
Oral Administration of Bovine Blood Peptide Generated No Adverse
Effect on Healthy Rats
Zhenghao Bao
1a
, Yuhao Zhang
1b
, Ao Wang
1c
, He Huang
1d
, Yuexin Xu
2e
, Hongpeng He
1, * f
,
Aqin Wang
3,* g
and Jun Yu
3,* h
1
Key Laboratory of Industrial Microbiology, Ministry of Education and Tianjin City, State Key Laboratory of Food
Nutrition and Safety, College of Biotechnology, Tianjin University of Science and Technology, 300457, Tianjin, China
2
Department of Pathology, Mentougou Hospital in Beijing, 102300, Beijing, China
3
Hangzhou BIBAU Biotechnology Co. Ltd, 310016, Hangzhou, China
Keywords: IDA, Rat, Bovine Blood Peptide, Heme Iron, Iron Supplement.
Abstract: IDA (iron deficiency anemia) is a disease with high incidence in many countries. Inorganic iron supplements,
typically ferrous sulfate, are widely utilized in the prevention and treatment of IDA. However, the
bioavailability of inorganic iron is only 2% to 20%. In addition, the gastrointestinal side effects occur
frequently. Heme iron which is isolated from animal blood is a promising choice for IDA patients. Before
extensive utilization of heme iron in clinic, safety evaluation is indispensable. In this study, with untreated
rats and ferrous sulfate-treated rats as controls, bovine blood peptide which carries heme iron was applied to
normal female rats. After 30 days of gavage feeding, no significant difference in body weight and organ
coefficient was observed. Serological examination revealed that oral administration of bovine blood peptide
did not disrupt iron metabolism nor caused adverse effects on liver and kidney functions. Pathological HE
staining of gastrointestinal tract showed that bovine blood peptide induced much less inflammatory irritation
than ferrous sulfate. These results suggest that bovine blood peptide is a kind of safe and reliable organic iron
supplement for IDA patients.
1 INTRODUCTION
Iron is one of the trace elements needed by the human
body. Due to some congenital or acquired factors, the
amount of stored iron in the body is too low to support
the synthesis of functional iron (hemoglobin, etc.)
and consequently resulted in iron deficiency anemia
(IDA) (Lin 2013). The pathogenic factors of IDA
include excessive iron loss, iron utilization disorder,
iron uptake deficiency, iron malabsorption, iron
transport disorder (Chen 2013). As reported, about
one billion people worldwide have some form of iron
deficiency, and more than half of them have
developed IDA (WHO 2001). The WHO report in
2011 mentioned that the prevalence of IDA in
children in China was 7.8% (WHO 2011). The
a
https://orcid.org/0000-0001-6267-0622
b
https://orcid.org/0000-0003-0693-7622
c
https://orcid.org/0000-0001-9147-8760
d
https://orcid.org/0000-0001-7660-4116
prevalence of IDA is more than 20% in women
(Zhang 2010). Generally, Infants, growing children
and women of childbearing age are vulnerable to IDA
(Liu 2012, Dalhøj 1991). Iron deficiency not only
leads to decreased red blood cells but also weaken the
activity of iron-containing enzyme in cells and
triggers clinical symptoms of IDA (Ge 2013).
Supplementation of iron increases the level of
hemoglobin (Hb). At present, there are two types of
iron supplements for IDA treatment (Ulas 2010). Oral
ferrous sulfate is the main drug used in clinical
treatment of IDA because of its low price and
significant effect. But side effects, such as irritation
of the gastrointestinal tract, constipation, or diarrhea
cannot be ignored. In addition, it has low
bioavailability (He 1995). Studies have shown that
e
https://orcid.org/0000-0002-2361-8597
f
https://orcid.org/0000-0002-5117-1091
g
https://orcid.org/0000-0002-4800-7637
h
https://orcid.org/0000-0002-0359-9210
Bao, Z., Zhang, Y., Wang, A., Huang, H., Xu, Y., He, H., Wang, A. and Yu, J.
Oral Administration of Bovine Blood Peptide Generated No Adverse Effect on Healthy Rats.
DOI: 10.5220/0011368100003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 313-317
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
313
excessive iron intake can cause changes in intestinal
microbiota and oxidative stress injury in the body
(Alexeev 2017, Zhang 2017).
