
Lycium barbarum Polysaccharides Protects against Strenuous
Exercise-induced Oxidative Damage in Rats
Lantao Liu and Weiqiang Zhang
†
Department of Physical Education, Central South University Changsha City, Hunan Province, 410083, China
Keywords: Lycium Barbarum Polysaccharides, Oxidative Damage, Exhaustive Running Exercise, Rats.
Abstract: Lycium barbarum contains a variety of nutrients and bioactive ingredients, which has multiple biological
and pharmacological effects. In the theory of Chinese medicine, Lycium barbarum as a common Chinese
herbal medicine can be used for the treatment of many diseases. The aim of the current study was to
evaluate the protective effects of Lycium barbarum polysaccharides (LBPs) on strenuous exercise-induced
oxidative damage in rats. The animals were divided into one control group (treated with distilled water) and
three LBPs groups (treated with 50, 100 and 200 mg/kg LBPs, respectively). After 28 days of treatment, the
exhaustive running exercise was performed, followed by the relevant biochemical parameter analysis. the
datas revealed that LBPs increases exhaustive running times, and levels of superoxide dismutase (SOD),
glutathione peroxidase (GPx), reduced glutathione (GSH), glutathione reductase (GR), and catalase (CAT)
in liver. LBPs decreases the levels of creatine kinase (CK), myoglobin (Mb), tumor necrosis factor-α (TNF-
α) and interleukin 1β (IL-1β) in serum, and also the levels of oxidized glutathione (GSSG),
malondialdehyde (MDA) and 8-hydroxy-2’-deoxyguanosine (8-OHdG) in liver. These results suggest that
LBPs has protective effects on oxidative damage induced by strenuous exercise.
1 INTRODUCTION
Reactive oxygen species (ROS) are the chemically
active oxygen free radicals and the substances that
can be converted to the free radicals, which are
generated during the aerobic metabolism of cells.
ROS mainly includes oxygen free radicals and some
non-free radicals substances such as hydrogen
peroxide (H
2
O
2
), hydroperoxide (ROOH), etc.
(Kurutas 2016). Under normal physiological
conditions, the body's antioxidant defense systemcan
remove ROS, maintaining a dynamic balance.
However, strenuous exercise can increase oxygen
intake, accompanied by the generation of ROS in
various tissues by mean of different ways, which
may exceed the capacity of defense system, resulting
in increased oxidative stress. Exercise-induced
endogenous ROS were produced by a variety of
sources, including mitochondrial respiratory chain
pathway, xanthine oxidase reaction pathway,
neutrophils respiratory burst pathway, hemoglobin
oxidation reaction pathway, and so on. It has been
reported that increased oxidative stress can bring
about various levels of oxidative damage to various
substances that make up cell tissue such as lipids,
sugars, proteins and DNA (Jówko et al. 2011).
Growing evidences show that exogenous
antioxidants from food and natural products
supplementation can be an effective means to cut
down exercise-induced oxidative damage (Chen et
al. 2013).
Lycium barbarum (L. barbarum) is a perennial
woody plant, mainly grown in some provinces of
northern China, such as Inner Mongolia, Ningxia
and Hebei. The fruits of L. barbarum, known as
wolfberry and Goqi, have been widely used as
traditional herbs and supplements more than 2500
years. In 2002, the fruits of L. barbarum were
identified as both food and medicine items by the
Chinese government departments. Many types of
components, such as polyphenols, polysaccharides,
alkaloids, carotenoids, vitamins, amino acids,
aminoethanesulfonic acids, and fatty acids in fruits
of L. barbarum have been isolated and identified,
which have some biological and health-related
activity (Tang et al. 2015). Numerous studies have
suggested that L. barbarum polysaccharides (LBPs)
is the most important ingredients of L. barbarum to
play many biological activities (Liu et al. 2015).
LBPs account for about 5 - 8% of the dry weight of
Liu, L. and Zhang, W.
Lycium barbarum Polysaccharides Protects against Strenuous Exercise-induced Oxidative Damage in Rats.
