
Metal-organic Frameworks: Preparation, Sensing, Drug Delivery,
Imaging and Therapy
Wei Huang
1,†
, Ruiqi Wang
2,†
and Yongli Zhang
3,* a
1
Department of Metallurgy and Materials
,
University of Birmingham Birmingham, U.K.
2
Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), Dalian, China
3
Critical Care Medicine, The First Affiliated Hospital of Dalian Medical University,Dalian, China
†
These authors contributed equally to this paper
*
Corresponding author
Keywords: Metal-Organic Frameworks, Preparation, Biomedicine, Application.
Abstract: Metal-organic frameworks (MOFs) materials have been widely used in biomedical field due to their unique
physical-chemical properties. This review summarized the preparation methods for MOFs, including
hydrothermal or solvothermal method, microwave synthesis, ultrasonic synthesis, mechanical method and
aerosol method. The MOFs synthesized by hydrothermal method exhibit uniform morphology. Moreover, the
properties of MOFs can be controlled by changing the concentration of precursors, the types of solvents and
catalysts. For the mechanical method, MOFs can be obtained by the mixing and grinding of raw materials in
a small amount of solvent without external heating. This method is applied for the large-scale production of
MOFs due to the simple operation, high yield and low energy consumption. With the advantages of large
specific surface area, high porosity, easy modification, low toxicity and biodegradability, this review also
focused in various biomedical applications of MOFs, such as fluorescence sensing, drug delivery, bioimaging
and tumor therapy.
1 INTRODUCTION
Metal-organic frameworks (MOFs) are crystalline
porous materials with three-dimensional periodic
structure constructed by coordination bonds between
metal ions or ion clusters and organic molecules
(Jiang, Alezi, Eddaoudi 2021). MOFs, firstly
proposed by O.M. Yaghi et al. in 1995, are also known
as porous coordination polymers (Yaghi, Li 1995).
MOFs have experienced a stage three generations
with rapid development. In the early preparation, the
pore size and stability were limited for the first
generation of MOFs. The significantly improved
stability of frameworks is achieved for the second
generation of MOFs, and the frameworks of MOFs
also remained their integrity even after removing the
guest molecules. For the third-generation MOFs, the
shrinkage and expansion of frameworks further is
attained. And the broad pore size of MOFs, from
micropore to mesopore are realized. Compared with
the traditional nanomaterials, MOFs offer the larger
a
https://orcid.org/0000-0002-4263-8382
specific surface area, high porosity, framework
flexibility and the controlled pore size by adjusting
the length of organic ligands (Lin, Zhang, Chen 2021,
Kim, Hong 2021, Pallach et al 2021). Moreover, the
uncoordinated unsaturated metal sites can be provided
for surface modification. This allows MOFs is easily
modified and functionalized. Due to their unique
characters, MOFs have been considered as a
promising absorbent or catalyst in gas storage and
separation, catalysis, membrane materials and other
fields (Wu, Lin, Ge, Wu, Xu 2013, Yang, Gates 2019,
Li, Wang, Sun, Lollar, Li, Zhou 2018). Moreover,
MOFs have good biodegradability and
biocompatibility, which can be used as drug carrier,
contrast agent as well as nano enzyme (Sun et al 2020,
Li et al 2020, Robison et al 2019). Therefore, MOFs
play a vital role in the biomedical field. This article
describes the preparation methods and various
applications in different branches of biomedical field
for MOFs.
230
Huang, W., Wang, R. and Zhang, Y.
Metal-organic Frameworks: Preparation, Sensing, Drug Delivery, Imaging and Therapy.
DOI: 10.5220/0011291000003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 230-237
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2 PREPARATION FOR MOFS
To obtain MOFs with various structures and functions
for various application, different synthesis methods of
MOFs have been proposed. The main methods
include hydrothermal or solvothermal method,
microwave synthesis, ultrasonic synthesis,
mechanical method and aerosol method and so on.
The synthesized MOFs have a variety of morphologic
features, including crystal and amorphous forms,
which makes MOFs play different functions in
various fields and have broad prospects.
2.1 Hydrothermal and Solvothermal
Methods
The hydrothermal or solvothermal method is one of
the most common preparation routes for MOFs.
Firstly, the raw materials including metal salts,
organic ligand and additives were dissolved in water
or organic solvent. Then the mixture was heated at a
certain temperature and pressure in reaction kettle and
MOFs products can be obtained. Kandiah et al.
synthesized the UiO-66-NH2 by dissolving ZrCl4 and
NH2-H2BDC in dimethylformamide (DMF). The
precursor solution was heated in an oven at 80 °C for
12 hours and kept at 100 °C for 24 hours. The final
product was obtained by washing and purifying
(Kandiah et al 2010). Besides reaction kettle
commonly used in MOFs preparation, Motegi et al.
synthesized MOFs of zirconium-based UiO-66 under
the nitrogen atmosphere using a standard reflux
device (Figure 1). (Motegi et al 2017) The synthesized
MOFs showed excellent hydrothermal stability. This
simple synthesis method can be scaled up to 1 L and
further scaled up to allow industrial production of
high-quality, uniform crystalline UiO-66 materials.
Figure 1: The schematic diagram of MOFs synthesis by
conventional reflux solvothermal method.
