
ECG Circuit: Analyzation and Application
Wenjun Ge
1,† a
, Junyang Sui
2,*,† b
and Pinzhang Wu
3,† c
1
Electrial and Mechanical Engineering, Jilin Institute of Chemical Technology, Jilin, Jilin, 132000, China
2
Bell Honors School, Nanjing University of Posts and Telecommunications, Nanjing, Jiangsu, 210000, China
3
Youpei College, Yancheng Institute of Technology, Yancheng, Jiangsu, 224007, China
*
Corresponding author
†
These authors contributed equally
Keywords: Electrocardiogram (ECG) Circuit, ECG Monitoring, Wearable ECG Sensors, Heart Rate Variability,
Wearable Devices.
Abstract: Cardiovascular disease has become the most common cause of death worldwide. According to the 2013
Global Burden of Disease Study, cardiovascular diseases are estimated to cause 17.3 million deaths
worldwide. As the primary technique for monitoring the heart's activity, the ECG plays an irreplaceable role
in the management of heart disease. Currently, there are two main types of devices used to measure ECGs.
One is the larger devices used in hospitals, such as medical ECG machines, and the other is smaller devices
for home use, mainly wearable devices such as the Samsung Galaxy Watch and Apple Watch. Compared to
traditional devices used in hospitals, wearable devices offer the advantages of small size, low energy
consumption and portability. In the future, lower noise, greater noise immunity, lower energy consumption
and higher ECG accuracy are necessary for developing wearable devices in the field. However, there are still
many difficulties at present. For example, as smart wearables need to minimize the device's size, the power
supply circuitry is also somewhat limited, and the device's battery life becomes a major issue. This review
introduces the application of ECG monitoring devices on wearable devices, introduces different wearable
ECG devices, and analyzes the performance of wearable ECG devices, points out the gap between current
wearable ECG and large medical ECG monitoring devices, analyses the causes of wearable ECG noise
generation, and proposes to reduce wearable ECG from electrodes, circuitry and other aspects monitoring and
improve the monitoring accuracy. Finally, the review summarizes the gap between wearable ECG and
medical-grade ECG monitoring devices, predicts the future challenges of wearable ECG, and expresses an
outlook on the future of wearable ECG devices.
1 INTRODUCTION
Cardiovascular diseases (CVD) have become the
most common cause of death worldwide. According
to the 2013 Global Burden of Disease study
estimation, CVD caused 17.3 million deaths globally.
CVD replaces 31.5% of all deaths and 45% of all non-
communicable disease deaths, more than twice that
caused by cancer, as well as more than all
communicable, maternal, neonatal, and nutritional
disorders combined (Townsend, Wilson, Bhatnagar,
Wickramasinghe, Rayner and Nichols ,2016)
Electrocardiogram (ECG), as the main technique for
a
https://orcid.org/0000-0002-5944-3215
b
https://orcid.org/0000-0001-7064-3659
monitoring cardiac activity, plays an irreplaceable
role in treating cardiac diseases. ECG diagnosis is one
of the most reliable methods for treating arrhythmia,
and it has great application value (R., B., and K. D.
2005). Currently, the devices that measure the ECG
are divided into two main categories. The one is the
large devices used in the hospital such as medical
ECG monitor, and the another is the home devices
that measure the ECG mainly with wearable devices
such as Galaxy Watch and Apple Watch.
The devices that measure the ECG are constantly
evolving with the development of technology.
Compared with conventional devices applied in
hospitals, the wearable takes advantage of small-size,
c
https://orcid.org/0000-0002-5511-0560
Ge, W., Sui, J. and Wu, P.
ECG Circuit: Analyzation and Application.
DOI: 10.5220/0011367500003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 283-290
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
283

low energy consumption, portable, etc. In the future,
Lower noise, stronger anti-noise ability, less energy
consumption and higher accuracy of an
electrocardiogram are necessary for wearable device
development. However, there are still many
difficulties remaining. For example, because smart
wearable devices need to minimize the device's size
and the power supply circuit is also limited to a
certain extent, the device's battery life has also
become an important issue.
