
Impact of Dopamine on Reward Related Behavior of Animals and
Therapeutic Role
Chenyiwei Fu
Beijing International Bilingual Academy, Hebei, 065201, China
Keywords: Dopamine, Experimental Conditioning, Reinforcement Learning, Parkinson’s Disease.
Abstract: Nowadays, some researchers proposed that human behavior are closely related to dopamine development.
The effect of dopamine on esthesia of reward of humans and animals is very significant. This paper will use
a literature review method to elaborate on the role of dopamine in experimental conditioning, animal reward
circuitry, and what role dopamine plays in human behavior, such as reinforcement learning. This paper will
also specifically illustrate the circuitry of dopamine secretion and the reinforcement process of the secretion
process. As it is difficult to achieve human experiments, a large number of animal experiments will be used
to demonstrate its effect on animals, then further apply some results on human. The paper found that the
secretion of dopamine played a significant role in reward related behavior under experimental conditioning.
This conclusion can also generalize to human behavior to a certain extent because of the genetic similarity
between mice and other animals and humans. Therefore, this paper, via literature review, will show the role
of dopamine in Parkinson's syndrome and the treatment and therapeutic effects of effective drugs on the
syndrome.
1 INTRODUCTION
Neurotransmitter dopamine plays a significant role in
human learning, motivation, and many other aspects.
This paper will further strengthen the irreplaceable
role of dopamine in reward circuitry by combining
past literature, presenting the trajectory of dopamine
in human brains and animal brains, and combine with
experiments under Experimental conditioning,
showing the degree that dopamine is practically
involved in reward related behaviors in animals. In
addition, dopamine is a neurotransmitter, and its
system regulation disorder is the main cause of
Parkinson's syndrome. Insufficient dopamine
secretion leads to Parkinson's syndrome. Thus, it can
be inferred that the secretion of dopamine is closely
related to Parkinson's disease. Therefore, this paper,
with a method of literature review, will also present
the role of dopamine in Parkinson's syndrome and the
treatment and therapeutic effects of effective drugs on
the syndrome.
2 DEFINITIONS
2.1 Midbrain Dopamine
The Midbrain dopamine, also known as
dopaminergic neurons in ventral mesodiencephalon
(mdDA), is responsible for several functions as
voluntary movements control, motivation behavior,
maintaining working memories, adjusting emotions
and more importantly, associations with rewarding
stimuli (Bissonette & Roesch 2016).
Midbrain dopamine(mdDA) has a great influence
on Reinforcement learning by adjusting strength of
synaptic connection between neurons (Bromberg-
Martin, Matsumoto & Hikosaka 2010). More
specifically, this would permit a individual to learn
the ideal choice of activities to pick up rewards, given
adequate trial-and-error involvement. According to
Montague et al (1996), such process could be
described as a modified Hebbian rule. When a cell
affect it’s neibors which eventually result in a reward
or punishment, brain would release dopamine in
order to reinforce the connection between two cells,
and eventually result in repeated behavior. Referring
to(Figure 1.)
134
Fu, C.
Impact of Dopamine on Reward Related Behavior of Animals and Therapeutic Role.