DOI: 10.5220/0011316100003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 255-260
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
255

fruits of L. barbarum, which is a
heteropolysaccharide containing protein, and
generally consists of 6 - 8 monosaccharides, 18
amino acids and a variety of trace elements. The
molecular weight is in the range of 24 to 241 kDa
(Chen et al. 2009). Modern pharmacological studies
indicated LBPs has multiple pharmacological and
biological functions, including anti-diabetic, anti-
hypoxia, anti-fatigue, hypolipidemic,
antihypertensive, anti-aging, anti-cancer, analgesic,
immune regulation and liver protection effects.
Especially, this compound exhibited strong
antioxidant activities in vitro by inhibiting different
types of free radicals (DPPH, ABTS, superoxide
anion and hydroxyl radical), reducing power
activities and metal ion chelating capability (Li and
Zhou 2007). Animal experiments also indicated that
LBPs can significantly lower lipid peroxidation and
improve antioxidant enzyme activities (Zhao et al.
2015). Based on the antioxidant activities of LBPs, it
can be hypothesized that strenuous exercise-induced
oxidative damage in animal model can be prevented
by LBPs pretreatment. Thence, the research was
implemented to investigate whether LBPs
administration could prevent strenuous exercise-
induced oxidative damage in rats.
2 EXPERIMENTAL
2.1 Plant Material
The dried fruits of L. barbarum were collected in
Zhong-ning County of Ning Xia Huizu Autonomous
Region and provided by Qinian Biological
Technology Co., Ltd. (Yinchuan, China). The plant
samples were authenticated by a biologist in the
college of chemistry and chemical engineering,
Central South University (Changsha, China). The
voucher specimen was laid in plants herbarium of
Central South University.
2.2 Chemicals and Reagents
Commercial diagnostic kit for creatine kinase (CK)
was provided by Suzhou Comin Technology Co.
(Suzhou, China). Commercial diagnostic kits for
reduced glutathione (GSH), oxidized glutathione
(GSSG) and glutathione reductase (GR) were
provided by Beyotime Biotechnology Institute
(Haimen, China). Commercial diagnostic kits for
catalase (CAT), superoxide dismutase (SOD),
malondialdehyde (MDA), and glutathione
peroxidase (GPX) were provided by Jiancheng
Research institutions (Nanjing, China). ELISA kits
for myoglobin (Mb) were provided by Huamei
Biological Engineering Co. (Wuhan, China). ELISA
kits for tumor necrosis factor-α (TNF-α), interleukin
1β (IL-1β) and 8-hydroxy-2’-deoxyguanosine (8-
OHdG) were provided by Huijia Biological
Technology Co. (Xiamen, China).
2.3 Experimental Animals
Male Wistar rats (weight 180 - 200 g) adapt to the
environment and diet for one week before the
experiment. During the experiment, four rats were
placed in an individual plastic cage under standard
feeding conditions (temperature of 22 ± 2 °C,
relative humidity of 50 ± 15%, and 12 h light: 12
hours dark cycle circulation). Animals ingested
commercial rodent food and free drinking purified
water. This animal experiment was approved by the
Ethics Committee of Central South University.
2.4 Preparation of L. barbarum
Polysaccharides
L. barbarum polysaccharides (LBPs) were extracted
according to the previously published method in the
literature (Zhao et al. 2005), and have been slightly
adjusted. Briefly, the dried samples were crushed to
fine powder with electric mill and passed the 200
mesh sieve. Then the powder was refluxed twice
with petroleum ether (1 h every time) to remove the
lipid, and then refluxed twice with 80% ethanol (1 h
every time) to remove the small molecule sugar. The
residue was extracted with 10 volumes of distilled
water at 90 °C for three times (2.5 h every time).
The filtrate from combined and filtered water
extracts was concentrated in a rotary evaporator
under reduced pressure at 50 °C. Then the
concentrate was centrifuged (3000 rpm, 15 min), and
the supernatant was mixed with 4 volumes of 95%
ethanol and stockpiled overnight at 4 °C. The
precipitation was washed in order with anhydrous
ethanol, acetone and ether, and the reagent was
evaporated. The resulting precipitate was dispersed
in distilled water, dialyzed and lyophilized to afford
crude polysaccharides.
