independent variables in the
dependent variable category, the mean, standard deviation and median, and the relative p-value of each independent variables.
Type no pancreatic
disease
(N=183)
benign
hepatobiliary
disease
(N=208)
Pancreatic ductal
adenocarcinoma
(N=199)
P-value
Age (years)
Mean (SD)
Median [Min, Max]
Continuous
56.3 (12.2)
57.0 [26.0, 89.0]
54.7 (13.3)
54.0 [26.0, 82.0]
66.2 (10.5)
67.0 [29.0, 88.0]
<0.001
Gender
Female
Male
Categorical
115 (62.8%)
68 (37.2%)
101 (48.6%)
107 (51.4%)
83 (41.7%)
116 (58.3%)
<0.001
Creatinine
Mean (SD)
Median [Min, Max]
Continuous
0.798 (0.559)
0.713 [0.0679,
3.45]
0.848 (0.616)
0.746 [0.0566,
3.34]
0.916 (0.724)
0.724 [0.0792,
4.12]
0.189
Lymphatic vessel
endothelial hyaluronan
receptor 1 (LYVE1)
Mean (SD)
Median [Min, Max]
Continuous
1.21 (1.92)
0.146 [0.000129,
8.32]
2.08 (2.37)
1.21 [0.000226,
11.0]
5.79 (3.78)
5.62 [0.00127,
23.9]
<0.001
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
320
Regenerating family
member 1 beta (REG1B)
Mean (SD)
Median [Min, Max]
Continuous
41.3 (61.9)
17.6 [0.00110,
544]
64.2 (116)
20.2 [0.00280,
864]
226 (277)
23 [1.65,
1400]
<0.001
Trefoil Factor 1 (TFF1)
Mean (SD)
Median [Min, Max]
Continuous
169 (278)
59.8 [0.00529,
1880]
448 (646)
210 [0.0132,
4460]
1150 (1430)
723 [0.0212,
13300]
<0.001
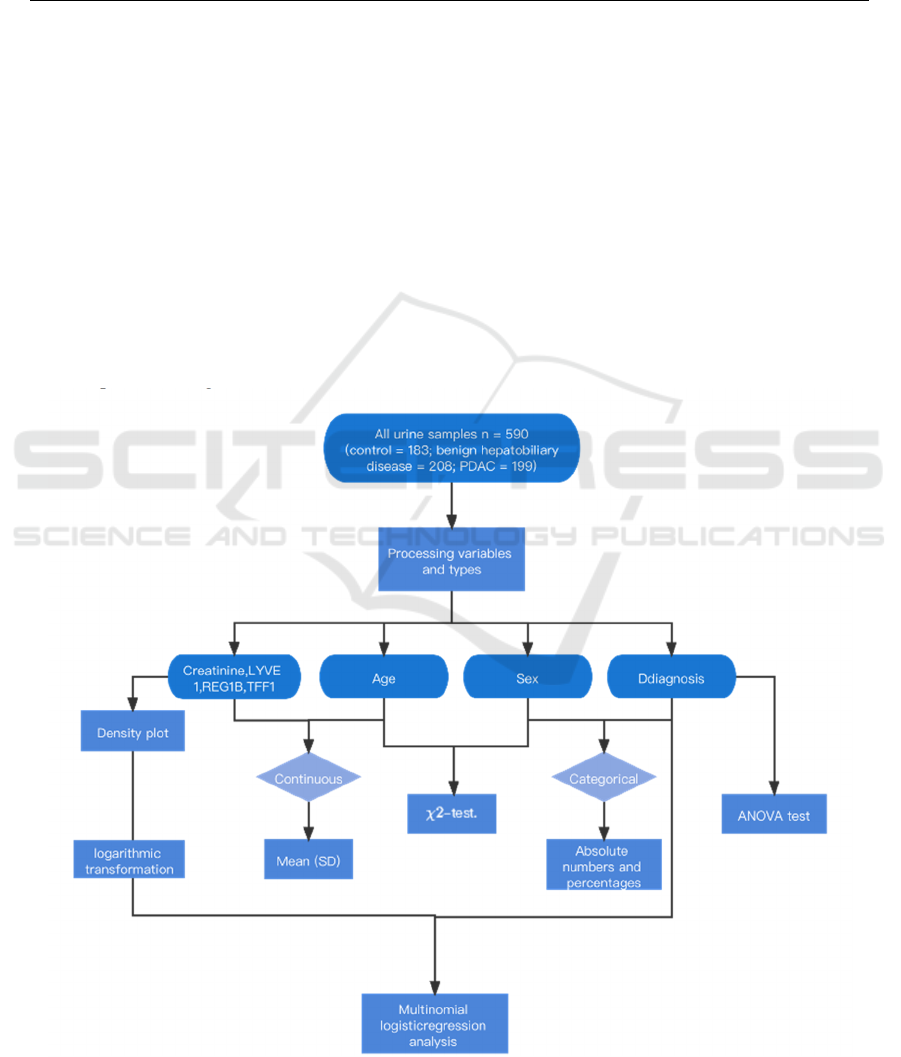
4 STATISTICAL ANALYSIS
We performed a preliminary processing of the data.