DOI: 10.5220/0011203200003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 134-140
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
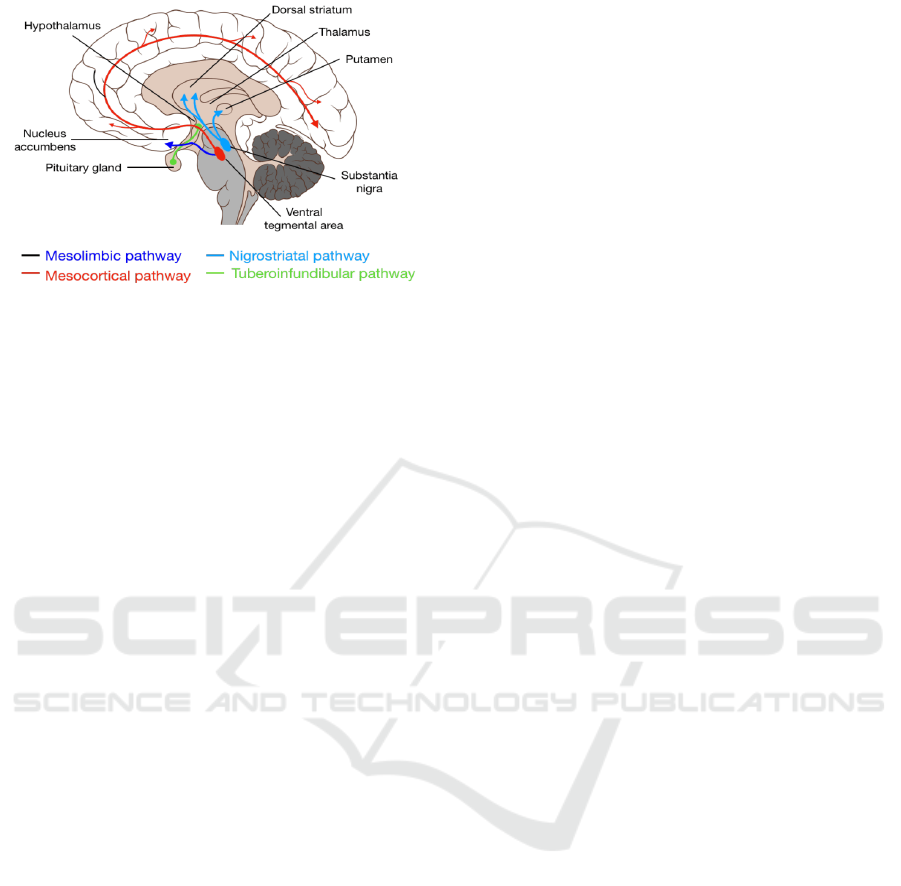
Figure 1: Nigrostriatal pathway and Mesocortical pathway
displaying release of dopamine.
Furthermore, Mesolimbic, Mesocortical, as well
as Nigrostriatal pathways are indispensable in the
connective process of midbrain dopamine cells. The
mesolimbic and nigrostriatal pathways are an integral
part of the basal ganglia through its reciprocal
connections to the ventral and dorsal striatum
respectively (Ikemoto 2007). The nigrostriatal
pathway is shown emitting mainly from SNc
(Substantial Nigra pars compacta) neurons, which
provide dopaminergic tone necessary for voluntary
movements and also carrying salience and PE
(predicted error) signal (Bissonette & Roesch 2016).
Simultaneously, VTA (Ventral tegmental area) is also
emanating from the Basal Ganglia through
Mesocortical pathway to both the ventral striatum as
well as Cortex. Corticostriatal input simultaneously
travels to Dorsal Striatum and ultimately result in
bodily movement, or behavior (Bissonette & Roesch
2016).
2.2 Reinforcement Learning
Reinforcement learning could be simply describe as
learning from past experiences, or Law of Effect
proposed by Edward Thorndike. Thorndike took
observation of cats in a puzzle box possessing certain
mechanism for cats to learn and ultimately, escape.
This is a traditional Instrumental conditioning, or
Operant conditioning experiment, a term used for
experiments which reinforcement is contingent upon
behavior (Sutton&Barto 2018). Furthermore,
researchers placed food, as primary Reinforcement
next to the confinement.
Qualitative data are taken during the observation.
“The cat that is clawing all over the box in her
impulsive struggle will probably claw the string or
loop or button so as to open the door. And gradually
all the other non-successful impulses will be stamped
out and the particular impulse leading to the
successful act will be stamped in by the resulting
pleasure, until, after many trials, the cat will, when
put in the box, immediately claw the button or loop
in a definite way” (Burnham 1972).