This paper summarizes the circuit used in
wearable devices which measure the ECG, including
the comparison with the devices applied in hospital,
as well as analysis of the typical ECG amplify circuit,
describing different types of ECG electrode, listing
some parameters about op-amp design, and the
current application of wearable devices which can
measure the ECG. Finally, the existing problems in
ECG research are discussed, and the future
development direction has prospected to bring some
references for related research.
2 THE APPLICATION OF ECG
CIRCUITS IN WEARABLE
DEVICES
The traditional medical ECG monitoring equipment
applied in hospitals (see Fig.1) usually uses the
common technique of 12-leads ECG to perform heart
analysis. It records heart electrical activity through
electrodes on the body surface and represents it into
a grid.
Figure 1: Medical ECG monitoring equipment and dry
electrodes used in traditional ECG monitoring.
Indeed, this technique offers different views of
heart electrical activity, allowing cardiologists to gain
a full, complete view of the patient heart, so some
anomalies of the ECG record symbolize pathologies,
can be easily detected, and lots of deaths prevented.
But this approach has two main drawbacks: one is
that patients and physicians should be in the same
place together with the electrocardiograph, which
means it cannot be detected anytime and anywhere.
As a result, this approach can’t predict and escape
some heart-related complications, such as cardiac
arrest, irregular heartbeat, congestive heart failure,
coronary artery disease, etc. Another is the traditional
disposable dry electrode which is often used for ECG
monitoring in hospitals at present (see Fig.1)
(
Beniczky, Conradsen, Henning 2005). The
conductive glue and other substances contained in the
dry electrode will penetrate the skin of patients,
which will cause skin allergies and other adverse
reactions in some patients. At the same time, using
the dry electrode for a long time will cause the
electrode strip to fall off and poor contact due to the
drying of conductive adhesive, so the measured ECG
signal will not be accurate (
Zhang, Bai, Zhou
1997
).
In the current year, wearable devices have
suddenly appeared on people's horizons. Compared
with large and heavy equipment in the hospital,
wearable devices take advantage of low energy
consumption, portability, and higher accuracy of
electrocardiogram, so people start to pay attention to
applying the wearable devices to monitor ECG. The
ECG circuits are the key portions of wearable
devices.
2.1 The Measurement Principle of
ECG Circuits in Wearable Devices
The biological electric change of the heart itself
passes the conductive organization and humoral fluid
around the heart, reflecting on coming up to the body
surface. It makes each part of the body also produce
regular electric change activity in each cardiac cycle.
ECG is a technology that uses an ECG circuit to
record the heart's electrical activity produced by each
cardiac cycle of the graph from the body surface.
2.2 The ECG Circuits in Wearable
Devices
2.2.1 A Wearable ECG Acquisition System
with Circuit Board-based Shirt
Fig.2 is a wearable ECG acquisition system based on
a planar-fashionable circuit board (P-FCB)-based
shirt. The system removes cumbersome wires from
the traditional Holter monitor system for
convenience. Dry electrode screen printing directly
on the fabric allows long-term monitoring without
skin irritation. The ECG monitor shirt uses a monitor
chip with a set of electrodes around the body.
Electrodes and interconnects are implemented using
P-FCB to enhance wearability and reduce production
costs (
Yoo, Yan, and Lee 2009).
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
284

Figure 2: (a) Clinical ECG monitoring system. (b)
Conventional Holter monitor system. (c) Proposed
ECG monitoring shirt with P-FCB electrodes.
Fig.3 shows the structure of the ECG monitoring
chip. It consists of an amplifier (1A) with a
programmable gain amplifier, a 10 b SAR ADC, a
compression accelerator, an AES-128 encryption
accelerator, an internal memory, an MCU, I/O
interface, and a pair of P-FCB electrodes. The
electrode is data compressed using a secondary
compression accelerator (
Randazzo, Ferretti, and
Pasero 2019
). Then encrypted and stored in internal
memory. To improve safety, the AES-128 accelerator
is used (
Kim and Yoo 2008). If the internal memory
is full, external memory can be used through the
external memory interface. Internal memory is
accessed through the fast-mode 𝐼
𝐶 interface.
Figure 3: ECG monitoring chip architecture.