As shown in Table 1, continuous variables are
expressed as mean (SD), while categorical variables
are reported as absolute numbers and percentages.
Firstly, the correlation between the number of
pancreatic disease-healthy, benign hepatobiliary
disease-noncancerous and PDAC—pancreatic
cancer) is evaluated by ANOVA test while
categorical variables like age and sex are done by χ
2
-
test. The level of significance, α was set at 0.05.
Next, we produced density plots of biomarkers
and then we performed logarithmic transformation on
the four urine biomarkers. At last, we established a
multinomial logistic regression model to predict
outcomes as we have more than 2 categorical
outcomes (healthy, benign, PDAC) that cannot be put
into meaningful orders.
Multinomial Logistic regression analysis was
used to estimate the odds ratio (OR) and 95%
confidence interval (95% CIs) are calculated. A 2-
sided P-value less than 0.05 was considered
significant. Data management and statistical analyses
were performed using R, version 4.1.1.
Figure 1: The process of data analysis.
The Investigation of the Correlation between Urine Biomarkers and Pancreatic Ductal Adenocarcinoma
321
5 RESULTS
According to table-1, P-value for creatinine is 0.189,
which is higher than the level of significance. This
indicates that there is no significant statistical
correlation between levels of creatinine and diagnosis
(cancerous, non-cancerous condition, healthy).
Therefore, we will remove creatinine in further
studies and continue to investigate the correlation
between the remaining 5 independent variables and
diagnosis. The p-value for age, sex, LYVE1, REG1B,
and TFF1 all have p-value way smaller than the alpha
level, indicating there is a correlation between these
biomarkers and confounders and diagnosis.
LYVE1's mean and median are slightly different
while the other 2 biomarkers having a relatively large
mean median difference and SD, indicating that
LYVE1 is less likely to be affected by individual
differences. The large difference will be discussed
further in the discussion session.
