
Real-time Monitoring Method for Carbon Dioxide and Residual
Oxygen in Medical Package
Xin Chen
1,2,1st
, Chunhong Zhang
3,1st
, Yuanlan Huang
3,1st
and Dan Li
3,*
1
Jinan Guoke Medical Technology Development Co., Ltd, Jinan, 250001, China
2
Shandong Engineering Technology Research Laboratory, Suzhou Institute of Biomedical Engineering and Technology,
Chinese Academy of Sciences, Suzhou, 215163, China
3
PLA Naval Medical Center, China
1st: Joint author
*
Corresponding author
Keywords: Vials, Medicine Packaging, Inert Gas Packaging, Nitrogen-Filled Packaging, Modified Packaging, Carbon
Dioxide Content, Residue Oxygen Content.
Abstract: Objective inert gas in vial is commonly used form of packaging which to prevent the drug deterioration.
Oxygen and carbon dioxide were remained in vial bottle is an important factor for medicine quality which be
strengthened monitoring. Methods The residue amount of carbon dioxide and oxygen were tested in different
kinds of vials, respectively. The test method of gas content in pharmaceutical packaging was introduced, and
the results were compared and analyzed. Results The results of experiment show that 1# gas contents in sample
is consistent with air, 2# and 3# samples are nitrogen-filled packaging, and oxygen and carbon dioxide content
in 3# sample changed little after a month. Therefore, 3# sample has the best preservation effect. Conclusion
The method with high test efficiency, can quickly and effectively reflect the gas component in samples.
According to the changes of gas component along with time in the packaging, preservation effect of 3# sample
is the best one in three kinds medicine samples.
1 INTRODUCTION
Most pharmaceutical products are sensitive to oxygen
and are easy to be oxidized causing deterioration,
discoloration, peculiar smell and the like, which not
only affects the curative effect of the products, but
also causes toxic and harmful substances generated
by oxidation to even endanger life, and drugs
containing hydroxide, calcium salt and other
components are easy to absorb carbon dioxide to
generate carbonate (Zhou, Mei 2011, Ma 2011, Zhou
2008). In order to reduce the amount of oxygen and
carbon dioxide contacted by drugs, oxygen and
carbon dioxide sensitive drugs are usually packaged
in the form of vacuum pumping, inert gas (generally
nitrogen) filling and the like.
As common medical packaging, vials are
mainly divided into soda-lime glass, borosilicate
glass, neutral borosilicate glass, plastic and other
material types, which can be used for packaging
powder injection, vaccine, lyophilized agent,
biological preparation and other drugs, most of
which adopt nitrogen-filled packaging form, and
are sealed with rubber, metal and plastic combined
cover (Huang, Zhu, Chen 2013, WANG, ZHAO
2007). Due to the limitation of inflation equipment
and process, the contents of nitrogen, oxygen and
carbon dioxide in the finished package of vials may
be different from expectations. In addition, under
the influence of packaging barrier and sealing (Fan
2014), the gases inside and outside the package
may exchange slowly, resulting in the changes of
the contents of the above gases with the extension
of storage time. Therefore, the timely detection and
dynamic monitoring of the gas composition in the
nitrogen-filled vials have certain guiding role in
preventing the deterioration of drugs and
determining the appropriate shelf life.
At present, many enterprises only rely on using
barrier packaging materials to hinder gas infiltration
/exudation of packaging materials, and cannot
eliminate the existing oxygen and other gases in the
Chen, X., Zhang, C., Huang, Y. and Li, D.
Real-time Monitoring Method for Carbon Dioxide and Residual Oxygen in Medical Package.
DOI: 10.5220/0011244800003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 209-213
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
209
packaging, and also lack real-time monitoring of the
internal gas composition. Instead, the monitoring of
the gas composition in the packaging should cover
any circulation link of the product, including just
completed packaging, storage process, transportation
process, sales process, expiration of the shelf life and
so on. The actual test results will also be used as
strong evidence for judging the quality of the goods.
(FAN 2012, WU, LIANG 2011) At present, GB/T
6285-2003 Determination of Trace Oxygen in
Gases—Electrochemical Method (Fan 2014) is the
standard for the test of oxygen content in gas, and no
method standard for the test of gas composition
content in packaging has been issued. In this paper,
according to the general testing methods and testing
experience in the industry, the oxygen and carbon
dioxide contents in several different packaging forms
of vials were tracked and tested.