2.2.2 ECG WATCH
The ECG WATCH (Fig.4) is developed for standard
techniques such as 12-lead electrocardiography
(ECG) or dynamic electrocardiogram systems that
are insufficient to fully resolve sporadic ECG
abnormalities such as atrial fibrillation. It is an
inexpensive, wearable, easy-to-use health device that
can monitor the heart activity of patients with
cardiovascular disease anytime and anywhere
without the need to go to the hospital or cardiologists.
The recording takes 10 seconds. It also embedded an
algorithm to detect possible atrial fibrillation
episodes.
Figure 4: The ECG WATCH.
Fig.5 shows the ECG WATCH PCB. A TI
MSP430 series low-power microcontroller collects
the ECG signal with a 10b 200kbps SAR ADC. ECG
signals are collected at 1kbps, which are sufficient to
obtain good temporal resolution. The external voltage
reference provides an accurate DC reference voltage
for the ADC. In order to identify the risk of atrial
fibrillation, a 10-second ECG acquisition is sufficient
for the implementation of the algorithm. On the other
hand, the flash memory on the microcontroller has
enough space to store a few seconds of collected data
on the board at 1kbps, eliminating the need for
external storage modules and thus reducing the size
of the circuit (Kim, Kim, and Yoo 2008).
Figure 5: ECG WATCH PCB.
2.2.3 Wearable Mobile Ear-based ECG
Monitoring Device
This work presents the design and evaluation of a
wearable mobile ear-based ECG monitoring system
based on a highly conductive material graphene
electrode. Smartphones and headphones are
ECG Circuit: Analyzation and Application
285
becoming more common across generations. Here, a
novel design aims to advance the development of an
ear-based graphene sensor that, via a mobile
connection, generates high-quality, long-term, real-
time ECG measurements in a system more familiar to
the end user.
A typical ECG monitoring system consists of
electrodes and a front-end data acquisition circuit. A
three-electrode system is used to collect ECG signals.
In a three-electrode system, two active electrodes are
used for differential input to the ECG amplifier. The
third electrode is connected to eliminate common-
mode interference and improve signal quality. In the
proposed work, two electrodes are placed near the
ear-the one is attached behind the ear, the other is
placed on the neck, and a third electrode is connected
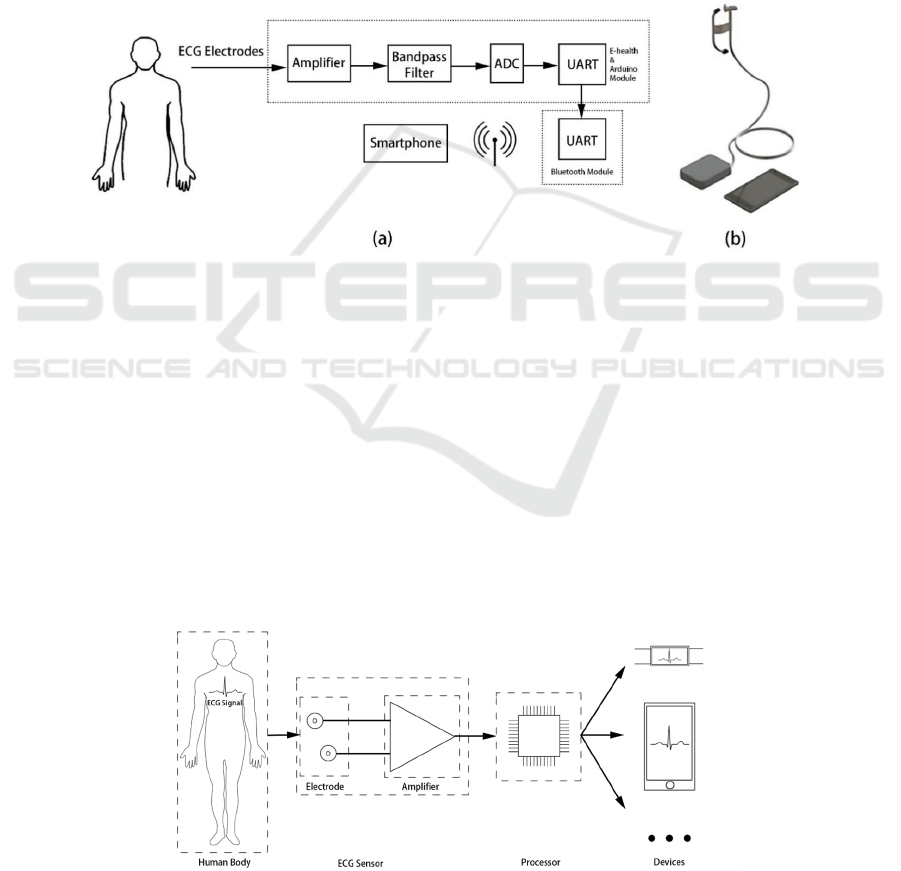
to the arm on the ground to obtain ECG signals, as
shown in Fig.6. a. The electrodes are tested in a
developed wearable device (see Fig.6b) that sends the
raw ECG signal from an ear-based electrode to a
measurement circuit mounted on the arm. The ECG
data is digitized, amplified, filtered in the
measurement circuit, and then transmitted to the
smartphone through Bluetooth for continuous
monitoring (
Celik, Balachandran, and
Manivannan 2019
).