3 CONNECTION BETWEEN
REFINFORCEMENT
LEARNING BEHAVIOR AND
FUNCTION OF DOPAMINE
Instead of conduct in-depth research directly from
human behavior, many experiments on reinforcement
learning are initiated with animals, especially mice,
due to similar brain structures and past knowledge on
circuit when dopamine neurons are triggered and
released. Two models of dopamine projection
systems released from ventral midbrain to ventral
stratum are responsible for reward related behavior
(Haber 2014).
3.1 Dopamine Projection System from
Ventral Midbrain
Recently, researchers have been utilizing Rats in
order to refine our understanding localization within
the ventral striatum and VTA that are responsible for
the rewarding effects of drugs of abuse (Ikemoto &
Wise 2004). Medial olfactory tubercle not only plays
an important role in relevant to drug reward, but also
shares a common function with the medial shell; they
further suggest that the accumbens shell is
functionally heterogeneous, as is the olfactory
tubercle (Ikemoto 2007).
Rats could learn to lever-press for cocaine or
amphetamine into the olfactory tubercle, although the
medial portion of the tubercle is more responsive to
the rewarding effects of these drugs than the lateral
portion (Ikemoto 2003). A representation of
dopamine signal circuitry has once been illustrated in
“precisely timed dopamine signals establish distinct
kinematic representations of skilled movements”
(Leventhal & Bova 2020). The aim of this study was
to determine the effects of precisely timed
dopaminergic manipulations on a relatively
unconstrained motor skill. This process effectively
illustrated the circuitry movement in relevant to
dopamine, with the use of experimental conditioning.
Rats were utilized considering ethical guidelines in
human is scarcely attainable. The procedure involves
stimulating or inhibiting midbrain dopamine neurons
in different time period of several groups while rats
Impact of Dopamine on Reward Related Behavior of Animals and Therapeutic Role
135
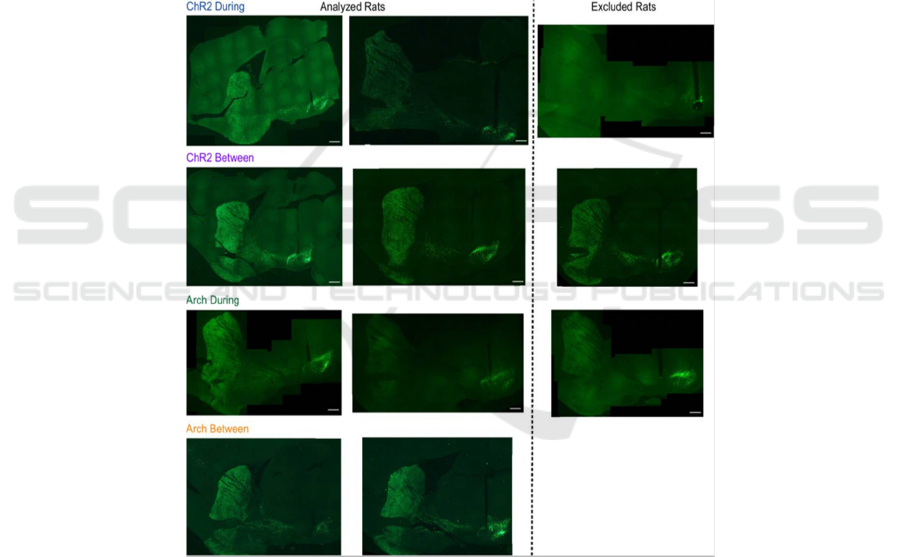
are performing a skilled reaching task, in which the
coordinated forelimb and digit movements to reach
for, grasp, and consume sugar pellets were involved.
In detail, researchers had stimulated or inhibited
substantia nigra pars compacta (SNc) dopamine
neurons at specific moments during rat skilled
reaching. Different Viruses as Tyrosine hydroxylase
(TH)-Cre+ rats were injected bilaterally with a
double-floxed channelrhodopsin (ChR2),
archaerhodopsin (Arch), or control EYFP construct
into SNc.