13
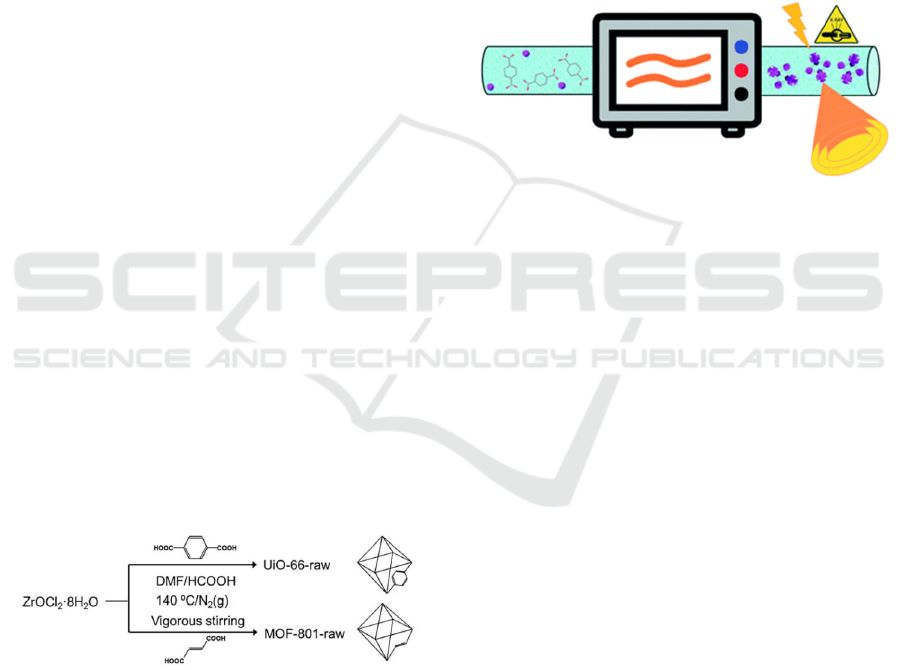
2.2 Microwave Synthesis
Microwave synthesis of MOFs refers to the reaction
process forming coordination bonds between metal
ions and organic ligands through the vibration and
friction of molecules of precursor solution under
microwave irradiation. In 2006, Zheng et al.
synthesized cubic Zn-MOF (MOF-5) with lengths
ranging from 200 nm to 4 µm by microwave assisted
method for the first time (Ni, Masel 2006). On the
basis of this work, many MOFs have been
successfully prepared by microwave synthesis. For
example, Li and colleagues reported the synthesis of
Zn-MOF (ZIF-7) using microwave assisted strategy,
in which diethylamine was added to further accelerate
the synthesis of ZIF-7, resulting in a significant
increase in the synthesis efficiency (Li et al 2010).
Taddei et al. reported the high-quality microwave-
assisted synthesis methods for large-scale preparation
of UiO-66, offering a possible way for industrial
production and commercialization of MOFs (Figure
2). (Taddei et al 2017)
Figure 2: The schematic diagram of MOFs synthesis by a
continuous-flow microwave reactor (Taddei et al 2017).
2.3 Ultrasonic Synthesis
In addition to microwave-assisted synthesis,
ultrasound-assisted synthesis is also widely used in
the preparation of MOFs. Ultrasonic wave can not
only facilitate the dissolution of metal salts, organic
ligand and additives, but also promote the binding
between metal ions and ligands due to the local
transient heating and vibration of solution caused by
its cavitation. Moreover, this reaction is only carried
out in very small individual reaction units, therefore,
MOF with small particle size were easily synthesized.
Jun Teng et al. designed and developed a novel small-
sized (about 95 nm) multilayer porous MOFs
(HPMOFs) by combining external ultrasound with the
inherent competitive binding between Co and Zn of
bimetallic Co/Zn-ZIF materials (Figure 3) (Teng et al
2018)
.
The competitive binding between two metal
ions in bimetallic MOFs was interfered with
ultrasonic in this strategy, in which the two metal ions
not only act as the building units of HPMOF, but also
the regulator of structure and size for HPMOF
nanocrystals. Thus, the small nanocrystals MOFs with
excellent selectivity and stability was achieved.
Metal-organic Frameworks: Preparation, Sensing, Drug Delivery, Imaging and Therapy
231
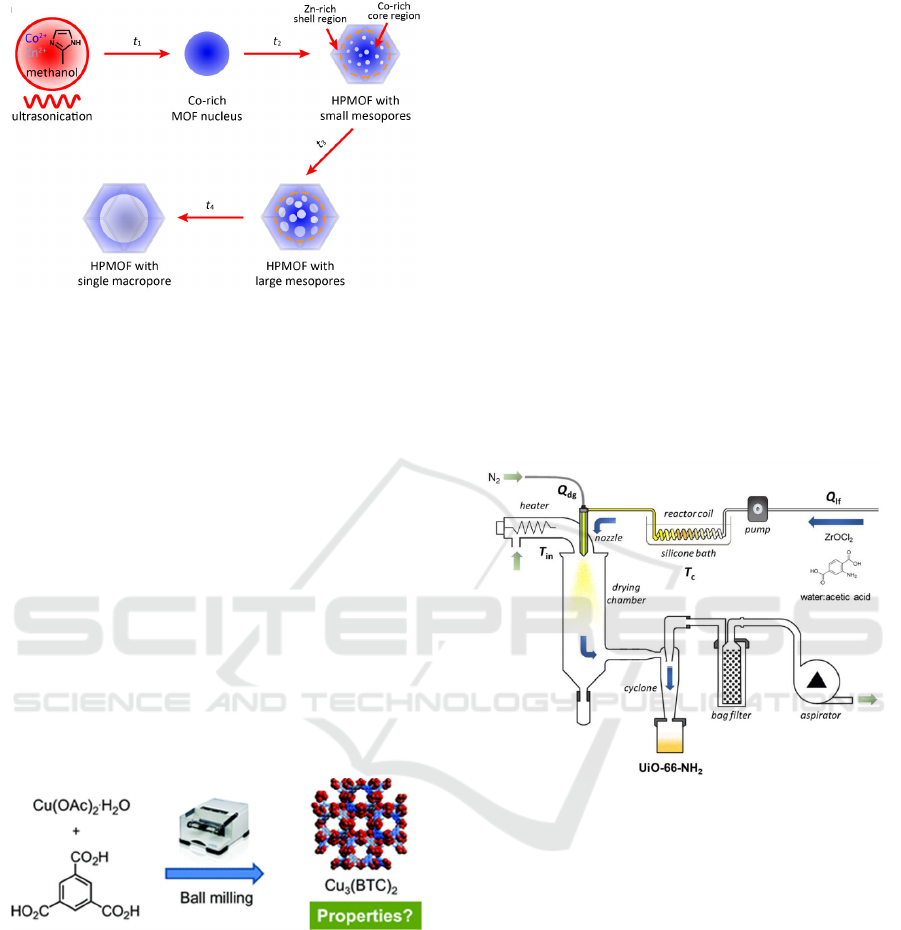
Figure 3: Growth strategy for the synthesis of MOFs (Teng
et al 2018).