2.5 Experimental Design
Animals had one week adaption period, and after
that, they were divided into four groups, each
consisting of 8 rats. LBPs were given to the rats at
doses of 0, 50, 100 and 200 mg/kg and the four
groups were accordingly named as the control (C)
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
256
group, the low-dose LBPs treatment (LBPL) group,
the medium-dose LBPs treatment (LBPM) group
and the high-dose LBPs treatment (LBPH) group.
LBPs were dissolved in 1.0 mL distilled water and
administered by oral gavage one time per day lasting
for 28 days. After 21 days, the rats were introduced
to the motor-driven treadmill (WI78059, Shanghai
Yuyan Scientific Instrument Co., Ltd.) and made to
run at 15 m/min and a 0° grade for 15 min one time
per day lasting for 7 days to accommodate running
exercise. At the final day of experiment (the 28th
day), the incremental running exercise to exhaustion
was conducted using methods previously described
(Huang et al. 2013) with some modifications. The
rats were introduced into a treadmill, started running
at 15 m/min and 0 °grade for 10 min, then at 20
m/min and 0 °grade for 10 min, and finally at 30
m/min and 10 ° grade to exhaustion. Exhaustion is
defined when the rats can't continue running on the
treadmills after 12 s of continuous electric shock,
and the exhaustive running time was measured.
2.6 Analysis of Biochemical
Parameters
After exhaustive running exercise, the rats were
sacrificed by decapitation under ether anesthesia.
Blood was collected and centrifuged (3000 rpm, 15
min) at 4 °C to obtain serum for CK, Mb, TNF-α
and IL-1β analysis. The liver samples were
immediately isolated, weighed, and homogenized for
GSH, GSSG, SOD, CAT, GPX, GR, MDA and 8-
OHdG determinations. The levels of CK, Mb, TNF-
α, IL-1β, SOD, CAT, GPX, GR, GSH, GSSG, MDA
and 8-OHdG were determined using commercial
assay kits and abiding by the procedures advised by
manufacturers.
2.7 Statistical Analysis
All data were presented as mean ± standard
deviation (SD), and SPSS software is used for
Statistical analysis.
3 RESULTS AND DISCUSSION
3.1 Effects of LBPs on the Exhaustive
Running Times
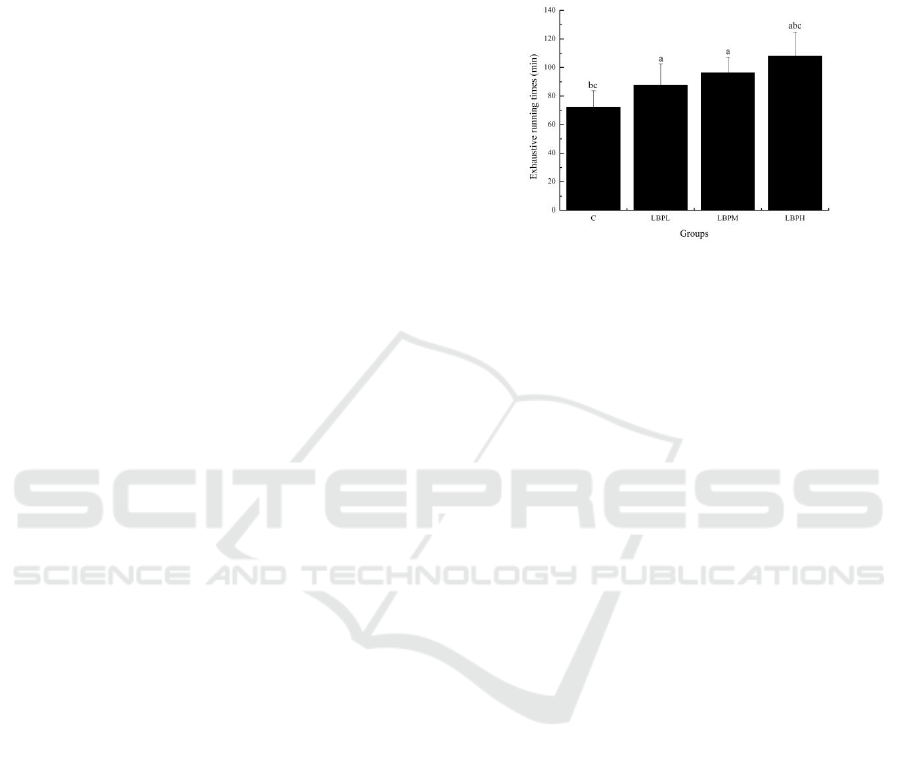
Figure 1: Effects of LBPs on the exhaustive running times.