We conclude that LYVE1 may be impacted more
by PDAC than the other 2, but since all 3 biomarkers
show statistical correlation with the diagnosis, we
should use 3 together when making predictions on a
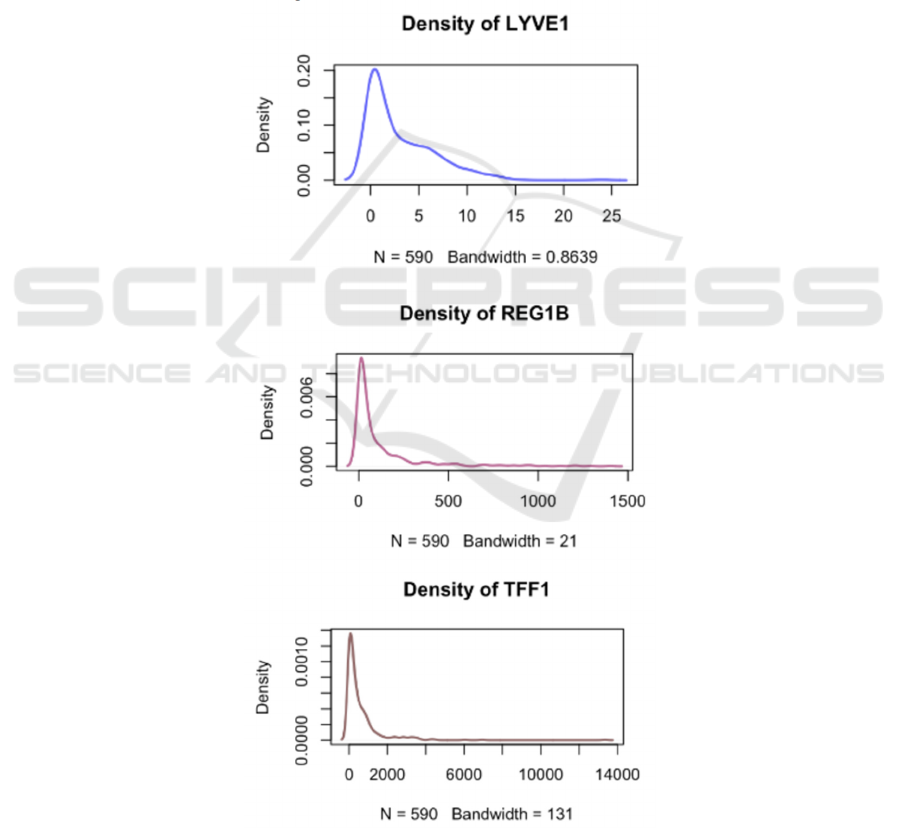
patient's health conditions. Through the density plot
of 3 urine biomarkers, it was found that the
distributions of the four urine biomarkers are all
lognormal distributions.
Figure 2: Diagrams of Density of 3 Urine Biomarkers.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
322
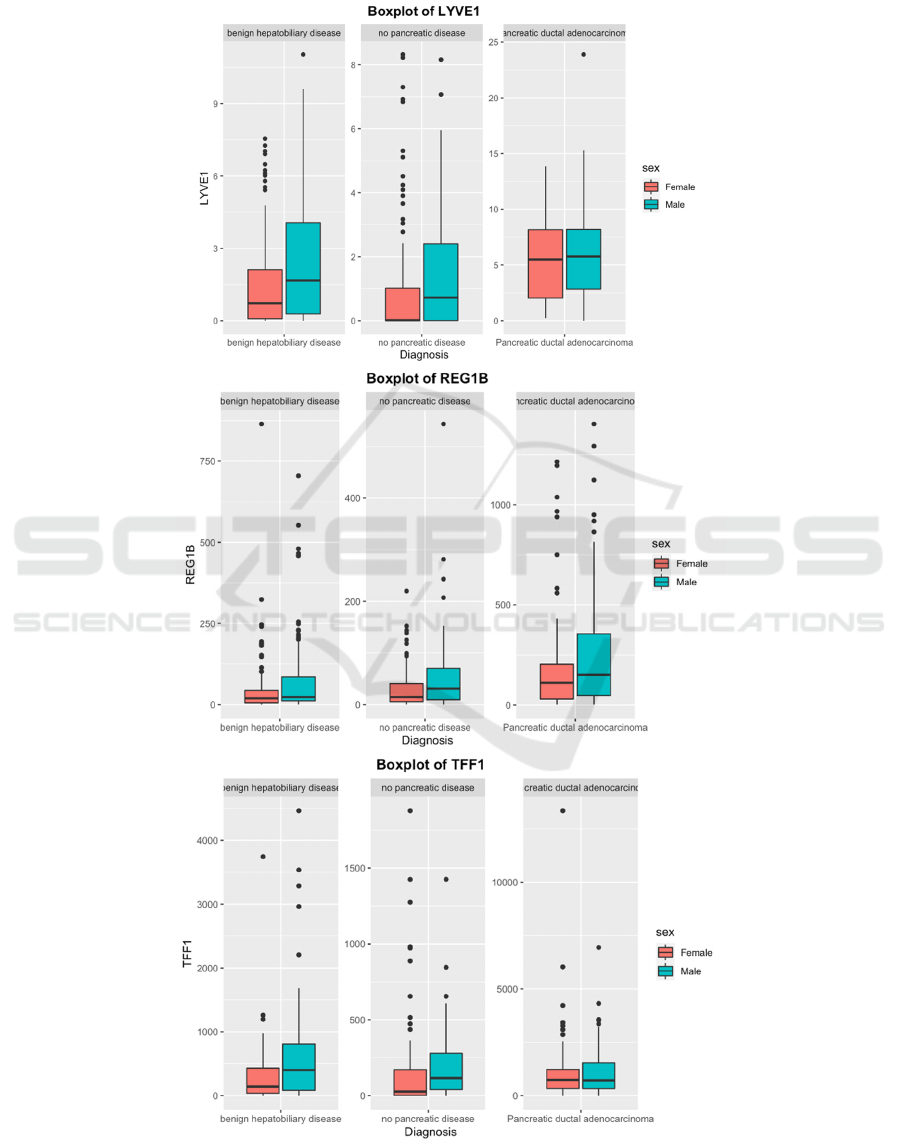
There are also a noticeable number of outliers for
samples under all 3 diagnoses, as can be seen among
3 box plots. Because we're investigating the possible
correlation between biomarkers and PDAC thus no
patients' samples, regardless of their underlying
health conditions, should be ignored.
Figure 3: Mean level of different urine biomarkers displayed by different sexes under 3 diagnoses.
The Investigation of the Correlation between Urine Biomarkers and Pancreatic Ductal Adenocarcinoma
323

As represented by TFF1 and REG1B in the box
plot (Figure 3), gender differences were shown under
the same diagnosis: male patients showed higher
levels of the 2 biomarkers than females, while both
sexes showed an increasing trend in the levels of the
2 biomarkers at the diagnosis of PDAC.
We also observed an increasing trend for LYVE1
when patients were diagnosed with PDAC. However,
unlike the other 2 biomarkers, the number of
extremes for this marker decreased when diagnosed
with PDAC.
We also noticed that for REG1B, dispersion of
data increased for both females and males when
diagnosed with PDAC while the dispersion for
creatinine remain similarly across diagnosis and
gender. Therefore, we assume that these data may not
follow a normal distribution and need to use a
different approach.
LYVE1 is also a significant indicator for health
(OR: 1.202, 95% CI: 1.099-1.315) and PDAC (OR:
2.418, 95% CI: 1.874-3.118). REG1B was identified
as a significant risk factor for health (OR: 0.834, 95%
CI: 0.716–0.973) and PDAC (OR: 1.247, 95% CI:
1.004–1.550). TFF1 was identified as a significant
risk factor for health (OR: 1.187, 95% CI: 1.080–
1.305) and PDAC (OR: 1.166, 95% CI: 1.013–1.343).
Formula 1: Summary of Multinomial Regression Model
Used.
6 DISCUSSION
Even though we concluded from our analysis on data
that LYVE 1 impacted most by PDAC, we should
still consider using all 3 biomarkers when analyzing
urine samples collected from clinics. The differences
of mean and standard deviation for REG1B and TFF1
among different diagnoses, according to several past
studies that also used urine biomarkers, were a likely
outcome of patients' other health conditions. Also, as
we discovered that the data obtained followed a log-
normal distribution, thus the extreme values we
thought to be outliers are normal under such
contribution.
LYVE1 itself was discovered to be a protein that
played a role in the autocrine regulation of cell
growth and tumor metastasis; in the meantime, the
other 2 biomarkers are more associated with other
organs and tissues. These urine biomarkers are not
unique to PDAC, thus could be affected by cancer or
disease. For instance, TFF1 is an indicator of urinary
canal's self-repair, but also present in normal breast
tissues; thus, situations like a male patient with
prostate carcinoma, a prostate cancer, near the male
urinary canal, may have a significantly higher level
of TFF1 than other patients. In the meantime, breast
cancer in women can also significantly increase the
TFF1 levels shown by a 2017 Japanese study. Serum
TFF1 and TFF3 but not TFF2 is higher in women
with breast cancer than in women without breast
cancer. Thus, the great differences in levels of TFF1
among individuals under the same pancreatic
diagnosis may not be due to PDAC we invested in but
other cancers not indicated in the study when samples
were collected. Therefore, the 3 biomarkers (L, R,
and T) should be analyzed together when testing and
predicting the diagnosis of PDAC for susceptible
patients in the clinic. Future studies could focus on
distinguishing between biomarkers or find
biomarkers that are uniquely correlated to PDAC
when making predictions in the clinics.