2.4 Mechanical Method
Mechanical method, first proposed by James et al. in
2006, refers that the solid phase precursor can be
extruded and grinded by the use of external
mechanical force, resulting in sufficient contact
between metal ions and ligands (Figure 4) (Yuan et al
2010)
.
With ball milling method, James et al. quickly
synthesized Cu-MOFs using copper acetate and 4-
picolinic acid as raw materials, which pioneered the
mechanical chemical synthesis of MOFs. Julien et al.
reported Zn-MOFs-74 by mechanical grinding (Julien
et al 2016). X-ray diffraction analysis (XRD) results
of products with different grinding time showed that
the longer the grinding time, the better the
crystallinity of products and the more significant the
characteristic peak.
Figure 4. The schematic diagram of Cu-MOFs synthesis by
ball milling (Yuan et al 2010).
2.5 Aerosol Method
For the spray pyrolysis or aerosol flow strategy, the
precursor solution was transformed from liquid to
nano/micro droplets or aerosols using spray tools, and
transported to the heating region by the mean of
flowing carrier gas. Under high temperature, final
nanomaterials can be prepared by the condensation
and reaction of reactants due to rapidly volatilization
of the solvent in the droplets. In this process, each
droplet can act as a single reactor in which do not
interfere with each other, leading to a good dispersion
of MOFs. In 2013, Arnau et al. synthesized Cu-MOF
(HKUST-1, MOF-199) with hollow sphere structure
by spray method.20 Additionally, based on this
method, Arnau et al. also synthesized NOTT-100,
MIL-88A, MOF-14, UiO-66 and other MOFs. In
2018, Ceren et al prepared a spherical porous Zr-
MOFs (UiO-66-NH2) by continuous-flow spray-
drying (Figure 5) (Avci-Camur et al 2018). By
adjusting the concentration of acetic acid as an
additive, the specific surface area and water
absorption values of the resulting microbeads were
comparable to those obtained using other methods. In
addition, this work demonstrates the possibility of
spray drying for large-scale production of high yield
UiO-66-NH2. Spray pyrolysis has become a green
method for rapid preparation of MOFs, offering a
bright prospect in the synthesis of MOFs and other
nanomaterials (Garzón-Tovar et al 2016).
Figure 5. The schematic diagram of the set-up for the
aqueous continuous-flow spray-drying synthesis of Zr-
MOFs
.
(Avci-Camur et al 2018).
3 APPLICATIONS IN SENSING,
DRUG DELIVERY, IMAGING,
AND THERAPY FOR MOFS
With the potential of adjustable pore structure, large
surface area, large internal pore volume, and
multifunctional surface modification, MOFs have
been widely used in biomedical applications, such as
fluorescence sensor, drug delivery, bioimaging, and
tumor therapy (Figure 6) (Lai et al 2021)
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
232
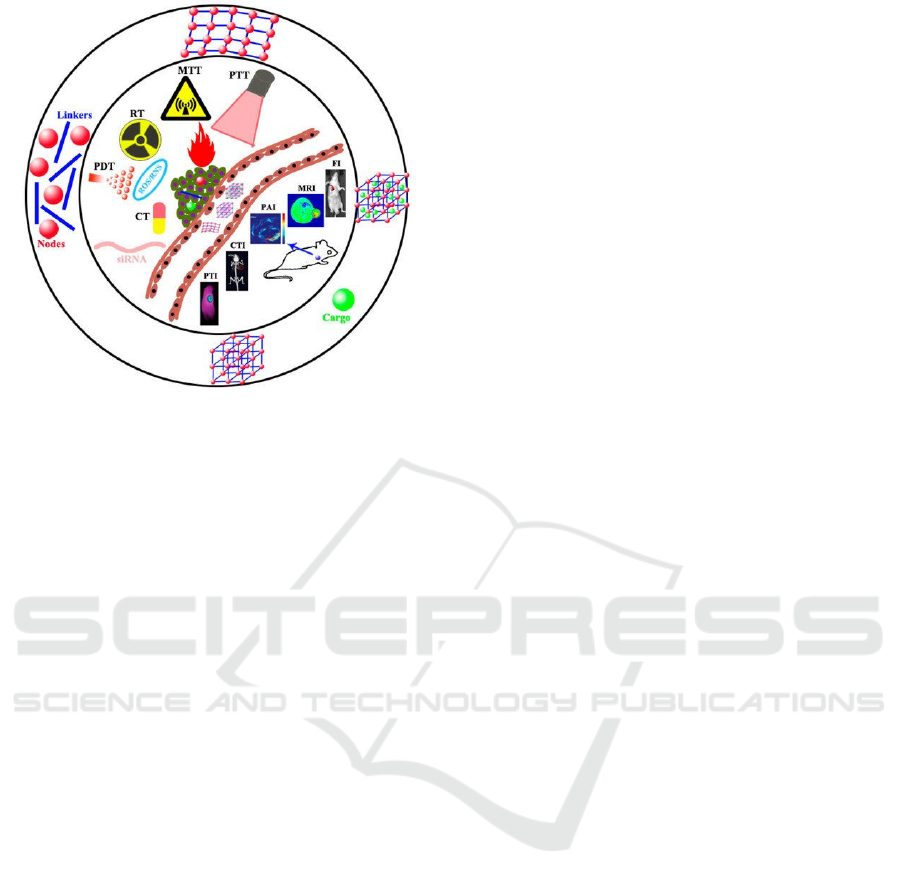
Figure 6: Biomedical applications of MOFs
.
