
Analysis on How CRISPR Technology Facilitates Anticancer
Therapeutics
Sitian Wang
Faculty of Medicine, Imperial College London, SW7 2AZ, London, U.K.
Keywords: Component, CRISPR, Immunotherapy, Cancer, TCR Therapy, CAR-T Therapy.
Abstract: As a novel treatment modality, immunotherapy, based on the principle of boosting the antitumour response,
help patients to fight against cancer. Out of many different types of immunotherapies, adoptive T cell therapy,
characterized by enhancing immunity and specificity by ex vivo manipulation on patient-derived T cells, has
aroused great attention of scholars, who expect to apply it to future cancer treatment. Several CAR-T therapies
have been officially approved in clinical use. Nevertheless, post-transfer T cell exhaustion and
immunosuppression within the tumour region still constitute the major technical limitations. As an
indispensable gene-editing tool with strong capacity in both biomedical research and clinical fields, CRISPR
technology has strong potentials in facilitating adoptive T cell therapies to overcome the current barriers
through full gene knockout on the engineered T cells. In this paper, the clinical feasibility and future prospect
of the combined use of CRISPR-Cas9 and adoptive T cell therapies are analyzed using two latest studies for
discussion and comparison. The studies indicate that CRISPR-Cas9 has facilitated to increase T cell
persistence and potency in TCR therapy and CAR-T therapy respectively with acceptable safety profile, and
it has shed the light for clinical use of CRISPR-Cas9 to increase the therapeutic effectiveness of adoptive T
cell therapy. Future investigations are still needed to further assess its clinical safety and to understand the
underlying mechanism of how CRISPR-Cas9 helps to extend the survival and increase anti-tumour response
of the T cells within the tumour region.
1 INTRODUCTION
CRISPR, which stands for Clustered Regularly
Interspaced Short Palindromic Repeats, is an
indispensable tool in biological research. It was
firstly found in archaea by Mojica et al. in 1995
(Mojica, Ferrer, Juez and Rodríguez-Valera 1995),
and then later experimentally verified by Barrangou
et al. in 2007 as the adaptive immune system of
bacteria to fight against the invading viruses
(Barrangou, Fremaux, Deveau, Richards, Boyaval
and Moineau et al 2007). After years of development,
CRISPR technology can now be modified to target
specific sequence of the genetic code and perform
gene-editing at a relatively precis location, and it has
been widely applied in various field including
biomedicine and agriculture. The function of
CRISPR technology was mainly dependent on the
CRISPR-associated (Cas) genes flanked by the
sequence of CRISPR. Out of the various types of
CRISPR-technology, the most widely applied one is
CRISPR-Cas9, where Cas9 is an endonuclease
guided by sgRNA (single guide RNA).
When Cas9 enters the nucleus, sgRNA facilitates
the recognition of the PAM sequence (protospacer
adjacent motif) and the target sequence, which
consequently leads to the activation of PAM-
dependent Cas9 nuclease. The consequent induced
DNA cleavage will generate double strand breaks
(DSB) and activate homologous recombination (HR)
or non-homologous end joining (NHEJ) to achieve
gene-editing (Sternberg, Redding, Jinek, Greene and
Doudna 2014). Compared with previous gene-
editing tools like Zinc Finger Nucleases (ZFNs) and
Transcription activator-like effector nucleases
(TALENs), CRISPR technologies allow rapid
retargeting of DNA sequence without the
requirement for manufacturing novel proteins for
each target site. Such advantage and the high
genome-editing efficiency of CRISPR allows it to
facilitate the progress in oncology research and
anticancer therapies through different modalities. In
addition to genetic screening to discover potential
128
Wang, S.
Analysis on How CRISPR Technology Facilitates Anticancer Therapeutics.
DOI: 10.5220/0011197300003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 128-133
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

therapeutic targets, CRISPR technologies can be
used to generate cell lines with specific gene
deletions or to manipulate multiple genes to explore
human malignancies. Compared with the traditional
method of manipulations of germline cells to
introduce driver mutation, CRISPR-Cas 9 can
establish cancers more directly in animal models,
which is less time consuming. More importantly, the
combination of CRISPR-Cas9 and cancer
immunotherapy can be a powerful therapeutic
strategy in increasing clinical safety and efficacy
(Yin, Xue and Anderson 2019). The aim of this
review is to analyse the clinical feasibility of using
CRISPR-Cas9 in cancer immunotherapy, especially
in adoptive T cell therapy, and discuss the current
limitation and the prospect of this direction in the
future. Two latest studies are analyzed in this review
to discuss how CRISPR-Cas9 has facilitated
adoptive T cell therapy, where they used CRISPR-
Cas9 to ex vivo knock out the genes of T cells to
increase its therapeutical effectiveness. With the
progress in gene-editing technologies and advances
in immunotherapy, the investigations so far show that
the combined use of CRISPR-Cas9 and
immunotherapy has high translational potential for
clinical use, and some of them have even been
proved safe and effective in incipient clinical pilot
study. Future studies still need to be carried out to
expand our understanding in the underlying
mechanism of the engineered T cells in tumour
microenvironment and further prove its feasibility
for wide clinical use.