2 TEST PRINCIPLE
Insert the sampler into the inside of the package to be
tested and collect enough sample gas from the top of
the package. The sample gas is introduced into the gas
analysis sensor, and the test data are recorded after a
certain test time interval or the gas concentration
output value of the gas analysis sensor is stable.
The corresponding gas analysis sensors are
needed for the test of different gas contents. When
testing the oxygen content in the sample gas, the
sample gas needs to be introduced into the oxygen
analysis sensor; when testing the carbon dioxide
content in the sample gas, the sample gas needs to be
introduced into the carbon dioxide analysis sensor.
For known packages filled with high purity nitrogen
inside, the nitrogen content in the packaging can be
obtained by subtracting oxygen content, carbon
dioxide content and other known gas content from the
total gas content.
3 DETECTION EQUIPMENT AND
METHOD
3.1 Test Instruments and Samples
3.1.1 Instruments and Their Performance
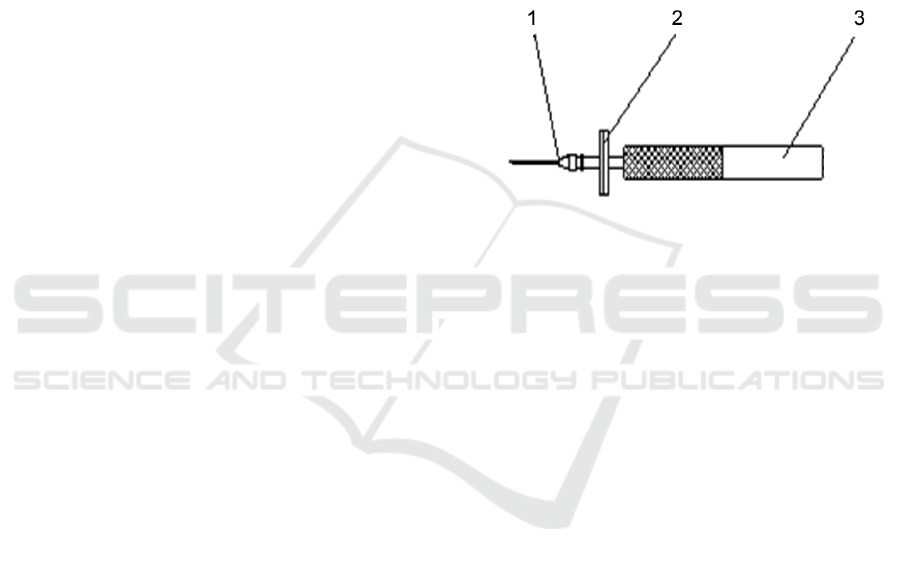
The test equipment used in this test is HGA-02
Headspace Gas Analyzer independently developed
and produced by Jinan Labthink Electromechanical
Technology Co., Ltd., which meets the requirements
of GB/T 6285 and is equipped with high-precision
oxygen and carbon dioxide sensors, a sliding test
head capable of testing samples at any height and a
high precision sampling device capable of absorbing
small volume gases (Fig.1), and is suitable for rapid
and accurate detection and analysis of the content and
mixing ratio of oxygen and carbon dioxide in flexible
plastic packaging bags and containers in production
sites, warehouses, laboratories and other occasions.
When the oxygen content is 0~2%, the test accuracy
is ±0.3% (absolute value), and ±0.5% (relative value)
when the oxygen content is 2~100%; the test range of
carbon dioxide is 0~100%, and the test accuracy is
±0.5%.
1. Sampling needle, 2. Filter, 3. Handle
Figure 1: Diagram of the Structure of High Precision
Sampling Device.
3.1.2 Samples
In this test, three kinds of vial powder injection
samples were used, numbered 1#, 2# and 3#
respectively, one of which was in ordinary air
packaging, and the other two were in nitrogen-filled
packaging. The number of samples for each kind of
sample should be sufficient to complete the entire
test, at least five, and take the average test as the test
result. The samples should be placed in the dryer for
more than 48 h under the sample condition
adjustment and standard environment specified in GB
/ T 2918.
3.2 Test Method
(1) Determine the appropriate test parameters
according to different equipments before verification.
The main factors affecting the stability and reliability
of the test results are sample gas extraction and
sample gas flow through the sensor. When the sample
gas extraction speed and the sample gas flow rate in
the instrument are constant, the sample gas extraction
and the sample gas flow through the sensor are related
to the sample gas extraction time and the sample gas
analysis time respectively. Therefore, it is necessary
to determine the optimal sample gas extraction time
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
210
and sample gas analysis time to prevent its impact on
the test results. Set the sampling time, analysis time
and other test parameters on the control panel of
Headspace Gas Analyzer, and the sampling time and
analysis time of this test are set to 12 s.