7 CONCLUSIONS
In summary, we performed secondary data analysis
with data obtained on Kaggle, including categorical
variables, chi-square test, and estimated dominance
ratio (OR) using multinomial logistic regression
analysis and calculated 95% confidence intervals
(95% CI). Ultimately, a correlation between the
number of biomarkers in urine (continuous variable)
and different diagnoses (no pancreatic disease health,
benign hepatobiliary disease non-cancer, and PDAC
pancreatic cancer) was successfully demonstrated
and urine biomarkers (LYVE1, REG1B, and TFF1)
could be used to screen for no pancreatic disease,
benign The urine biomarkers (LYVE1, REG1B, and
TFF1) can be used to screen for no pancreatic disease,
benign hepatobiliary disease, and pancreatic ductal
adenocarcinoma, cancer, providing a completely non-
invasive and convenient method for detecting PDAC.
REFERENCES
Abdou, A. G., Aiad, H. A., & Sultan, S. M. (2008). pS2
(TFF1) expression in prostate carcinoma: correlation
with steroid receptor status. APMIS: acta pathologica,
microbiologica, et immunologica Scandinavica,
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
324

116(11), 961–971. https://doi.org/10.1111/j.1600-
0463.2008.01009.x
Caputo, D., & Caracciolo, G. (2020). Nanoparticle-enabled
blood tests for early detection of pancreatic ductal
adenocarcinoma. Cancer letters, 470, 191–196.
https://doi.org/10.1016/j.canlet.2019.11.030
Davis, V. W., Schiller, D. E., Eurich, D., Bathe, O. F., &
Sawyer, M. B. (2013). Pancreatic ductal
adenocarcinoma is associated with a distinct urinary
metabolomic signature. Annals of surgical oncology,
20(3), S415–S423. https://doi.org/10.1245/s10434-
012-2686-7
Debernardi, S., O'Brien, H., Algahmdi, A. S., Malats, N.,
Stewart, G. D., Plješa-Ercegovac, M., Costello, E.,
Greenhalf, W., Saad, A., Roberts, R., Ney, A., Pereira,
S. P., Kocher, H. M., Duffy, S., Blyuss, O., &
Crnogorac-Jurcevic, T. (2020). A combination of
urinary biomarker panel and PancRISK score for earlier
detection of pancreatic cancer: A case-control study.
PLoS medicine, 17(12), e1003489.
https://doi.org/10.1371/journal.pmed.1003489
Ishibashi, Y., Ohtsu, H., Ikemura, M., Kikuchi, Y., Niwa,
T., Nishioka, K., Uchida, Y., Miura, H., Aikou, S.,
Gunji, T., Matsuhashi, N., Ohmoto, Y., Sasaki, T., Seto,
Y., Ogawa, T., Tada, K., & Nomura, S. (2017). Serum
TFF1 and TFF3 but not TFF2 are higher in women with
breast cancer than in women without breast cancer.
Scientific reports, 7(1), 4846.
https://doi.org/10.1038/s41598-017-05129-y
Li, J., Liu, F., Fang, X., Cao, K., Meng, Y., Zhang, H., Yu,
J., Feng, X., Li, Q., Liu, Y., Wang, L., Jiang, H., Shao,
C., Lu, J., & Bian, Y. (2021). CT Radiomics Features
in Differentiation of Focal-Type Autoimmune
Pancreatitis from Pancreatic Ductal Adenocarcinoma:
A Propensity Score Analysis. Academic radiology,
S1076-6332(21)00209-9. Advance online publication.
https://doi.org/10.1016/j.acra.2021.04.014
Mayerle, J., Kalthoff, H., Reszka, R., Kamlage, B., Peter,
E., Schniewind, B., González Maldonado, S., Pilarsky,
C., Heidecke, C. D., Schatz, P., Distler, M., Scheiber, J.