The most abundant protein in blood is
hemoglobin which can be purified and processed to
produce heme iron supplements. Heme iron is better
than inorganic iron. Bovine blood peptide also
provides human body with useful peptides and amino
acids. Therefore, it is a potential substitute of ferrous
sulfate. The advantages of blood peptide and heme
iron have been extensively reported while the safety
and reliability remain to be further clarified.
This study aimed to evaluate the safety of bovine
blood peptide in normal female rats. Results of the
present study will provide useful information for the
clinical application of bovine blood peptide in IDA
treatment.
2 MATERIALS AND METHODS
2.1 Animals and Reagents
Forty-eight female SD rats with body weight between
60-80g (purchased from SiPeiFu, Beijing, China)
were randomly divided into 6 groups, namely control
group, bovine blood peptide low-dose group (2
mg/kg/d), bovine blood peptide medium dose group
(20 mg/kg/d), bovine blood peptide high-dose group
(100 mg/kg/d), ferrous sulfate low-dose group(100
mg/kg/d) and ferrous sulfate high-dose group(200
mg/kg/d). Bovine blood peptide powder and ferrous
sulfate tablets were provided by BIBAU, Hangzhou,
China. After adaptive feeding for 5 days, rats were
continuously feed with different iron supplements as
indicated by gavage for 30 days.
2.2 Measurement of Blood
After gavage administration, rats were sacrificed and
blood was collected for the measurement of liver and
kidney functions with biochemical or ELISA kits
(from Jiancheng, Nanjing, China) on an automatic
biochemical analyzer.
2.3 Pathological Examination
Stomach tissues were collected, embedded with
paraffin and then sliced for HE staining to examine
tissue damage and the infiltration of inflammatory
cells.
2.4 Statistical Analysis
All data were expressed as mean ± standard
deviation. Prism 8.0 was used for statistical analysis.
One-way analysis of variance (ANOVA) with P <
0.05 was used to consider the difference statistically
significant.
3 RESULTS
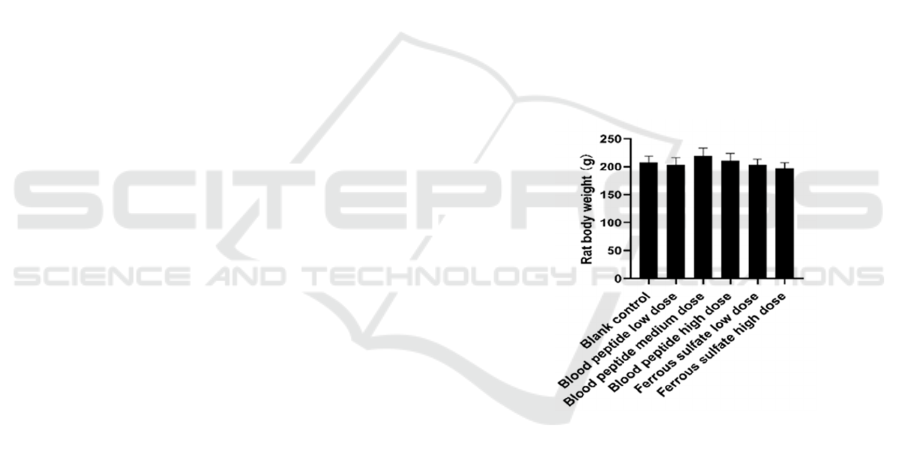
3.1 Body Weight of Rats Was Not
Affected
The body weight of rats after 30 days of iron
supplementation was shown in Fig. 1. At the end of
the experiment, the body weight of rats in each group
was about 200g, and there was no significant
difference among different groups (P > 0.05). The
results showed that bovine blood peptide did not
affect the normal growth of rats, which was consistent
with the results of traditional iron supplementation
agent ferrous sulfate.
Figure 1: Rat body weight post 30-day feeding.