(Lai et al
2021).
3.1 Fluorescence Sensor
Fluorescent sensing materials, as the core materials of
light-emitting diodes and various solid-state sensors,
are widely applied in our daily life. It is known that
many small organic molecules can emit fluorescence.
The introduction of these small molecules or
functional groups into the pore structure of MOFs can
not only control the fluorescence properties of
materials, but also explore the luminescence and
electron transport mechanism of materials. Therefore,
the design and synthesis of MOFs with fluorescence
properties have attracted more attention in recent
years. Researches have shown that amino
functionalized UiO-66 can selectively probe
phosphate ions in aqueous solution, with obvious
fluorescence response in the range of 5-150 µM, and
a good linear relationship between fluorescence
intensity and phosphate ion concentration (Zhao et al
2015). The theoretical detection line can reach up to
1.25 µM, which is far below the emission standard of
phosphate ions. Similarly, azide (-N3) and nitro (-
NO2) functionalized UiO-66 (UiO-66-N3 and UiO-
66-NO2) can also be used as fluorescent probes for
rapid and selective detection of H2S, even below the
concentration of H2S in the human body (Bai et al
2016)
.
3.2 Drug Delivery
MOFs, as a drug delivery platform, have attracted
considerable attention in recent years due to their
unique properties. The stable porous structure and
large specific surface area favor the load of a large
number of drug molecules by physical adsorption or
bonding reactions. The toxicity of MOFs can be
controlled by changing the type of ions. In addition,
the coordination bonds can degrade in a low pH
environment. MOFs can be passively targeted at
tumor sites by enhanced permeability and retention
(EPR) effect. Moreover, the targeting of MOFs can be
increased by functional surface modification, such as
cell membranes, proteins, organic polymers and so on.
For the MOFs-based drug delivery system, the
selectivity to lesions and selective aggregation of
drugs can be improved, which can reasonably control
the distribution of drugs in vivo, and avoid the toxic
and side effects caused by unnecessary drug diffusion.
In addition, the usage of nano-drug delivery platform
can also regulate the biological metabolism of drugs
and control drug release by chemical switch, leading
to the improved efficiency of drug treatment. It is
expected to achieve controlled release of drugs when
some small drug molecules are encapsulated in MOFs
with low toxicity.
Furthermore, some studies have shown that the
combination of different organic functional groups in
the structure of MOFs can achieve the regulation of
the types of drug loading and sustained release effects.
Cunha et al. found that the loading capacity of UiO-
66-X (X = H, CH
3
, NH
2
, NO
2
, Cl, Br) modified by
different organic functional groups was very different
for different drug small molecules (Cunha et al 2013).
In 2017, Deng group found that the type and content
of functional groups modified on the organic ligand of
MIL-101 (Fe) can affect the slow-release effect of the
drug by changing the interaction between small
molecules and pore channels (Dong et al 2017).
3.3 Bioimaging
MOFs can be used as contrast agents in biological
imaging to enhance the imaging signal of magnetic
resonance (MRI), fluorescence imaging (FOI) and X-
ray computed tomography (CT). The high-resolution
images for the structure of the living body structure
can be provided by the detection the radiofrequency
signals of protons inside organisms using external
magnetic fields, gradient fields and radio waves. The
contrast ratio of images can be further improved due
to the change in the transverse and longitudinal
relaxation rates of protons by the usage of MOFs.