(2) Place the finished packing sample of vial in
the gas collection device, carefully remove the
stopper of the vial, and the gas in the bottle will be
collected in the gas collection device. Stably place the
sample, then insert the sampling needle into the inside
of the package through the middle part of the sealing
gasket. The puncturing force shall be appropriate to
prevent the sampling needle from sticking into the
contents of the package, causing the needle to become
blocked or broken.
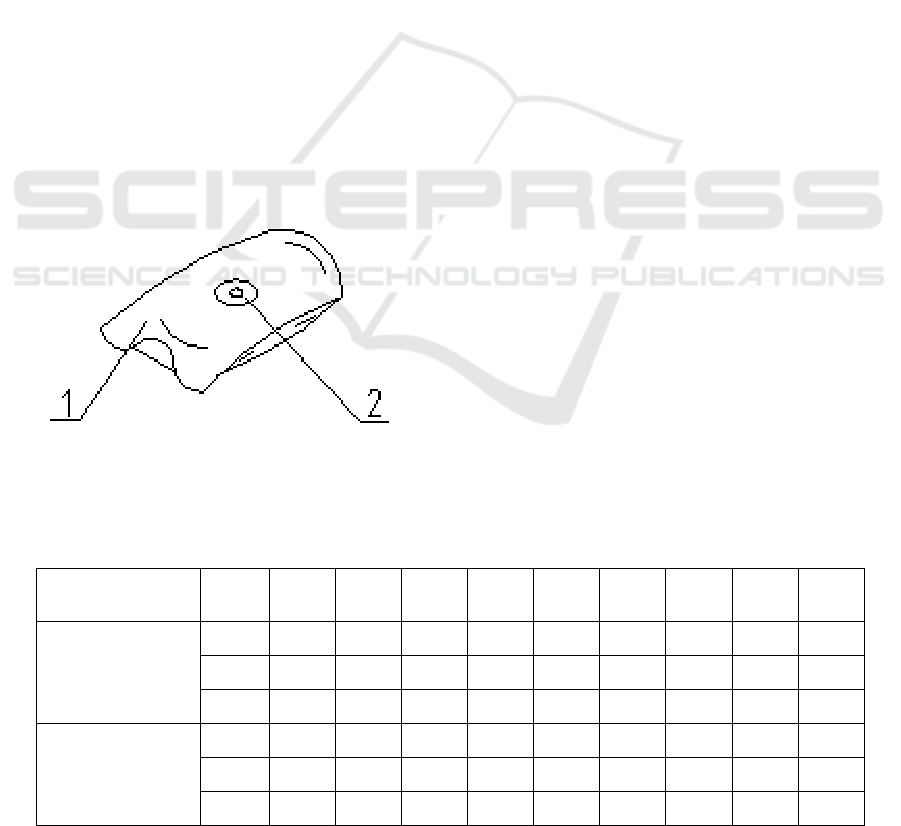
(3) Carefully insert the sampling needle into the
gas collection device (Fig.2). Click the test button to
start the test. The sampling needle shall not be pulled
out from the gas collection device. The sample gas to
be tested in the sampler shall be introduced into the
detection device through the sample injection port.
The sample gas will enter the gas analysis sensor
through the sample injection port and pipeline. The
instrument collects the sample gas in the gas
collection device and analyzes it. After the test, the
test results, i.e. oxygen content and carbon dioxide
gas content, are automatically displayed. Five
specimens of each sample were tested in parallel.
1. Samples, 2. Sealing gasket
Figure 2: Schematic diagram of gas sampling with gas
collection device.
4 RESULTS AND DISCUSSION
4.1 Gas Sampling Time
The analysis time of sample gas is fixed at 12 s. The
optimum time of gas sampling time is determined by
measuring the gas content in three kinds of samples
with the sampling time changed in the range of 4s -
12s. The test results are shown in Table 1.
The data in Table 1 show that when the sampling
time is 8 s - 12 s, the oxygen content test results of the
three samples are stable and independent of the
sampling time, with the maximum error <2% and
standard deviation RSD <0.5%. Therefore, the
sampling time of this equipment is fixed at 12 s to
ensure that sufficient sample gas is drawn.
4.2 Gas Analysis Time
The gas sampling time is fixed at 12 s. The optimal
analysis time of sample gas is obtained by testing the
gas content in three kinds of samples with the gas
analysis time varied within the time range of 8 s - 18
s. The test results are shown in Table 2.