Figure 6: (a) Electrode placement and block diagram of the ECG monitoring system; (b) Prototype design of ear-based ECG
monitoring system.
3 METHODS TO OPTIMIZE THE
PERFORMANCE OF ECG
SENSOR
In the ECG monitoring of wearable devices, the
accuracy of ECG signal detection for the wearer has
become an important index to measure the
performance of wearable ECG monitoring devices,
which is one of the main reasons why wearable ECG
monitoring devices cannot replace medical-grade
ECG monitoring devices at present. For well-known
reasons, the interference of ECG signals collected by
wearable devices is significantly greater than that of
medical-grade ECG monitoring devices.
The basic structure of major wearable ECG
designs is shown in Fig.7. To detect the ECG signal
from the human body, the system needs a sensor that
consists of 2 parts (electrode and amplifier). The
sensor can get the ECG signal and convert it to an
electronic signal that the signal processor can
process.
Figure 7: The basic structure of major wearable ECG designs.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
286
Every step of these processes causes problems
(consuming power, generating noise etc.),
influencing the system's performance. Therefore, it is
important to find the methods to optimize the
performance of the ECG sensor.
3.1 Electrodes of Wearable ECG
Equipment
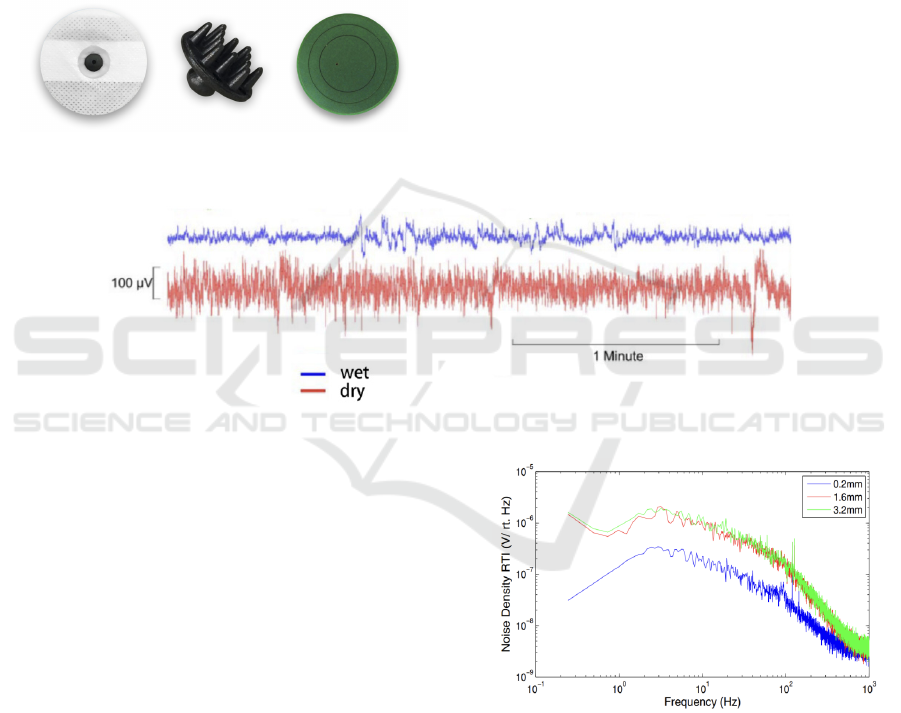
Fig.8 shows various bio electrodes: wet electrode, dry
electrode, non-contact electrode, which are widely
used in ECG equipment.