According to Figure 1, the exhaustive running time
of different doses of LBP groups (LBPL, LBPM and
LBPH) were significantly longer than that of C
group (p < 0.05). Compared with LBPL group,
exhaustive running time of LBPH group was
significantly prolonged (p < 0.05). Compared with
LBPM group, the exhaustive running time of LBPH
group was significantly prolonged (p < 0.05). The
above data showed that LBPs had a strong anti-
fatigue effect.
3.2 Effects of LBPs on the CK and Mb
in Serum
Strenuous exercise leads to increased muscle
membrane permeability or muscle cell damage,
causing creatine kinase (CK) and myoglobin (Mb)
and other proteins to escape from the cell and into
the blood circulation.
According to Figure 2, the CK and Mb levels of
the different doses of LBP groups (LBPL, LBPM
and LBPH) were significantly reduced compared
with the C group (p < 0.05). The CK levels of the
LBPH groups, as well as the Mb levels of the LBPM
and LBPH groups were significantly reduced
compared with the LBPL group (p < 0.05). The CK
and Mb levels of the LBPH groups were
significantly reduced compared with the LBPM
group (p < 0.05). The above data showed that LBPs
might prevent muscle damage or promote rapid
regeneration of damaged muscle
.
Lycium barbarum Polysaccharides Protects against Strenuous Exercise-induced Oxidative Damage in Rats
257
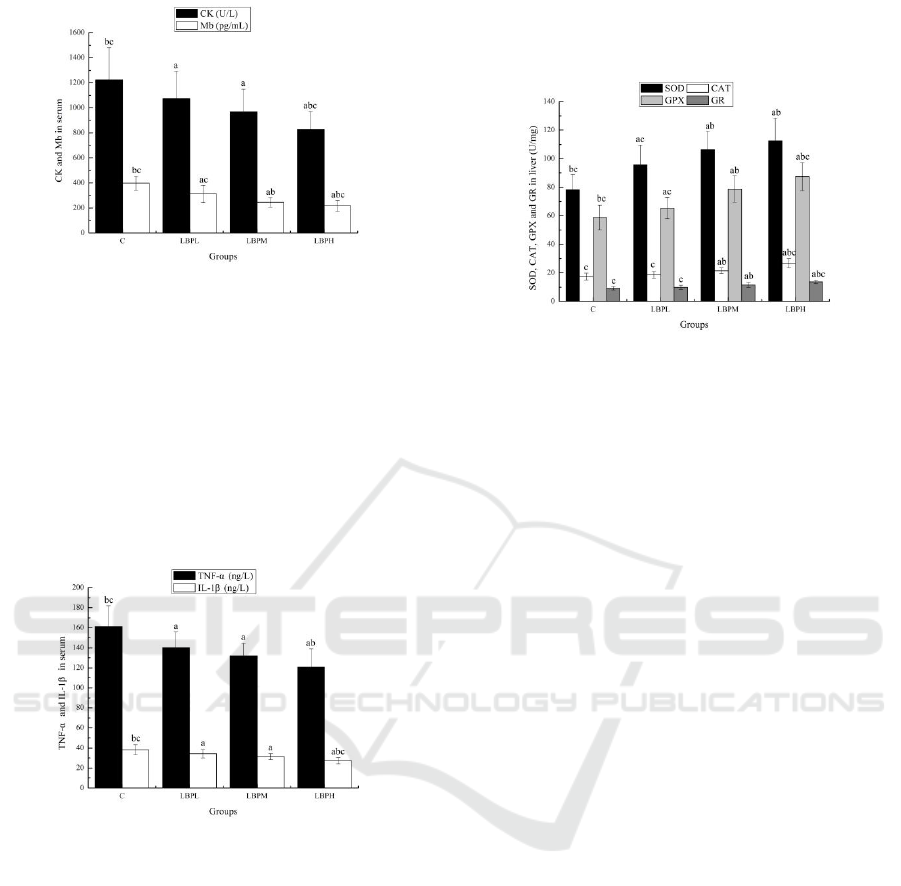
Figure 2: Effects of LBPs on the CK and Mb in serum.