3.2 Liver and Kidney Organ
Coefficients and Functions Were
Unaffected
To evaluate the safety of bovine blood peptide, size
of liver and kidney was measured. As shown in Table
1, compared with the blank control, liver and kidney
organ coefficients of various experimental groups
were not significantly different (P > 0.05). By
detecting ALT, bilirubin and Glo (Figure 2), it was
found that there was no significant difference in liver
function in each group supplemented with iron (P >
0.05). The results showed that the functions of liver
and kidney were not affected by bovine blood peptide
or ferrous sulfate.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
314
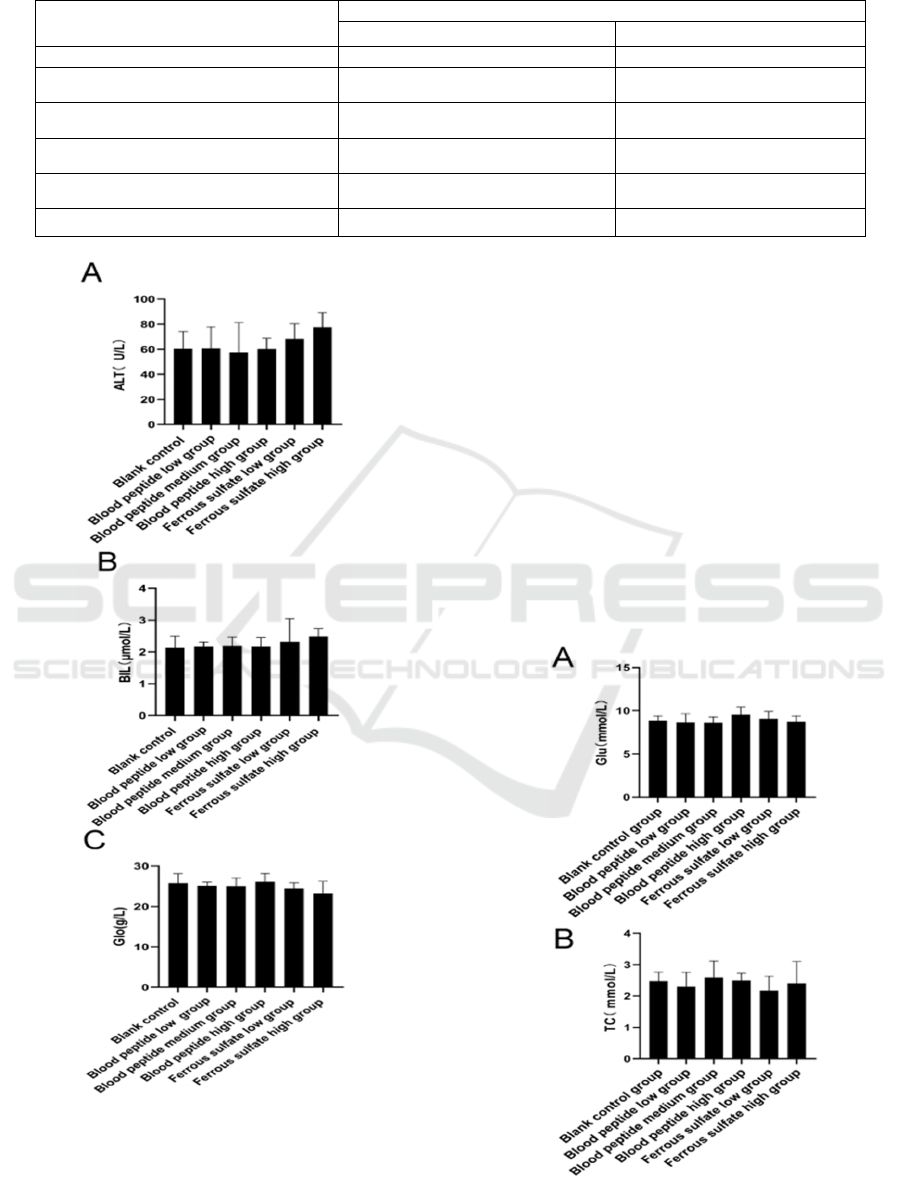
Table 1: Organ coefficient of rats fed with iron supplements.