Figure 8: Some ECG electrodes.
Wet electrodes are a kind of electrode of common
ECG equipment used by hospitals because it uses gel
to keep good contact of skin to make sure the signal
is good. However, most wearable ECG sensors do not
use this kind of electrode. The reason is simple:
Wearable devices should be comfortable and
portable. But the gel of wet electrodes is
inconvenient. People feel bad when putting gel on
them, and it is hard to replace gel after old gel
becomes useless.
The dry electrode is a replacement for the wet
electrode. They do not need gel to keep the
connection between electrode and skin at the expense
of the performance. Fig.9 shows the noise of a wet
electrode and a dry electrode. We can find that dry
electrodes generate more noise than wet electrodes,
which means the result of dry electrodes is more
imprecise.
Figure 9: The noise of a wet electrode (blue) and a dry electrode (red).
Meanwhile, using dry electrodes is
uncomfortable, too. People need pressure on the
electrode and skin to keep the electrode on the skin,
which causes a strong pressing feeling. Using a non-
contact electrode can solve this problem. However, it
brings some problems: Firstly, non-contact electrodes
generate more noise than wet and dry electrodes,
making a huge challenge in the amplifier design.
Secondly, it is hard to keep the distance between
electrodes and skin, especially using wearable
devices like smartphones or T-short sensors. As a
result, the distance of the electrode will change
rapidly and causes more noise. Fig.10 shows the
effect of sensor separation distance on input-referred
noise of an ECG device. When the distance between
the electrode and skin increases, the input-referred
noise increases.
Figure 10: The effect of sensor separation distance on
input-referred noise of an ECG device (Brain Support.
2020).
In conclusion, every electrode has some
advantages and disadvantages. And a better user-
friendliness electrode has lesser accuracy. So, it is
important to select an appropriate electrode by
considering all working situations of the design.
ECG Circuit: Analyzation and Application
287
3.2 Typical ECG Amplify Circuit
Table 1 is the comparison of some parameters of the
ECG signal. The voltage range of the ECG signal is
0.5-4 mV. It is too small to be processed. As a result,
we need an amplifier, whose gain is recommended
larger than 40 dB, to amplify the signal. And the
signal frequency range is 0.01-250 Hz. So, a filter is
recommended to decrease another useless signal.
Table 1: Some parameters of ECG signal.
Parameter or Measuring
Technique
Principal
Measurement
Range of
Paramete
r
Signal
Frequency
Range
Standard
Sensor or
Method
Electrocardiography
(
ECG
)
0.5-4 mV 0.01-250 Hz
Skin
electrodes
Wearable ECG sensors always use typical
instrumentation amplifiers (INAs) and common-
mode feedback circuits, which connect to the human
body, to solve the biasing problem.
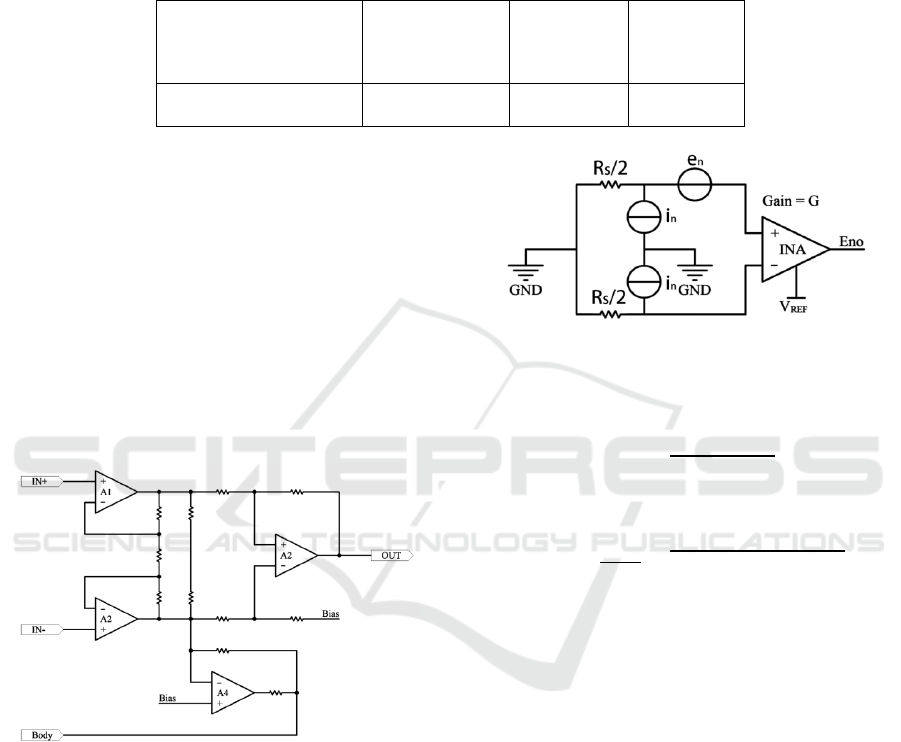
Fig.11 is a typical INA circuit for ECG devices.