Explain further into the skilled reaching task,
training and testing were carried out in custom-built
skilled reaching chambers housed within soundproof,
ventilated cabinets (Leventhal & Bova 2020). Trials
were initiated with rats breaking a photobeam at the
back of the chamber, which caused a pellet to be
delivered in front of the reaching slot. Rats could
make multiple reaches until the pellet delivery arm
descended 2 s after the video trigger event. Following
training, optical fibers were implanted over SNc
contralateral to the rat ’ s preferred reaching paw.
Immunohistochemistry confirmed that opsin
expression was restricted to TH-expressing neurons
in SNc projecting to striatum (Leventhal & Bova
2020).
As a result, activity of SNc dopamine neuron
which was altered gradually changes skilled reaching
outcomes. Furthermore, dopamine neuron
stimulation caused a trend of decline in performance.
This shows that dopamine plays an indispensable role
in reinforcement learning and similar skill
acquisition.
Figure 2: Examples of immunohistochemistry from rats for each group.
3.2 Dopamine Projection from Ventral
Tegmental Area
Lichtenburg et al (2018) illustrated inner,
dopaminergic circuitry, or movement while rats are
encountering rewarding tasks or decision making
(Lichtenberg, etc. Reward related behaviors are often
signaled by DA system. Studies have shown that
Dopamine neurons in the ventral tegmental area
(VTA) and subsequent DA release into the NAc
increases and decreases in response to events that are
better or worse than expected. In the appetitive
context, release of dopamine are usually determined
by prediction of potential reward. In contrast, in
aversive contexts, unavoidable aversive events as
shocks or air puff, significantly reduce DA release,
whereas unexpected omission of aversive events or
the cues that predict avoidable shock reliably elicit
phasic DA release (McCutcheon 2012).
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
136
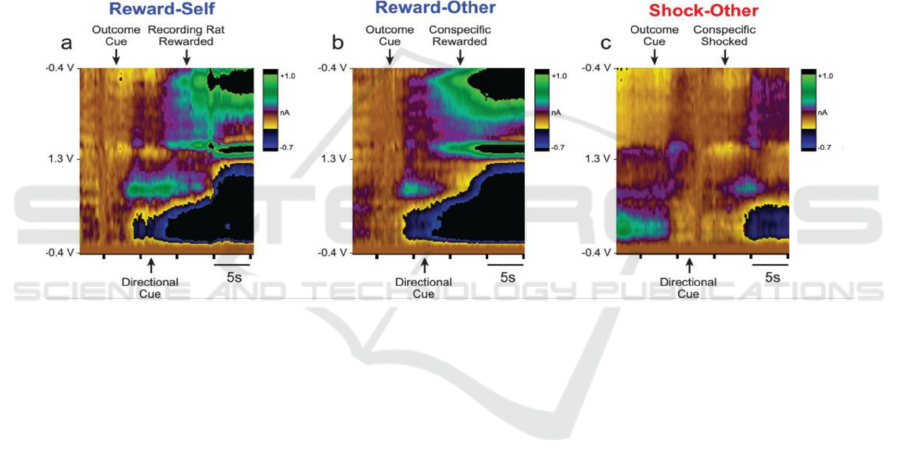
In order to detect and obtain information on how
Dopamine signals are modulated by appetitive and
aversive events from conspecific (opponent), the
research further recorded accumbal, dopamine
release utilizing Fast-Scan Cyclic Voltammetry
(FSCV), which is useful in detection of release of DA
in response to different contexts.
Eight rats were observed during performance of
Pavlovian Social Distress Paradigm. Each group
involved two rats, with the recording rat and
Conspecific, the opponent. They were separated by a
guillotine door which is transparent, allowing visual,
smelly, and vocal action permeate through. One of
each directional light, food cup, and shock grid was
placed in each room with a house light in the middle,
placed right above the delineated line. To initiate each
trial, the researcher first turn on the houselight; after
5 seconds, three different stimuli was randomly
applied to each room correlated to reward, neutral or
shock(punishment) outcomes., with no precursors to
rats but display as which light would be on. Outcomes
was eventually displayed while rats would then
experience the FSCV session mentioned above.