3.3 Effects of LBPs on the TNF-α and
IL-1β in Serum
Previous studies have shown that strenuous exercise
can induce proinflammatory cytokines and
pleiotropic cytokine secretion to increase. TNF-α
and IL-1β are inflammatory cytokines secreted by
monocyte-macrophages and have proinflammatory
effects, which could also stimulate the production of
pleiotropic cytokine IL-6.
Figure 3: Effects of LBPs on TNF-α and IL-1β in serum.
According to Figure 3, the TNF-α and IL-1β
levels of LBPL, LBPM and LBPH were
significantly reduced compared with the C group (p
< 0.05). Compared with the LBPL group, the TNF-α
and IL-1β levels of the LBPH groups were
significantly reduced (p < 0.05); Compared with the
LBPM group, the IL-1β levels of the LBPH groups
were significantly reduced (p < 0.05). The above
data showed that LBPs could attenuate strenuous
exercise-induced inflammatory responses.
3.4 Effects of LBPs on the SOD, CAT,
GPX and GR in Liver
Antioxidant enzymes play an important protective
role in exercise-induced free radical oxidative
damage, and the decrease in the activity of these
enzymes means that the tissue is more susceptible to
free radical damage (Lee et al. 2009).
Figure 4: Effects of LBPs on the SOD, CAT, GPX and GR
in liver.
According to Figure 4, the SOD and GPX levels
of the LBPL, LBPM and LBPH groups; the CAT
and GR levels of the LBPM and LBPH groups were
significantly prolonged compared with the C group
(p < 0.05). Compared with the LBPL group, the
SOD, CAT, GPX and GR levels of the LBPM and
LBPH groups were significantly prolonged (p <
0.05). Compared with the LBPM group, the CAT,
GPX and GR levels of the LBPH groups were
significantly prolonged (p < 0.05). The above data
showed that LBPs could up-regulate the expression
of antioxidant enzymes to prevent strenuous
exercise-induced oxidative damage.
3.5 Effects of LBPs on the GSH and
GSSG in Liver
Glutathione is a tripeptide consisting of glutamine,
cysteine and glycine, which has two forms of
reduced (GSH) or oxidized (GSSG) (Masella et al.
2005). As a main intracellular antioxidant, GSH
plays an important role in preventing exercise-
induced oxidative damage by removing free radicals
and preventing the accumulation of hydroperoxides.
Under the action of GPx, GSH can reduce the H
2
O
2
to produce H
2
O, while GSH is oxidized to GSSG in
cells. GSSG also produces GSH under the catalysis
of GR. Strenuous exercise can cause severe
oxidative stress, leading to accumulation of GSSG
and reduction of GSH. GSH depletion can increase
the formation of hydroxyl radicals, which would
further lead to DNA damage (Muñoz et al., 2010).