MRI contrast agents include two types: one is a
positive contrast agent that shortens the longitudinal
relaxation time (T1) of the water proton, and the other
is a negative contrast agent that reduces the transverse
relaxation time (T2) of the water proton (Wu, Jiang,
Roy 2016). MRI contrast agents were evaluated by the
Metal-organic Frameworks: Preparation, Sensing, Drug Delivery, Imaging and Therapy
233

longitudinal and transverse relaxation rates of protons
(r1 and r2), and the type of contrast agents can be
determined by the ratio of r2/r1. The contrast agent is
positive or T1 relaxation when r2/r1 is small, which
usually contains paramagnetic transition metal ions
(such as Gd
3+
or Mn
2+
). In the case of large r2/r1, the
contrast agent is negative or called T2 relaxation, and
often contain superparamagnetic materials. Lin et al.
reported a Gd-based MOFs as a T1 imaging contrast
agent with a r1 of 35.8 mM
-1
s
-1
.(Rieter et al 2006)
Moreover, such nanoparticle also exhibits T2 imaging
capability with a r2 of 55.6 mM
-1
s
-1
. Horcajada et al.
synthesized Fe
3+
-MOFs (MIL-88A) with a r2 of about
50 mM
-1
s
-1
, which reveals the promising application
for T2 magnetic resonance imaging as contrast agent
(Lin, Rieter, Taylor 2009).
FOI has been extensively used in the diagnosis of
tumors and diseases because of its non-invasive
ability to distinguish diseased tissues. At present, FOI
using MOFs can be achieved by recombination with
fluorescent particles or by linking and adsorbing
fluorescent molecules. Tang et al. designed a MOFs
coated upconverting nanoparticles (NaYF
4
: Yb,
Er@Fe-MIL) that exhibit both the fluorescence
characteristics of the core and the T2-weighted
magnetic resonance imaging characteristics of the
MOFs shell (Tang et al 2015). In addition, MOFs also
have the inherent fluorescence properties. It is
reported that Mn
3
[Co(CN)
6
]
2
@SiO
2
exhibits green
fluorescence at 488 nm single photon excitation, and
blue fluorescence at 720 nm two-photon excitation.
This two-photon fluorescence imaging show a greater
penetration depth, less photobleaching and light
damage and higher resolution than single-photon
fluorescence imaging (Huang et al 2013).
CT imaging refers that the fault or cross section
image of the detected object can be drawn using the
attenuation signal of X-ray in different beam paths.
The improved contrast of CT imaging is achieved by
using the contrast agent with high X-ray attenuation.
Lin et al. prepared two MOFs with high Zr (37 wt%)
and Hf (57 wt%), respectively (Dekrafft et al 2012).
Zr with an atomic number of 40 and Hf with an atomic
number as high as 72 can be used as a component of
CT contrast agent. MOFs, modified with PEG with an
enhanced biocompatibility, showed negative
enhancement of CT signal in liver and spleen after
intravenous injection of 15 minutes on in vivo CT
imaging of mice. Zhang et al. reported a MOFs
nanocrystal (UiO-PDF) with iodine-boron
dipyrrolimethylene (I
2
-BDP) that can be used as CT
contrast agent. In addition, the CT imaging
capabilities of MOFs by combining with other CT
contrast agents such as noble metal material can be
realized (Zhang et al 2017).
The performance of contrast agent with the high
ordinal number metal can be effectively improved.
Meanwhile, MOFs with controllable particle size can
be selectively enriched in tumor sites by EPR effect,
which can further improve the diagnostic efficiency
of tumor.
3.4 Photothermal, Photodynamic,
Microwave Hyperthermia, And
Synergistic Therapy
3.4.1 Photothermal Therapy
Photothermal therapy mean that the thermal damage
and apoptosis of tumor cells due to the local warming
of tumor sites occur by the usage of near-infrared
(NIR) laser irradiation. The photothermal agents that
easily accumulate in tumor sites are usually selected
as auxiliary agents in clinical practice, because of
simple tumors is insensitive to the absorption of near
infrared golden light. Common photothermal agents
include metal compounds (MoS2, Co9Se8), noble
metal nanomaterials (gold nanoparticles), carbon
nanomaterials (graphene), organic fluorescent dyes
(IR825, ICG) and MOFs. Particularly, MOFs have
attracted wide attention due to their functionalization
and biodegradation. Cai et al. developed a MIL-100
(Fe) nMOF using hyaluronic acid (HA) as a surface
modification for targeted therapy of tumors.35 The
MOFs loaded with the indocyanine green (ICG) also
was used for image-guided photothermal tumor
therapy, which exhibited high ICG loading (40%),
strong NIR absorption and photostability. In vitro and
in vivo studies clearly revealed that
MOFs@HA@ICG with a good photothermal
therapeutic effect showed high cellular uptake in
MCF-7 cells and increased accumulation in xenograft
tumors.36 In addition, Wang et al. reported a polymer-
MOF hybrid that is Zr MOF (UiO-66) particle
modified by polyaniline (PAN) (UiO-66@PAN) as a
nanoplatform for photothermal therapy of tumors.
Under laser irradiation, the temperature of UiO-
66@PAN solution at a concentration of 100 µg mL-1
increased to 57.2 °C, which was sufficient to
effectively kill malignant tumor cells. In cell
experiments, this platform shows non-cytotoxicity in
mouse colon cancer CT26 and human colon cancer
HTC116 cell lines. However, the cell death rate
reached 70 % after laser irradiation. In vivo
experiments shows that tumors treated with UiO-
66@PAN and NIR radiation completely retreated
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
234

after 10 days, demonstrating the promising prospect
of UiO-66@PAN for photothermal therapy of tumors.