A., Mahajan, U. M., Weiss, F. U., Grützmann, R., &
Lerch, M. M. (2018). Metabolic biomarker signature to
differentiate pancreatic ductal adenocarcinoma from
chronic pancreatitis. Gut, 67(1), 128–137.
https://doi.org/10.1136/gutjnl-2016-312432
Nakamura, S., Sadakari, Y., Ohtsuka, T., Okayama, T.,
Nakashima, Y., Gotoh, Y., Saeki, K., Mori, Y., Nakata,
K., Miyasaka, Y., Onishi, H., Oda, Y., Goggins, M., &
Nakamura, M. (2019). Pancreatic Juice Exosomal
MicroRNAs as Biomarkers for Detection of Pancreatic
Ductal Adenocarcinoma. Annals of surgical oncology,
26(7), 2104–2111. https://doi.org/10.1245/s10434-
019-07269-z
Poruk, K. E., Gay, D. Z., Brown, K., Mulvihill, J. D.,
Boucher, K. M., Scaife, C. L., Firpo, M. A., &
Mulvihill, S. J. (2013). The clinical utility of CA 19-9
in pancreatic adenocarcinoma: diagnostic and
prognostic updates. Current molecular medicine, 13(3),
340–351.
https://doi.org/10.2174/1566524011313030003
Princivalle, A., Monasta, L., Butturini, G., Bassi, C., &
Perbellini, L. (2018). Pancreatic ductal
adenocarcinoma can be detected by analysis of volatile
organic compounds (VOCs) in alveolar air. BMC
cancer, 18(1), 529. https://doi.org/10.1186/s12885-
018-4452-0
Radon, T. P., Massat, N. J., Jones, R., Alrawashdeh, W.,
Dumartin, L., Ennis, D., Duffy, S. W., Kocher, H. M.,
Pereira, S. P., Guarner posthumous, L., Murta-
Nascimento, C., Real, F. X., Malats, N., Neoptolemos,
J., Costello, E., Greenhalf, W., Lemoine, N. R., &
Crnogorac-Jurcevic, T. (2015). Identification of a
Three-Biomarker Panel in Urine for Early Detection of
Pancreatic Adenocarcinoma. Clinical cancer research:
an official journal of the American Association for
Cancer Research, 21(15), 3512–3521.
https://doi.org/10.1158/1078-0432.CCR-14-2467
Sahni, S., Pandya, A. R., Hadden, W. J., Nahm, C. B.,
Maloney, S., Cook, V., Toft, J. A., Wilkinson-White,
L., Gill, A. J., Samra, J. S., Dona, A., & Mittal, A.
(2021). A unique urinary metabolomic signature for the
detection of pancreatic ductal adenocarcinoma.
International Journal of Cancer, 148(6), 1508.
https://doi.org/10.1002/ijc.33368
Seifert, A. M., Reiche, C., Heiduk, M., Tannert, A.,
Meinecke, A. C., Baier, S., von Renesse, J., Kahlert, C.,
Distler, M., Welsch, T., Reissfelder, C., Aust, D. E.,
Miller, G., Weitz, J., & Seifert, L. (2020). Detection of
pancreatic ductal adenocarcinoma with galectin-9
serum levels. Oncogene, 39(15), 3102–3113.
https://doi.org/10.1038/s41388-020-1186-7
Yu, S., Li, Y., Liao, Z., Wang, Z., Wang, Z., Li, Y., Qian,
L., Zhao, J., Zong, H., Kang, B., Zou, W. B., Chen, K.,
He, X., Meng, Z., Chen, Z., Huang, S., & Wang, P.
(2020). Plasma extracellular vesicle long RNA
profiling identifies a diagnostic signature for the
detection of pancreatic ductal adenocarcinoma. Gut,
69(3), 540–550. https://doi.org/10.1136/gutjnl-2019-
318860
The Investigation of the Correlation between Urine Biomarkers and Pancreatic Ductal Adenocarcinoma
325