Therefore, depletion of GSH in tissues has been used
as a sensitive index of exercise-induced ROS
production.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
258
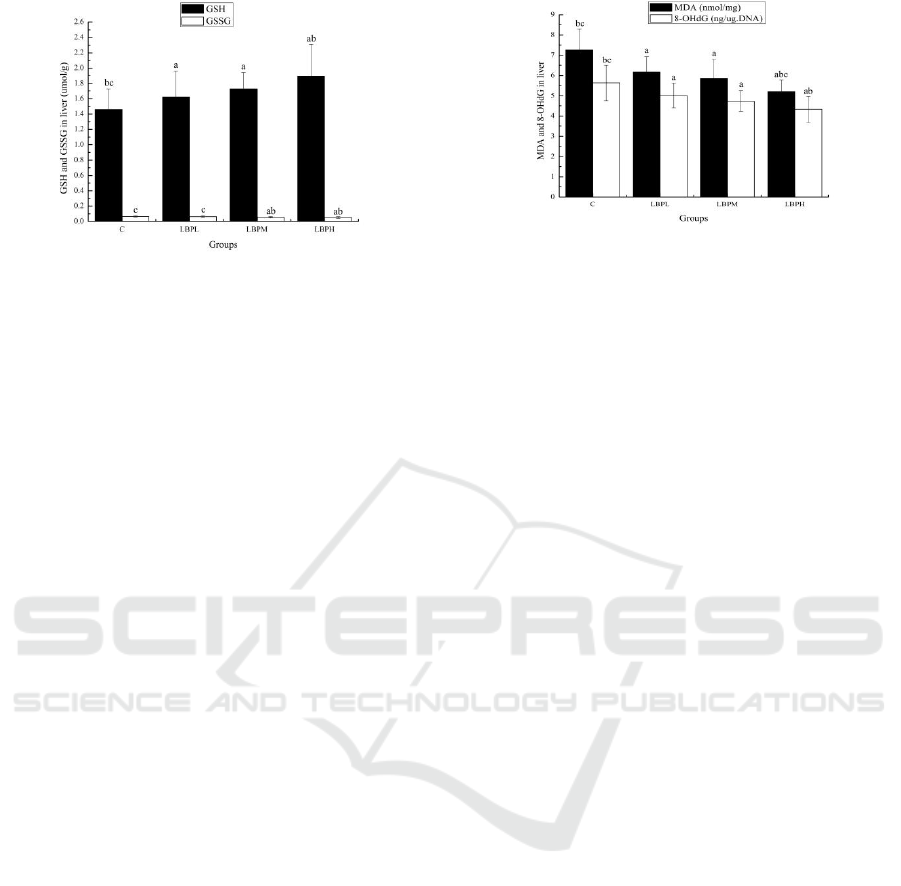
Figure 5: Effects of LBPs on the GSH and GSSG in liver.
According to Figure 5, compared with the C
group, the GSH levels of the LBPL, LBPM and
LBPH groups were significantly prolonged (p <
0.05); the GSSG levels of the LBPM and LBPH
groups were significantly reduced (p < 0.05).
Compared with the LBPL group, the GSH levels of
the LBPH groups were significantly prolonged (p <
0.05); the GSSG levels of the LBPM and LBPH
groups were significantly reduced (p < 0.05). The
above data showed that LBPs are adequate
protection against exercise-induced ROS generation.
3.6 Effects of LBPs on the MDA and
8-OHdG in Liver
MDA is the end product of peroxidative
decomposition of polyenic fatty acids and has been
often used as a marker of lipid peroxidation. 8-
OHdG is one of the major products of DNA
oxidation. When ROS attacks the guanine in DNA,
it causes deoxyguanosine oxidation to form 8-
OHdG. 8-OHdG can be measured with high
sensitivity and it is extensively investigated in
human and animal exercise studies, and is thus used
as a biomarker of oxidative DNA damage (Hamurcu
et al. 2010).
According to Figure 6, the MDA and 8-OHdG
levels of the LBPL, LBPM and LBPH groups were
reduced compared with the C group (p < 0.05).
Compared with the LBPL group, the MDA and 8-
OHdG levels of the LBPM and LBPH groups were
significantly reduced (p < 0.05). Compared with the
LBPM group, the MDA levels of the LBPH groups
were significantly reduced (p < 0.05). The above
data showed that LBPs could attenuate lipid
peroxidation and oxidative DNA damage induced by
exhaustive exercise.
Figure 6: Effects of LBPs on the MDA and 8-OHdG in
liver.
4 CONCLUSIONS
The results of this study provide strong evidence that
LBPs have protective effects on oxidative damage
induced by strenuous exercise in rats due to
increased the levels of SOD, CAT, GPX, GR and
GSH in liver, simultaneously decreased the levels of
CK, Mb TNF-α and IL-1β in serum, and the levels
of GSSG, MDA and 8-OHdG in liver. The
protective effects on oxidative damage of LBPs was
dose-dependent in rats, which might be related to the
per se antioxidant activities of LBPs.