3.4.2 Photodynamic Therapy
Photodynamic therapy (PDT) is an important method
in clinical treatment of tumor. In the PDT, oxygen can
be converted into reactive oxygen species (ROS)
using photosensitizers under laser irradiation,
resulting the tumor cell death. PDT, with high
selectivity, small side effects, no trauma and
restorability, has attracted more attention in the
clinical. Lu et al. synthesized Al-Mn mixed MOFs
(Mn-MOF) with Mn as the active center to enhance
the photodynamic effect.37 The ROS produced by
Mn-MOF under light irradiation can be detected using
ROS detection reagent, DCFH as a probe. It is
confirmed that the fluorescence intensity of ROS
probe increased threefold after irradiation, indicating
that Mn-MOF has good photosensitivity and can be
used as a photosensitizer of PDT.
Based on the easy modification and
functionalization of MOFs materials, Zhang et al.
reported a simple and universal strategy for the
enhancement of PDT (Zhang et al 2018). The
platinum nano-enzymes with high catalase activity
and stability can be uniformly decorated in the
photosensitizer MOF. Therefore, the formation of
singlet oxygen for hypoxic tumor sites under laser
irradiation is promoted by the release of O
2
activated
by H
2
O
2
catalyzed by platinum nano-enzymes,
resulting tumor cells death.
3.4.3 Microwave Hyperthermia
Microwave hyperthermia of tumor (MWT) also is a
treatment method that induces apoptosis by means of
local heating at the tumor site. Compared with
photothermal therapy, a lot of heat can be generated
by the high-speed oscillatory friction between
polarized ions and dipoles in the radiation zone
induced by high-speed alternating electric field
generated by microwave (MW) as a heat source in the
microwave hyperthermia. Microwave hyperthermia
has the advantages of low cost, low toxicity and small
wound. However, the tumor cannot be accurately
located by a single microwave therapy. Meanwhile,
the temperature changes of the edge zone caused by
the gradient of the thermal field is not enough to
eliminate the tumor cells, resulting in the occurrence
of recurrence. To solve this problem, the concept of
microwave sensitizer was proposed. Microwave
sensitizer exhibit high microwave-heat conversion
efficiency, which is based on the ion domain
limitation. Compared with inorganic nanomaterials,
many studies have proved that MOFs materials can be
used as excellent microwave sensitizer in clinical
microwave hyperthermia of tumors. Zhou et al.
prepared Zr-MOF-PEG-TPP@DOX with
mitochondrial targeting ability as MW sensitizer, by
loaded chemotherapy drug doxorubicin (DOX) with a
porous zirconium-based MOF nanocubes (Zr-MOF,
UiO-66) modified by triphenyl phosphate (TPP) and
polyethylene glycol (PEG).39 The local temperature
of H22 tumor-bearing mice treated with Zr-MOF-
PEG-TPP@DOX increase to 50.8 °C after 5 min
microwave irradiation, which meets the requirements
of local temperature rise for thermal therapy. Tumor
growth also proved the good tumor inhibition effect
of this nanoplatform.
3.4.4 Synergistic Therapy
Besides the catalytic activity, photosensitivity or
microwave sensitization of MOFs, combine with
different therapies, the good loading performance and
functionalization of MOF materials can be used to
achieve the synergistic treatment, which can
effectively enhance the lethality for tumors. For
instance, MOF materials loaded with chemotherapy
drugs (such as adriamycin, cisplatin), cooperated with
chemotherapy, hyperthermia and kinetic therapy,
become an effective synergistic treatment. Ma et al.
reported that Zr-MOF with degradation and release of
terephthalic acid in acidic tumor microenvironment,
was used to inhibit carboxylic anhydrase (CAIX)
induced by hypoxic factor HIF-1α.40 Moreover, Zr-
MOF loaded with the chemotherapy drug quercetin
(QU) can also improve the radiotherapy sensitivity of
QU used for the inhibition of hypoxic factor, which
realizes the inhibition of hypoxic and chemotherapy.
As one of the important research fields in clinical
tumor treatment, the combination of immunotherapy
and MOF materials has attracted more attention. Lin
et al. reported a novel dihydroporphyrin-based MOF
nanomaterial (TBC-Hf), which encapsulated with an
inhibitor of IDOi for the immunomodulatory enzyme
IDO within the framework (Figure 7).41 The
synthesized IDOi@TBC-Hf can be used for the
synergistic treatment of photodynamic therapy and
immunotherapy. Based on this treatment, the effective
tumor suppression in colorectal cancer models is
achieved, and increased T cells in the tumor
microenvironment were detected after inhibiting IDO
and activating the immune system, which provides a
new idea for the clinical treatment of cancers.
Metal-organic Frameworks: Preparation, Sensing, Drug Delivery, Imaging and Therapy
235

Figure 7: In vivo anticancer efficacy of IDOi@TBC-Hf.41.
4 CONCLUSIONS
The unique properties of MOF, such as tunable pore
structure, large surface areas, high drug loading, easy
modification and functionalization, make it a potential
candidate in the biomedical field. This review
provides a more systematic understanding for the
preparation methods and bio-applications of MOFs.
However, limitation and challenges still exist for
MOF, such as standardization of preparation methods,
large-scale preparation, biocompatibility and
biodegradability. These factors limit the application of
MOF in biological field. Therefore, a simple and
stable preparation strategy with uniform size and high
yield of biocompatible MOFs is urgently developed.
Additionally, compared to biomedical application of
MOFs, the research on the biological effect of MOF
materials have received less attention. Therefore, it is
of great significance to clarify the biological effect of
MOF materials for safe application. In summary,
although the research based on MOF has made great
progress, it still faces challenges related to its
toxicology, clinical application and large-scale
production technology.