There are 3 parts to it: The First part is the full
differential input buffer, which consists of A1 and
A2. The second part is the common-mode feedback
circuit. The voltage of the human body is unknown,
so using it to set the biasing voltage to the body
voltage. In the third part, a differential amplifier
converts the differential input into a single end
output.
Figure 11: Typical INA circuit for ECG devices.
Instrumentation amplifiers amplify small input
signals accurately. It is appropriate to build an ECG
sensor. But, because of the small input signal,
reducing noise is very important. However, building
the noise model of the whole circuit is very complex.
But using an integrated INA noise model is simpler.
Fig.12 is the noise model of integrated INAs. In this
figure, Eno is the noise of the output stage, and en is
the RMS sum of the noise of the input and output
stages. G is the gain of INA. in is the noise current.
Figure 12: Integrated INA noise model.
Sometimes, manufacturers may give eni, which is
the noise of the input stage. In this situation, we can
use (1) to calculate en:
𝑒
n
=
𝑒
ni
2
+(𝑒
no
/G)
2
(1)
Then we can find the result of output noise of the
circuit is Eno:
Eno=G∙
√
𝑁𝐸𝐵
∙
𝑒
()
+2𝑖
2
(𝑅
/2)
2
(2)
In equation (2) (Sullivan, T. J., Deiss, S. R.,
Cauwenberghs 2008), NEB is the noise equivalent
bandwidth. According to (2), we can reduce the
signal bandwidth through filters or use lower resistor
values to reduce noise. But it also brings some
problems: It is impossible to reduce the bandwidth to
a very small value. Because the frequency of the ECG
signal is 0.01-250 Hz, we cannot make the bandwidth
lower than 250 Hz. Secondly, using lower resistors
cost higher power consumption. As a result, users
should recharge their devices frequently. In
conclusion, every method to reduce noise leads to
other problems. So, selecting values according to the
product requirements is the best choice.
3.3 Pretreatment of ECG Signal
In the preprocessing of ECG signal, the baseline drift
caused by power line interference and interference of
body breathing movement can be removed first.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
288

The interference amplitude of the power line is
usually with volt magnitude, which is far larger than
that of the ECG signal with millivolt magnitude
(
Renesas 2020). Therefore, some methods, such as
adaptive zero-phase shift notch filter based on least
mean square (LMS) algorithm, are used to remove
power line interference and avoid phase distortion of
signals (
Gu, Hu, Zhang, Ding, Yan 2020).
And the baseline drift caused by breathing and
body movement is also an inevitable interference in
ECG signal acquisition by wearable devices. To
eliminate the baseline drift, we can estimate or extract
the baseline component, remove the component
caused by the drift by subtraction (Kuo, Morgan
1995
), or use a high-pass filter (Blanco-Velasco,
Weng, Barner 2008
).
4 CONCLUSION
After years of development, the gap between
wearable ECG devices and medical-grade ECGs is
getting smaller and smaller. Today, wearable ECG
devices can be found everywhere, such as mobile
phones, smartwatches, and headphones, which can
measure your heart rate, pressure, and blood oxygen
saturation. We can use this wearable ECG data to
analyze your physical health. to prevent disease. But
it must be acknowledged that although wearable
ECGs have developed rapidly over the years, they
still cannot replace large medical ECG machines. In
some cases, wearable ECG monitors are not as
accurate as medical-grade devices. For example,
noise and voltage. The increasingly miniaturized
wearable ECG devices are also becoming more and
more problematic in terms of battery life. Of course,
we have a lot to look forward to in the future of
wearable ECG devices.