The data in Table 2 show that when the analysis
time of the three kinds of sample gases exceeds 11 s,
the oxygen content test results are stable regardless of
the change of analysis time, with maximum error
<3% and standard deviation RSD <0.5%. In order to
ensure the stability of the test results and reduce the
test time, the preferred gas analysis time of this
equipment is set to be 12 s.
4.3 Sample Testing and Analysis
In this test, the oxygen and carbon dioxide contents
of the three samples before and after the interval of 1
month are tested respectively. The test results are
shown in Table 3.
Table 1: Gas Content Analysis Results for Different Gas Sampling Times
Sampling time (s) Sample 4 5 6 7 8 9 10 11 12
Oxygen content (%)
1# 19.31 19.57 21.17 20.49 20.36 20.47 20.35 20.38 20.41
2# 1.83 1.96 1.81 1.92 2.09 2.11 2.09 2.10 2.12
3# 0.85 0.92 1.09 0.95 1.19 1.19 1.20 1.18 1.20
Carbon dioxide
content (%)
1# 0.02 0.02 0.03 0.02 0.02 0.02 0.02 0.03 0.02
2# 0.01 0.00 0.01 0.02 0.01 0.00 0.00 0.01 0.01
3# 0.00 0.01 0.00 0.01 0.00 0.00 0.00 0.01 0.00
Real-time Monitoring Method for Carbon Dioxide and Residual Oxygen in Medical Package
211

Table 2: Gas Content Analysis Results for Different Gas Analysis Times.
Sampling time
(s)
Sample 8 9 10 11 12 13 14 15 16 17 18
Oxygen
content (%)
1# 19.05 21.00 20.46 20.53 20.49 20.39 20.46 20.37 20.41 20.50 20.47
2# 1.73 1.80 1.91 2.07 2.09 2.09 2.11 2.10 2.12 2.10 2.09
3# 1.05 1.12 1.07 1.20 1.19 1.17 1.19 1.21 1.18 1.20 1.20
Carbon
dioxide
content (%)
1# 0.03 0.02 0.03 0.03 0.02 0.02 0.02 0.02 0.03 0.02 0.02
2# 0.01 0.01 0.02 0.00 0.00 0.01 0.00 0.00 0.01 0.00 0.00
3# 0.01 0.00 0.01 0.00 0.00 0.00 0.00 0.01 0.00 0.01 0.00
Table 3: Test results of oxygen and carbon dioxide content in three samples.
Gas content/%
1# sample 2# sample 3# sample
Before 1
month
After 1
month
Before 1
month
After 1
month
Before 1
month
After 1
month
Oxygen
content
1 20.31 20.76 2.16 16.31 1.26 2.99
2 20.56 20.23 2.03 16.49 1.18 3.04
3 19.70 20.31 2.07 16.08 1.20 3.09
4 20.64 20.51 2.11 15.94 1.21 3.13
5 20.11 20.57 2.17 16.18 1.17 3.06
Average 20.26 20.48 2.11 16.20 1.20 3.06
Standard
deviation
0.38 0.21 0.059 0.21 0.035 0.052
Carbon
dioxide
content
1 0.03 0.02 0.01 0.01 0.00 0.00
2 0.02 0.02 0.01 0.02 0.00 0.01
3 0.03 0.03 0.00 0.02 0.00 0.01
4 0.02 0.02 0.02 0.02 0.00 0.00
5 0.02 0.02 0.00 0.01 0.00 0.00
Average 0.02 0.02 0.01 0.02 0.00 0.004
Standard
deviation
0.0055 0.0045 0.0084 0.0055 0.00 0.0055
As can be seen from Table 1, the standard
deviation of the 12 groups of data is low, indicating
that the dispersion degree between the test data of
each group is small, and the test method for testing
the oxygen and carbon dioxide content in the package
adopted in this paper has good stability and
repeatability.
The detection data of the three samples before one
month showed that the contents of oxygen and carbon
dioxide in 1# sample bottle were high, which were
basically the same as those in air, indicating that the
gas in 1# sample was ordinary air. The contents of
oxygen and carbon dioxide in 2#and 3# samples are
obviously lower than those in 1# sample, which are
nitrogen-filled packaging, and the contents of oxygen
and carbon dioxide in 3# sample are the lowest. The
difference of oxygen and carbon dioxide content in
the nitrogen-filled packaging is related to the nitrogen
filling process and the purity of the filled nitrogen. In
order to ensure the quality guarantee effect of the
nitrogen-filled packaging, the content of nitrogen in
the package should be increased, and the contents of
oxygen and carbon dioxide in contact with the
medicine should be reduced. Therefore, the air in the
package should be removed as far as possible before
nitrogen filling, the volume of residual gas should be
reduced, and the purity of the filled nitrogen should
be ensured.