REFERENCES
Avci-Camur, C.; Troyano, J.; Pérez-Carvajal, J.; Legrand,
A.; Farrusseng, D.; Imaz, I.; Maspoch, D. Aqueous
production of spherical Zr-MOF beads via continuous-
flow spray-drying. Green chemistry 2018, 20, 873-878.
Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou,
H.-C. Zr-based metal–organic frameworks: design,
synthesis, structure, and applications. Chemical Society
Reviews 2016, 45, 2327-2367.
Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.;
Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering
phototheranostic nanoscale metal–organic frameworks
for multimodal imaging-guided cancer therapy. ACS
applied materials & interfaces 2017, 9, 2040-2051.
Carné-Sánchez, A.; Imaz, I.; Cano-Sarabia, M.; Maspoch,
D. A spray-drying strategy for synthesis of nanoscale
metal–organic frameworks and their assembly into
hollow superstructures. Nature Chemistry 2013, 5, 203-
211.
Cunha, D.; Gaudin, C.; Colinet, I.; Horcajada, P.; Maurin,
G.; Serre, C. Rationalization of the entrapping of
bioactive molecules into a series of functionalized
porous zirconium terephthalate MOFs. Journal of
Materials Chemistry B 2013, 1, 1101-1108.
Dekrafft, K. E.; Boyle, W. S.; Burk, L. M.; Zhou, O. Z.; Lin,
W. Zr-and Hf-based nanoscale metal–organic
frameworks as contrast agents for computed
tomography. Journal of materials chemistry 2012, 22,
18139-18144.
Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate
metal–organic frameworks for dialing-in the binding
and programming the release of drug molecules. Journal
of the American Chemical Society 2017, 139, 14209-
14216.
Garzón-Tovar, L.; Cano-Sarabia, M.; Carné-Sánchez, A.;
Carbonell, C.; Imaz, I.; Maspoch, D. A spray-drying
continuous-flow method for simultaneous synthesis and
shaping of microspherical high nuclearity MOF beads.
Reaction Chemistry & Engineering 2016, 1, 533-539.
Huang, Y.; Hu, L.; Zhang, T.; Zhong, H.; Zhou, J.; Liu, Z.;
Wang, H.; Guo, Z.; Chen, Q. Mn3[Co(CN)6]2@SiO2
Core-shell Nanocubes: Novel bimodal contrast agents
for MRI and optical imaging. Scientific Reports 2013,
3, 2647.
Jiang, H.; Alezi, D.; Eddaoudi, M. A reticular chemistry
guide for the design of periodic solids. Nature Reviews
Materials 2021, 6, 466-487.
Julien, P. A.; Užarević, K.; Katsenis, A. D.; Kimber, S. A.;
Wang, T.; Farha, O. K.; Zhang, Y.; Casaban, J.;
Germann, L. S.; Etter, M. In situ monitoring and
mechanism of the mechanochemical formation of a
microporous MOF-74 framework. Journal of the
American Chemical Society 2016, 138, 2929-2932.
Kandiah, M.; Nilsen, M. H.; Usseglio, S.; Jakobsen, S.;
Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E. A.;
Bonino, F.; Lillerud, K. P. Synthesis and stability of
tagged UiO-66 Zr-MOFs. Chemistry of Materials 2010,
22, 6632-6640.
Kim, H.; Hong, C. S. MOF-74-type frameworks: tunable
pore environment and functionality through metal and
ligand modification. CrystEngComm 2021, 23, 1377-
1387.
Lai, X.; Jiang, H.; Wang, X. Biodegradable Metal Organic
Frameworks for Multimodal Imaging and Targeting
Theranostics. Biosensors 2021, 11, 299.
Li, H.; Wang, K.; Sun, Y.; Lollar, C. T.; Li, J.; Zhou, H.-C.
Recent advances in gas storage and separation using
metal–organic frameworks. Materials Today 2018, 21,
108-121.
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
236

Li, Y. S.; Bux, H.; Feldhoff, A.; Li, G. L.; Yang, W. S.; Caro,
J. Controllable synthesis of metal–organic frameworks:
From MOF nanorods to oriented MOF membranes.
Advanced Materials 2010, 22, 3322-3326.
Li, Y.; Zhou, J.; Wang, L.; Xie, Z. Endogenous hydrogen
sulfide-triggered MOF-based nanoenzyme for synergic
cancer therapy. ACS Applied Materials & Interfaces
2020, 12, 30213-30220.
Lin, R.-B.; Zhang, Z.; Chen, B. Achieving High
Performance Metal–Organic Framework Materials
through Pore Engineering. Accounts of Chemical
Research 2021, 141-144.
Lin, W.; Rieter, W. J.; Taylor, K. M. Modular synthesis of
functional nanoscale coordination polymers.
Angewandte Chemie International Edition 2009, 48,
650-658.
Lu, J.; Yang, L.; Zhang, W.; Li, P.; Gao, X.; Zhang, W.;
Wang, H.; Tang, B. Photodynamic therapy for hypoxic
solid tumors via Mn-MOF as a photosensitizer.
Chemical Communications 2019, 55, 10792-10795.
Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Weichselbaum,
R. R.; Lin, W. Chlorin-based nanoscale metal–organic
framework systemically rejects colorectal cancers via
synergistic photodynamic therapy and checkpoint
blockade immunotherapy. Journal of the American
Chemical Society 2016, 138, 12502-12510.
Ma, T.; Liu, Y.; Wu, Q.; Luo, L.; Cui, Y.; Wang, X.; Chen,
X.; Tan, L.; Meng, X. Quercetin-modified metal–
organic frameworks for dual sensitization of
radiotherapy in tumor tissues by inhibiting the carbonic
anhydrase IX. Acs Nano 2019, 13, 4209-4219.