Group Or
g
an coefficient
(
g
/100
g
)
, mean ± SD
liver kidney
Blank control group 4.67±0.47 0.57±0.06
Blood peptide low dose group 4.92±0.25 0.69±0.03
Blood peptide medium dose group 4.57±0.33 0.71±0.08
Blood peptide high dose group 4.76±0.41 0.58±0.05
Ferrous sulfate low dose group 4.87±0.27 0.51±0.08
Ferrous sulfate high dose group 4.75±0.14 0.46±0.01
A. Alanine aminotransferase, B. total bilirubin, C. globulins
Figure 2: Rat liver function.
3.3 Glycolipid Metabolism Were Not
Affected by Iron Supplements
Bovine blood peptide used in this study was a mixture
of peptide in different length prepared from blood
protein digestion. It was previously reported that
some peptide might affect blood glucose. To test
whether the blood glucose and lipid of normal rats
altered after ingestion of bovine blood peptide, rat
sera were examined. There was no significant
difference in serum glucose, total cholesterol and
triglyceride levels among groups supplemented with
iron and the control group (Figure. 3) (P > 0.05). The
results showed that bovine blood peptide did not
affect the glycolipid metabolism of normal rats after
intragastric administration.
Oral Administration of Bovine Blood Peptide Generated No Adverse Effect on Healthy Rats
315
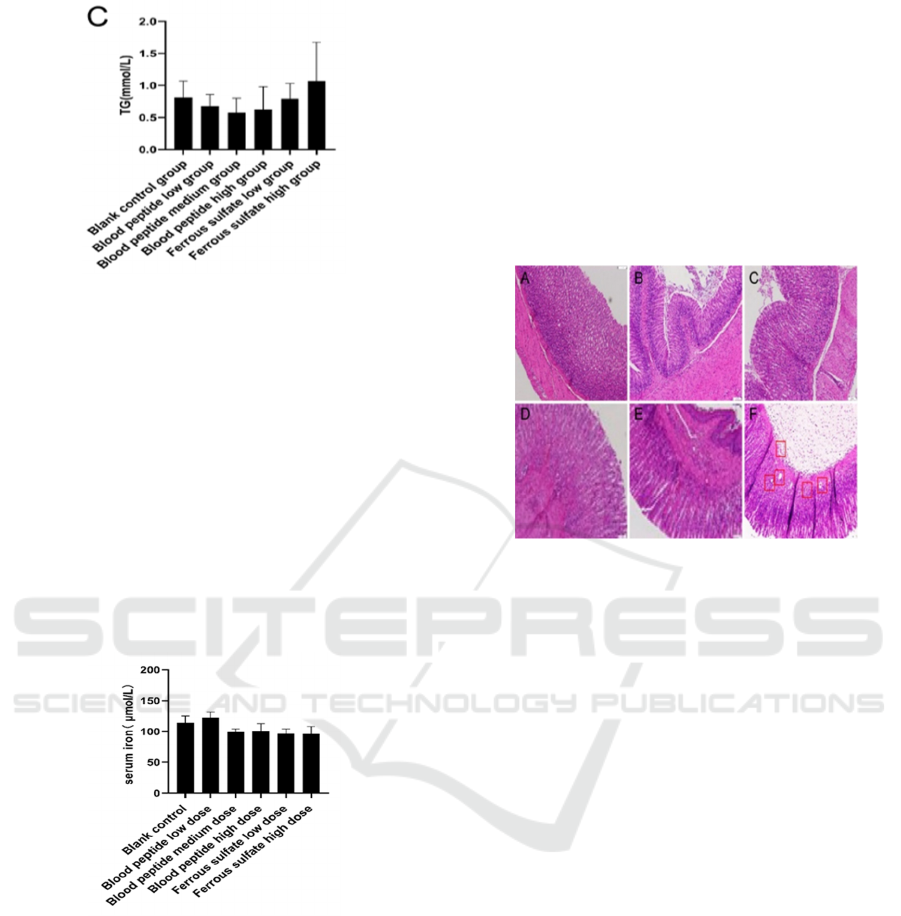
A. glucose, B. total cholesterol, C. triglyceride
Figure 3: Rat Glucose and Lipid Metabolism Index
3.4 Iron Metabolism Was Not Affected
by Iron Supplements
To test whether bovine blood peptide would increase
the iron concentration in serum in normal rats, serum
iron was determined. As show in Figure 4, there was
no difference in serum iron concentration among
different groups (P > 0.05), and there was no
significant dose-response relationship. The results
indicate that the supplementation of blood peptide
iron or ferrous sulfate did not significantly change the
status of iron metabolism in healthy rats fed normal
diet.