REFERENCES
Chen, Z., Li, S., Wang, X., Zhang, C. L. (2013). Protective
effects of Radix Pseudostellariae polysaccharides
against exercise-induced oxidative stress in male rats.
Exp. Ther. Med. 5, 1089-1092.
Chen, Z., Lu, J., Srinivasan, N., Tan, B. K., Chan, S. H.
(2009). Polysaccharide-protein complex from Lycium
barbarum L. is a novel stimulus of dendritic cell
immunogenicity. J. Immunol. 18, 3503–3509.
Hamurcu, Z., Saritas, N., Baskol, G., Akpinar, N. (2010).
Effect of wrestling exercise on oxidative DNA
damage, nitric oxide level and paraoxonase activity in
adolescent boys. Pediatr. Exerc. Sci. 22, 60–68.
Huang, K. C., Wu, W. T., Yang, F. L., Chiu, Y. H., Peng,
T. C., Hsu, B. G, Liao, K .W., Lee, R.P. (2013).
Effects of freshwater clam extract supplementation on
time to exhaustion, muscle damage, pro/anti-
inflammatory cytokines, and liver injury in rats after
exhaustive exercise. Molecules. 18, 3825–3838.
Jówko, E., Sacharuk, J., Balasińska, B., Ostaszewski, P.,
Charmas, M., Charmas, R. (2011). Green tea extract
supplementation gives protection against exercise-
induced oxidative damage in healthy men. Nutr. Res.
31, 813-821.
Kurutas, E. B. (2016). The importance of antioxidants
which play the role in cellular response against
Lycium barbarum Polysaccharides Protects against Strenuous Exercise-induced Oxidative Damage in Rats
259

oxidative/nitrosative stress: current state. Nutr. J. 15,
71–73.
Lee, S. P., Mar, G. Y., Ng, L. T. (2009). Effects of
tocotrienol-rich fraction on exercise endurance
capacity and oxidative stress in forced swimming rats.
Eur. J. Appl. Physiol. 107, 587–595.
Li, X. L. & A.G. Zhou (2015). Effect of drying methods
on physicochemical properties and antioxidant
activities of wolfberry (Lycium barbarum)
polysaccharide. Carbohydr. Polym. 127, 176–181.
Liu, L., Lao, W., Ji, Q. S., Yang, Z. H., Yu, G. C., Zhong,
J. X. (2015). Lycium barbarum polysaccharides
protected human retinal pigment epithelial cells
against oxidative stress-induced apoptosis. Int. J.
Ophthalmol. 8, 11–16.
Masella, R., Benedetto, R. D., Varì, R., Filesi, C.,
Giovannini, C. (2005). Novel mechanisms of natural
antioxidant compounds in biological systems:
involvement of glutathione and glutathione-related
enzymes. J. Nutr. Biochem. 16, 577–586.
Muñoz, M. E., Galan, A. I., Palacios, E., Diez, M. A.,
Muguerza, B., Cobaleda, C. (2010). Effect of an
antioxidant functional food beverage on exercise-
induced oxidative stress: a long-term and large-scale
clinical intervention study. Toxicology. 278, 101–111.
Tang, H. L., Chen, C., Wang, S. K., Sun, G. J. (2015).
Biochemical analysis and hypoglycemic activity of a
polysaccharide isolated from the fruit of Lycium
barbarum L. Int. J. Biol. Macromol. 77, 235–242.
Zhao, Q., Dong, B., Chen, J., Zhao, B., Wang, X., Wang,
L., Zha, S., Wang, Y., Zhang, J., Wang, Y. (2015).
Effect of drying methods on physicochemical
properties and antioxidant activities of wolfberry
(Lycium barbarum) polysaccharide. Carbohydr.
Polym. 127, 176–181.
Zhao, R., Li, Q., Xiao B. (2005). Effect of Lycium
barbarum polysaccharide on the improvement of
insulin resistance in NIDDM rats. Yakugaku Zasshi.
125, 981–98.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
260