Motegi, H.; Yano, K.; Setoyama, N.; Matsuoka, Y.; Ohmura,
T.; Usuki, A. A facile synthesis of UiO-66 systems and
their hydrothermal stability. Journal of Porous Materials
2017, 24, 1327-1333.
Ni, Z.; Masel, R. I. Rapid production of metal− organic
frameworks via microwave-assisted solvothermal
synthesis. Journal of the American Chemical Society
2006, 128, 12394-12395.
Pallach, R.; Keupp, J.; Terlinden, K.; Frentzel-Beyme, L.;
Kloß, M.; Machalica, A.; Kotschy, J.; Vasa, S. K.;
Chater, P. A.; Sternemann, C. Frustrated flexibility in
metal-organic frameworks. Nature Communications
2021, 12, 1-12.
Rieter, W. J.; Taylor, K. M.; An, H.; Lin, W.; Lin, W.
Nanoscale metal− organic frameworks as potential
multimodal contrast enhancing agents. Journal of the
American Chemical Society 2006, 128, 9024-9025.
Robison, L.; Zhang, L.; Drout, R. J.; Li, P.; Haney, C. R.;
Brikha, A.; Noh, H.; Mehdi, B. L.; Browning, N. D.;
Dravid, V. P. A bismuth metal–organic framework as a
contrast agent for X-ray computed tomography. ACS
Applied Bio Materials 2019, 2, 1197-1203.
Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang,
Z.; Yan, H.; Cui, C.; Tan, W. Metal–organic framework
nanocarriers for drug delivery in biomedical
applications. Nano-Micro Letters 2020, 12, 1-29.
Taddei, M.; Casati, N.; Steitz, D. A.; Dümbgen, K. C.; van
Bokhoven, J. A.; Ranocchiari, M. In situ high-resolution
powder X-ray diffraction study of UiO-66 under
synthesis conditions in a continuous-flow microwave
reactor. CrystEngComm 2017, 19, 3206-3214.
Tang, J.; Chen, L.; Li, J.; Wang, Z.; Zhang, J.; Zhang, L.;
Luo, Y.; Wang, X. Selectively enhanced red
upconversion luminescence and phase/size
manipulation via Fe 3+ doping in NaYF 4: Yb, Er
nanocrystals. Nanoscale 2015, 7, 14752-14759.
Teng, J.; Chen, M.; Xie, Y.; Wang, D.; Jiang, J.-J.; Li, G.;
Wang, H.-P.; Fan, Y.; Wei, Z.-W.; Su, C.-Y.
Hierarchically Porous Single Nanocrystals of
Bimetallic Metal–Organic Framework for Nanoreactors
with Enhanced Conversion. Chemistry of Materials
2018, 30, 6458-6468.
Wang, W.; Wang, L.; Li, Y.; Liu, S.; Xie, Z.; Jing, X.
Nanoscale polymer metal–organic framework hybrids
for effective photothermal therapy of colon cancers.
Advanced Materials 2016, 28, 9320-9325.
Wu, B.; Lin, X.; Ge, L.; Wu, L.; Xu, T. A novel route for
preparing highly proton conductive membrane
materials with metal-organic frameworks. Chemical
Communications 2013, 49, 143-145.
Wu, W.; Jiang, C. Z.; Roy, V. A. Designed synthesis and
surface engineering strategies of magnetic iron oxide
nanoparticles for biomedical applications. Nanoscale
2016, 8, 19421-19474.
Yaghi, O.; Li, H. Hydrothermal synthesis of a metal-organic
framework containing large rectangular channels.
Journal of the American Chemical Society 1995, 117,
10401-10402.
Yang, D.; Gates, B. C. Catalysis by metal organic
frameworks: perspective and suggestions for future
research. Acs Catalysis 2019, 9, 1779-1798.
Yuan, W.; Garay, A. L.; Pichon, A.; Clowes, R.; Wood, C.
D.; Cooper, A. I.; James, S. L. Study of the
mechanochemical formation and resulting properties of
an archetypal MOF: Cu3 (BTC) 2 (BTC= 1, 3, 5-
benzenetricarboxylate). CrystEngComm 2010, 12,
4063-4065.
Zhang, T.; Wang, L.; Ma, C.; Wang, W.; Ding, J.; Liu, S.;
Zhang, X.; Xie, Z. BODIPY-containing nanoscale
metal–organic frameworks as contrast agents for
computed tomography. Journal of Materials Chemistry
B 2017, 5, 2330-2336.
Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang,
Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme decorated
metal–organic frameworks for enhanced photodynamic
therapy. ACS nano 2018, 12, 651-661.
Zhao, J.; Li, H.; Han, Y.; Li, R.; Ding, X.; Feng, X.; Wang,
B. Chirality from substitution: enantiomer separation
via a modified metal–organic framework. Journal of
Materials Chemistry A 2015, 3, 12145-12148.
Zhou, H.; Fu, C.; Chen, X.; Tan, L.; Yu, J.; Wu, Q.; Su, L.;
Huang, Z.; Cao, F.; Ren, X. Mitochondria-targeted
zirconium metal–organic frameworks for enhancing the
efficacy of microwave thermal therapy against tumors.
Biomaterials science 2018, 6, 1535-1545.
Metal-organic Frameworks: Preparation, Sensing, Drug Delivery, Imaging and Therapy
237