False color plot from FSCV indicates that DA
(dopamine) from rats merely released when outcome
cue was displayed in contrast with little, or declined
reaction after the directional cue. This indicates a
correlation between DA release and reaction in
response to a reward circuitry, or experimental
conditioning. Some researchers also argue a depletion
of DA neuron while individual encounter a reward-
punishment process (Willard, etc. 2019, Bouchard
2015, Morita 2018, Lindahl & Hellgren 2017).
Figure 3: Fast-Scan Cyclic Voltammetry (FSCV) image presenting release of dopamine within rats.
4 FUNCTIONS OF DOPAMINE
4.1 Role in the Prefrontal Cortex of the
Brain
Researchers believe that the role of dopamine is not
only to use rewards to learn the value of past
behaviors, but also that dopamine plays an
indispensable role in the prefrontal cortex of the
brain, enabling us to learn new tasks efficiently,
quickly and flexibly (Wang, Kurth-Nelson, Kumaran
2018).
These researchers from London (deepmind
organization) tested their theories by simulating six
meta-learning experiments in the field of
reconstructed neuroscience-each experiment requires
an agent to perform tasks that use the same basic
principles (or the same set of Skills), but different in
some ways.
An experiment they replicated is called the
Harlow experiment, which was a psychology
experiment in the 1940s to explore the concept of
meta-learning. In the original test, a group of
monkeys were shown two unfamiliar objects, and
only one of them would give them food rewards. The
two objects were displayed 6 times in total, each time
they were placed randomly, so the monkey must
know which one will give them food rewards. Then,
they were shown two other new objects again, and
again, only one of them would give them food.
During this training process, the monkey develops
a strategy to select objects that can be rewarded: it
learns to choose randomly the first time, and then, the
next time it chooses specific objects based on reward
feedback, instead of from left to right. Right
selection. This experiment shows that monkeys can
internalize the basic principles of tasks and learn an
abstract structure of rules—in fact, they learn how to
learn.
Impact of Dopamine on Reward Related Behavior of Animals and Therapeutic Role
137
In fact, researchers found that the meta-RL
(reinforcement learning) agent can learn how to
quickly adapt to various tasks with different rules and
structures. Moreover, since the network has learned
how to adapt to various tasks, it has also learned
general principles on how to learn effectively (Wang,
Kurth-Nelson, Kumaran 2018, Wang, Smith &
Delgado 2016). They also found that most of the
learning takes place in the recurrent network, which
supports the view that the role of dopamine in the
learning process is more important than previously
thought. While traditionally, dopamine is thought to
strengthen the synaptic connections of the prefrontal
system, thereby strengthening specific behaviors.
4.2 Role in the Parkinson’s Disease
Another function of dopamine is reflected in the
concept of Parkinson’s disease (Opara, Małecki &
Socha 2017, Radhakrishnan & Goyal 2018, Seppi &
Ray Chaudhuri 2019). With modern development,
brain detection instruments like CT scan, MRI, and
FMRI have been widely used in psychology,
neuroscience and even medical fields (Tocchio,
Kline-Fath, Kanal, Schmithorst & Panigrahy 2015,
Villanueva-Meyer, Mabray & Cha 2017). Among
them, FMRI has been heavily invested in the research
on the reinforcement learning process and reward
circuitry described in this article (Wang, Smith &
Delgado 2016, Glover 2011). Parkinson's disease
causes a characteristic combination of motor
symptoms due to progressive neurodegeneration of
dopaminergicneurons in the substantia nigra pars
compacta (Glover 2011).
Currently, although there is no cure for
Parkinson's disease with good results, we still cannot
deny the existence of effective drugs (Seppi & Ray
Chaudhuri 2019). In addition to being used for
patients, these drugs can also test the change in
dopamine and it's influence on parkinson's disease
(Wang, Kurth-Nelson, Kumaran, et al 2018).