Figure 4: The serum iron content of rats in different groups.
3.5 Bovine Blood Peptide Caused Less
Gastrointestinal Side Effects than
Ferrous Sulfate
A major disadvantage of ferrous sulfate is severe
gastrointestinal side effects after taken orally. To
determine the effects of iron supplements on
gastrointestinal tract, HE staining was performed.
Compared with the normal control group, the
number and distribution of inflammatory cells in the
low dose and medium dose blood peptide groups were
not obvious (Figure 5). In the high-dose group, the
mild inflammatory reaction was mainly in the mucosa
layer, and there was no increase of inflammatory cells
in the submucosa. Whereas gastric tissues from low
and high dose ferrous sulfate groups displayed
infiltration of neutrophils and lymphocytes in the
mucous and the submucosa layers. In ferrous sulfate
high dose group, interstitial edema and vascular
dilatation and congestion were observed. These results
demonstrated that bovine blood peptide is less
irritating to the gastrointestinal tract than ferrous
sulfate.
Figure 5: Changes in the stomach tissue in rats (HE
staining, 100×): (A) blank control; (B) blood peptide low
dose; (C) blood peptide medium dose; (D) blood peptide
high dose; (E) ferrous sulfate low dose; (F) ferrous sulfate
high.
4 CONCLUSIONS
In this study, the body weight, liver and kidney
function, liver and kidney size, glucose and lipid
metabolism, and iron metabolism of normal rats were
not significantly affected by the supplementation of
bovine blood peptide or ferrous sulfate. Different
from ferrous sulfate, bovine blood peptide didn’t lead
to obvious inflammatory change in gastrointestinal
mucosa. Histological examination demonstrated that
compared with ferrous sulfate, bovine blood peptide
is a type of safer iron supplement for the treatment of
IDA and can avoid the gastrointestinal irritation
caused by inorganic iron supplements.
CONFLICT OF INTEREST
We have no conflicts of interest to disclose.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
316

REFERENCES
Alexeev, E. E., He, X., Slupsky, C. M., & Lnnerdal, B..
(2017). Effects of iron supplementation on growth, gut
microbiota, metabolomics and cognitive development
of rat pups. Plos One, 12(6), e0179713.
Chen H.Z, G.W. Lin, J.Y. Wang. (2013), Practical Internal
Medicine (People's Health Publishing House, Beijing).
Dalhøj J, Wiggers P. (1991) Haemoglobinkoncentration og
jerndepoter hos kvindelige bloddonorer [Hemoglobin
concentration and iron stores in female blood donors].
Ugeskr Laeger. 1991;153(9):643-645.
Ge, J. B., Y.J. Xu. (2013). Internal medicine (People's
Health Publishing House, Beijing).
He, F. P. . (1995). Treatment of 58 cases of iron deficiency
anemia with low dose ferrous sulfate. Clinical Focus,
10, 552-553.
Lin G.W. (2013), Modern clinical hematology (Fudan
University Press, Shanghai).
Liu, K., & Kaffes, A. J. . (2012). Iron deficiency anaemia:
a review of diagnosis, investigation and management.
European Journal of Gastroenterology & Hepatology,
24(2), 109-116.
Ulas, D, Bayraktar, Soley, Bayraktar, & Division, et al.
(2010). Treatment of iron deficiency anemia associated
with gastrointestinal tract diseases. World Journal of
Gastroenterology.
WHO. (2001). Iron deficiency anemia: assessment
prevention and control. A guide for programme
managers. Geneva Switzerland Who, 21,42.
WHO. (2011). The global prevalence of anaemia in 2011.
Geneva Switzerland Who, 126, 5409-5418.
Zhang Y, Y.M. Hu. (2010) Experimental study on the effect
of heme iron on iron deficiency anemia in rats. Practical
Preventive Medicine, 12, 2503-2504.
Zhang, Z. N., T. Shen. (2017). Diagnostic and therapeutic
criteria of hematological diseases (Science Press,
Beijing).
Oral Administration of Bovine Blood Peptide Generated No Adverse Effect on Healthy Rats
317