After a comprehensive analysis of the test data of
the three samples before and after one month's
interval, the oxygen and carbon dioxide contents in
1# sample did not change significantly; the content of
both gases in the 2# sample increased after being
placed for one month, and the oxygen content
increased by about 7 times; the oxygen and carbon
dioxide contents of 3# sample only increased slightly.
This shows that among the three samples, 3# sample
has good barrier property and sealing property, which
can effectively prevent the gas exchange inside and
outside the bottle; 2# sample has poor barrier
property or sealing property, and the gas in the
environment permeates into the sample at a high
speed, while 1# sample is ordinary air, so it is
impossible to infer the barrier property or sealing
effect from the change of oxygen and carbon dioxide
content. From the point of view of the preservation
effect of the sample on the internal gas, if the same
drug is packaged, the quality guarantee effect of 3#
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
212

sample on the packaged drug is better, and the quality
guarantee period can be relatively prolonged.
5 CONCLUSIONS
The content of oxygen and carbon dioxide in
packaging is an important factor affecting the quality
of pharmaceuticals, and the residual oxygen is one of
the basic requirements of GMP for inert gas
packaging of sterile drugs. In this paper, the contents
of oxygen and carbon dioxide in three kinds of vial
powder injection samples were measured by
Headspace Gas Analyzer, and the contents of residual
oxygen and carbon dioxide in 3# sample were the
lowest and the gas preservation effect was the best.
From the test process, the test method used in this
paper is simple, short-time, high-efficiency, and can
quickly and effectively reflect the gas content in the
samples, also reduce the number of defective
products with unqualified internal gas composition in
the production line, effectively control the proportion
of oxygen and carbon dioxide in the gas-modulated
packaging of drugs, promote the overall quality of
domestic packaging products to pursue the goal of
high standards and high quality, and accelerate the
large-scale use of innovative and convenient modern
test equipment in domestic drugs and other industries.
At present, the testing technology both at home
and abroad have no uniform standard test methods,
but the test principle, test structure, test indicators and
other specific content has been initially formed. Due
to the lack of standard support, versatility and data
comparability are not high. Therefore, there is an
urgent need for the development and publication of
relevant standards in the industry, and only with
testing standard specification can this method and
quantitative indicators be effective in product
standards.
REFERENCES
FAN Jun. The Relation between Headspace Gas Analysis
and Shelf Life of Package[J]. Packaging Engineering,
2012,33(5):146.
Fan Y. (2014) Air Tightness Inspection of Vial Container
[J]. Heilongjiang Medicine Journal, (3): 593-595.
GB/T 6285-2003, Determination of Trace Oxygen in Gases
- Electrochemical Method [S]. Fan Y. (2014) Air
Tightness Inspection of Vial Container [J].
Heilongjiang Medicine Journal, (3): 593-595.
Huang W.C., Zhu L., Chen Z., et al. (2013) The Application
of Neutral Borosilicate Vial in Freeze-Dried Hepatitis
A Vaccine Production [J]. International Journal of
Epidemiology and Infectious Disease, 40(2): 139-140.
Ma C.L. (2011) Discussion on the Deterioration of Chinese
Herbal Medicine and its and Prevention [J]. Chinese
and Foreign Medical Research, 23 (9): 147-148.
WANG Xing-dong, ZHAO Jiang. Packing Efficiency and
Correlation detection of Nitrogen[J]. Packaging
Engineering, 2007, 28(9):213-214.
WU Shu-yi, LIANG Yi. Observation and Discussion of the
Latest In-line Headspace Oxygen Monitoring[J].
Mechanical and Electrical Information,2011, (32):42-
44.
Zhou J.Q., Mei D. (2011) Effects of Drug Packaging
Materials on Drug Quality and Safety [J]. Adverse
Drug Reactions, 13(1): 27-31.
Zhou Y. (2008) Discussion on Factors of Drug
Deterioration and its Control Measures [J]. Chinese
Journal of Modern Drug Application, 10 (2): 116-117.
Real-time Monitoring Method for Carbon Dioxide and Residual Oxygen in Medical Package
213