Approaches in relevance to capturing dopamine
variation in cerebral function, is to test patients in two
conditions under FMRI scan; one is after dopamine
withdrawal with relatively low levels of dopamine in
a pragmatic OFF-medication state and once after
dopamine intake with relatively high levels of
dopamine in an ON-medication state. The differences
in the patient's behaviour and neural activation
between the ON- and OFF-medication state,
considered together with the behaviour and activation
patterns of healthy control participants, is then used
to infer the functional effects of dopamine in the
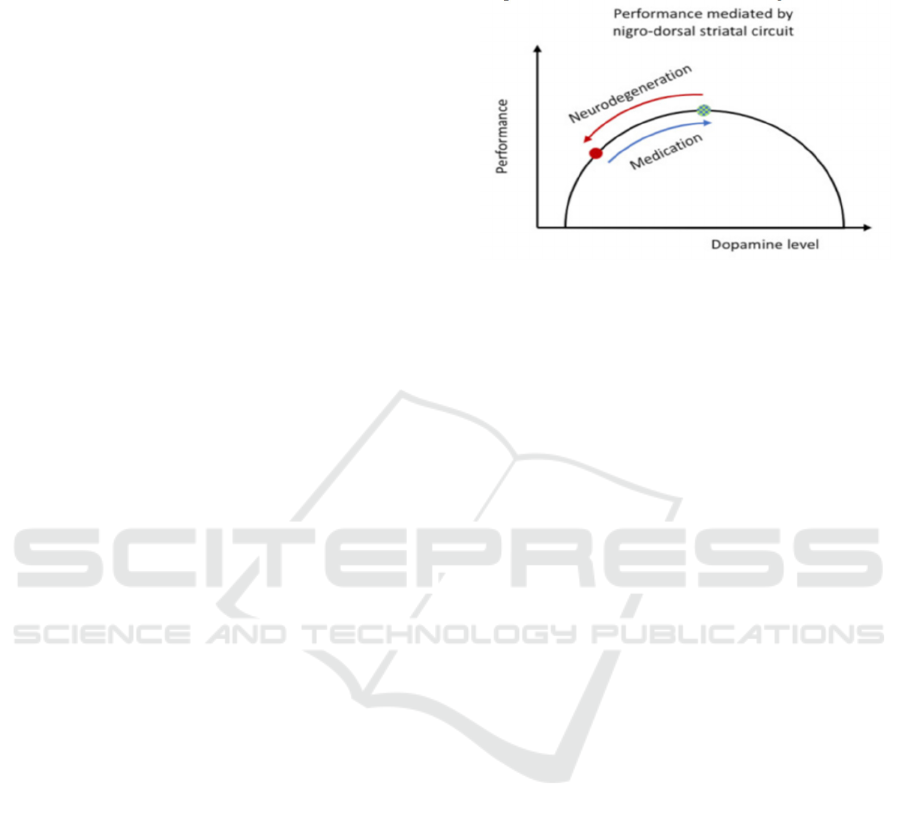
human brain. As shown in the figure, with the
application of medicines, the overall performance of
the patient has risen to a plateau, which is the top of
the performance, and then decline. (figure 4)
Figure 4: Performance mediated by nigro-dorsal stratal
circuit.
5 CONCLUSIONS
From the analysis of theory and actual cases, when
animals encounter certain tasks, the secretion of
dopamine plays a significant role. When the hub
tissues in animal brains encounter the same results or
similar stimuli again and again, the secretion
channels of dopamine are narrowed again and again
until a fixed circuitry is formed. Furthermore, such
discovery also further contributes to modern
education or animal domestication. Human education
is more from the perspective of students, similar to
analogy, to promote students' learning. Unlike
humans, although animals are far inferior to humans
in their cognitive and observational abilities, animal
trainers can also ensure that animals learn and
understand certain tasks through such methods that
have been used extensively, assimilated and similar
to reinforcement learning. Simutaneously, one still
need to be skeptical of dopamine effect on human.