In the future, wearable ECG devices will become
even smaller and more accurate. It may also rely on
the body's energy to provide a long-life span, with
functionality not limited to ECG monitoring but even
whole-body health monitoring. Although there is still
a long way to go, we expect this day to come.
REFERENCES
Beniczky S, Conradsen I, Henning O, et al. Automated real-
time detection of tonic-
Blanco-Velasco M, Weng B, Barner KE. ECG signal
denoising and baseline wander correction based on the
empirical mode decomposition. Comput Biol Med
2008;38(1):1–13.
Brain Support. (2020) Wet, dry, active and passive
electrodes. What are they, and what to choose?
https://www.brainlatam.com/blog/wet-dry-active-and-
passive-electrodes.-what-are-they-and-what-to-
choose-413
clonic seizures using a wearable EMG device. [J].
Neurology, 2018, 90(5)
Gu, X._ Hu, J._ Zhang, L._ Ding, J._ Yan, F. - An Improved
Method with High Anti-interference Ability for R Peak
Detection in Wearable Devices (2020)
H. Kim, Y. Kim, and H. Yoo, "A low-cost quadratic level
ECG compression algorithm and its hardware
optimization for body sensor network system," in Proc.
IEEE Eng. Med. Biol. Conf. (EMBC), Aug. 2008, pp.
5490–5493.
H. Kim, Y. Kim, and H.-J. Yoo, "A 1.12 mW continuous
healthcare monitor chip integrated on a planar-
fashionable circuit board," in Proc. IEEE Int. Solid-
State Circuits Conf. (ISSCC) Dig. Tech. Papers, Feb.
2008, pp. 150–151.
Hyeonjeong Lee, Jaewon Lee, and Miyoung Shin. "Using
Wearable ECG/PPG Sensors for Driver Drowsiness
Detection Based on Distinguishable Pattern of
Recurrence Plots". Electronics 2019, 8, 192;
doi:10.3390
Jearld Yoo, Long Yan, and Seulki Lee. "A Wearable ECG
Acquisition System with Compact Planar-Fashionable
Circuit Board-Based Shirt". IEEE. Novembei, 2009.
Pp:1089-7771
Köhler BU, Hennig C, Orglmeister R. The principles of
software QRS detection. IEEE Eng Med Biol
2002;21(1):42–57
Kuo SM, Morgan D. Active noise control systems:
algorithms and DSP implementations. John Wiley &
Sons, Inc; 1995.
N. Townsend, L. Wilson, P. Bhatnagar, K.
Wickramasinghe, M. Rayner and M. Nichols,
"Cardiovascular disease in Europe: epidemiological
update 2016," European Heart Journal, pp. 1–14,
August 2016.
Numan Celik, Wamadeva Balachandran, and Nadarajah
Manivannan. "Wearable Mobile Ear-based ECG
Monitoring Device Using Graphene-Coated Sensors".
IEEE. July. 2017, PP. 978-1-5090-1012.
R., B. and K. D. Telemetric ECG evaluation using
einthoven-leads. in Computers in Cardiology, 2005.
2005.
Renesas. (2020) Noise Calculations of Instrumentation
Amplifier Circuits.
https://www2.renesas.cn/us/en/document/apn/r13an00
11-noise-calculations-instrumentation-amplifier-
circuits-rev100
Sullivan, T. J., Deiss, S. R., Cauwenberghs, G.. (2008). A
Low-Noise, Non-Contact EEG/ECG Sensor.
Biomedical Circuits & Systems Conference. 154-157.
Vincenzo Randazzo, Jacopo Ferretti, and Eros Pasero.
“ECG WATCH: a real time wireless wearable ECG”.
IEEE. Mar, 2019. Pp:978-1-5386-8428.
ECG Circuit: Analyzation and Application
289

Zhang Y, Bai J, Zhou X, et al. First trial of home ECG
and blood pressure telemonitoring system in Macau[J].
Telemedicine Journal the Official Journal of the
American Telemedicine Association, 1997, 3(1):67.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
290