Although many experiments, including diseases as
Parkinson's disease, have demonstrated the role of
dopamine in human brain control and learning, too
many experiments, especially those related to
injection, violate the ethical guidelines of human
experiments, and it is difficult for researchers to reach
one solid conclusion stating a directly, causal effect
between the two.
REFERENCES
Bissonette, G. B., & Roesch, M. R. (2016). Development
and function of the midbrain dopamine system: what
we know and what we need to. Genes, brain, and
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
138

behavior, 15(1), 62–73.
https://doi.org/10.1111/gbb.12257
Bova A, Gaidica M, Hurst A, Iwai Y, Hunter J, Leventhal
DK. (2020). Precisely timed dopamine signals establish
distinct kinematic representations of skilled
movements. Elife. Nov 27; 9: e61591. doi:
10.7554/eLife.61591. PMID: 33245045; PMCID:
PMC7861618.
Bromberg-Martin, E. S., Matsumoto, M., & Hikosaka, O.
(2010). Dopamine in motivational control: rewarding,
aversive, and alerting. Neuron, 68(5), 815–834.
https://doi.org/10.1016/j.neuron.2010.11.022
Burnham J. C. (1972). Thorndike's puzzle boxes. Journal of
the history of the behavioral sciences, 8, 159–167.
https://doi.org/10.1002/1520-
6696(197204)8:2<159::aid-jhbs2300080202>3.0.co;2
Glover G. H. (2011). Overview of functional magnetic
resonance imaging. Neurosurgery clinics of North
America, 22(2), 133–vii.
https://doi.org/10.1016/j.nec.2010.11.001
Haber S. N. (2014). The place of dopamine in the cortico-
basal ganglia circuit. Neuroscience, 282, 248–257.
https://doi.org/10.1016/j.neuroscience.2014.10.008
Ikemoto S, Wise RA. (2004). Mapping of chemical trigger
zones for reward. Neuropharmacology. 47 Suppl
1:190-201. doi: 10.1016/j.neuropharm.2004.07.012.
PMID: 15464137.
Ikemoto S. (2003). Involvement of the olfactory tubercle in
cocaine reward: intracranial self-administration
studies. The Journal of neuroscience: the official
journal of the Society for Neuroscience, 23(28), 9305-
9311. https://doi.org/10.1523/JNEUROSCI.23-28-
09305.2003
Ikemoto S. (2007). Dopamine reward circuitry: two
projection systems from the ventral midbrain to the
nucleus accumbens-olfactory tubercle complex. Brain
research reviews, 56(1), 27–78.
https://doi.org/10.1016/j.brainresrev.2007.05.004
Kashtelyan, V., Lichtenberg, N. T., Chen, M. L., Cheer, J.
F., & Roesch, M. R. (2014). Observation of reward
delivery to a conspecific modulates dopamine release
in ventral striatum. Current biology : CB, 24(21), 2564–
2568. https://doi.org/10.1016/j.cub.2014.09.016
Leventhal D, Bova A. (2020). Precisely-timed dopamine
signals establish distinct kinematic representations of
skilled movements. figshare.
Lichtenberg, N. T., Lee, B., Kashtelyan, V., Chappa, B. S.,
Girma, H. T., Green, E. A., Kantor, S., Lagowala, D.
A., Myers, M. A., Potemri, D., Pecukonis, M. G.,
Tesfay, R. T., Walters, M. S., Zhao, A. C., Blair, R.,
Cheer, J. F., & Roesch, M. R. (2018). Rat behavior and
dopamine release are modulated by conspecific
distress. eLife, 7, e38090.
https://doi.org/10.7554/eLife.38090
Lindahl, M., & Hellgren Kotaleski, J. (2017). Untangling
Basal Ganglia Network Dynamics and Function: Role
of Dopamine Depletion and Inhibition Investigated in a
Spiking Network Model. eNeuro, 3(6),
ENEURO.0156-16.2016.
https://doi.org/10.1523/ENEURO.0156-16.2016
McCutcheon, J. E. etc. (2012). Encoding of aversion by
dopamine and the nucleus accumbens. Frontiers in
neuroscience, 6, 137.
https://doi.org/10.3389/fnins.2012.00137
Meder, D., Herz, D. M., Rowe, J. B., Lehéricy, S., &
Siebner, H. R. (2019). The role of dopamine in the brain
- lessons learned from Parkinson's disease.
NeuroImage, 190, 79–93.
Morita, K., & Kato, A. (2018). A Neural Circuit
Mechanism for the Involvements of Dopamine in
Effort-Related Choices: Decay of Learned Values,
Secondary Effects of Depletion, and Calculation of
Temporal Difference Error. eNeuro, 5(1),
ENEURO.0021-18.2018.
https://doi.org/10.1523/ENEURO.0021-18.2018
Oleson, E. B., etc. (2012). Subsecond dopamine release in
the nucleus accumbens predicts conditioned
punishment and its successful avoidance. The Journal
of neuroscience: the official journal of the Society for
Neuroscience, 32(42), 14804–14808.
Opara, J., Małecki, A., Małecka, E., & Socha, T. (2017).
Motor assessment in Parkinson`s disease. Annals of
agricultural and environmental medicine: AAEM,
24(3), 411–415.
https://doi.org/10.5604/12321966.1232774
Radhakrishnan, D. M., & Goyal, V. (2018). Parkinson's
disease: A review. Neurology India, 66(Supplement),
S26–S35. https://doi.org/10.4103/0028-3886.226451
Seppi, K., Ray Chaudhuri, K., & the collaborators of the
Parkinson's Disease Update on Non-Motor Symptoms
Study Group on behalf of the Movement Disorders
Society Evidence-Based Medicine Committee (2019)
Movement disorders : official journal of the Movement
Disorder Society, 34(2), 180-198.
https://doi.org/10.1002/mds.27602
Sveinbjornsdottir S. (2016). The clinical symptoms of
Parkinson's disease. Journal of neurochemistry, 139
Suppl 1, 318–324. https://doi.org/10.1111/jnc.13691
Tocchio, S., Kline-Fath, B., Kanal, E., Schmithorst, V. J.,
& Panigrahy, A. (2015). MRI evaluation and safety in
the developing brain. Seminars in perinatology, 39(2),
73–104. https://doi.org/10.1053/j.semperi.2015.01.002
Villanueva-Meyer, J. E., Mabray, M. C., & Cha, S. (2017).
Current Clinical Brain Tumor Imaging. Neurosurgery,
81(3), 397–415. https://doi.org/10.1093/neuros/nyx103
Wang, J.X., Kurth-Nelson, Z., Kumaran, D. et al. (2018).
Prefrontal cortex as a meta-reinforcement learning
system. Nat Neurosci 21, 860–868.
https://doi.org/10.1038/s41593-018-0147-8
Wang, K. S., Smith, D. V., & Delgado, M. R. (2016). Using
fMRI to study reward processing in humans: past,
present, and future. Journal of neurophysiology,
115(3), 1664–1678.
https://doi.org/10.1152/jn.00333.2015
Willard, A. M., Bouchard, R. S., & Gittis, A. H. (2015).
Differential degradation of motor deficits during
gradual dopamine depletion with 6-hydroxydopamine
in mice. Neuroscience, 301, 254–267.
https://doi.org/10.1016/j.neuroscience.2015.05.068
Impact of Dopamine on Reward Related Behavior of Animals and Therapeutic Role
139

Willard, A. M., Isett, B. R., Whalen, T. C., Mastro, K. J.,
Ki, C. S., Mao, X., & Gittis, A. H. (2019). State
transitions in the substantia nigra reticulata predict the
onset of motor deficits in models of progressive
dopamine depletion in mice. eLife, 8, e42746.
https://doi.org/10.7554/eLife.42746
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
140