2 CRISPR-CAS9 IN TCR
THERAPY AND CAR-T
THERAPY
2.1 CRISPR-Cas9 Knockout of the
Gene Encoding PD-1 And
Endogenous TCR Increases the
Persistence of the Engineered T
Cells in TCR Therapy
Adoptive T cell therapy is a type of immunotherapy
that involves the direct extraction of T cells from the
patient and conduct certain manipulation to increase
its anti-tumour effectiveness. One of the adoptive T
cell therapy called engineered T cell receptor (TCR)
therapy, where T cells from the patients are isolated
and genetically manipulated in vitro to express the
synthetic T cell receptor that specifically target the
cancer cells. However, previous studies have
illustrated that the expression of α and β chains in
endogenous TCR is related with the reduced the
expression of therapeutic TCR due to the competitive
expression, and programmed cell death protein 1
(PD-1) is negatively associated with the antigen
response and persistence of the engineered T cells in
the tumour region (Hamilton, Doudna 2020). The
consequently reduced therapeutic efficacy
constituted the major limitation of TCR therapy, and
CRISPR-Cas9 may be a promising approach to
overcome it through disrupting the genes associated
with T cell exhaustion and the reduced antigen
response.
In an article published in Science in 2020,
Stadtmauer et al. conducted the first-in-human phase
1 clinical trial, where they aimed to investigate the
use of CRISPR-Cas9 in improving the effectiveness
and safety of TCR therapy on patients with advanced
and refractory cancer (Stadtmauer, Fraietta, Davis,
Cohen, Weber and Lancaster et al 2020). They
hypothesised that the deletion of the genes encoding
PD-1 and the α and β chain in endogenous TCR
would improve the persistence of the engineered T
cells and increase the feasibility of the initial TCR
therapy. Referring to the graphical abstract (see
Fig.1), CRISPR-Cas9 was used to knock out TRAC,
TRBC, and PDCD1 over isolated T cells from the
patients, and then they introduced the synthetic TCR
transgene NY-ESO-1, which can specifically target at
myeloma, melanoma, and sarcoma, through
lentiviral transduction into the cells. The CRISPR-
Cas9-engineered T cells were later infused back into
the 3 patients with advanced and refractory cancer.
The T cells were then tracked and monitored in vivo
to determine if they could persist longer with better
safety profile after the CRISPR-Cas9 modification.
The results of the In vitro assessment indicated that
cells modified by CRISPR-Cas9 has higher
cytotoxicity than those retained the endogenous
TCR, suggesting a higher potency. After the cell
infusion, there is no evidence of T cell genotoxicity
or overt side effect observed in the patients, and high
level and sustained persistence of the engineered
cells were found with antigen-specific cytotoxicity.
Overall, the results of this trial overall indicated the
acceptable safety profile of the CRISPR-modified
transgenic T cells with higher sustained level and
higher specificity targeting the tumour. However, the
potential mechanism of the extended survival of the
T cells was not investigated in this study, and whether
the longer half-life of the engrafted transgenic T cells
is attributed to PD-1 deficiency was not explained.
Hence the transcriptional state of the modified T cells
Analysis on How CRISPR Technology Facilitates Anticancer Therapeutics
129

within the tumour micro-environment can be
investigated next step to see how the cytotoxicity and
persistence are increased. In addition, only one
patient out of the total three had the highest level of
engraftment, which restricted the in vivo single-cell
analysis, herein more patients for infusion of the cells
with higher editing efficiencies and engraftment
level are needed to fully assess the safety and
feasibility in the use of CRISPR-Cas9 in TCR
therapy in the future.
Figure 1: Graphical Abstract of using CRISPR-Cas9 to knock out the gene encoding PD-1 and endogenous TCR (Stadtmauer,
Fraietta, Davis, Cohen, Weber and Lancaster et al 2020).
2.2 CRISPR-Cas9 Improves the
Efficacy of CAR-T Therapy via
Knockout of Adenosine Receptor
Similarly, CRISPR-Cas9 also shows great potential
in improving the effectiveness of CAR-T therapy,
which is another type of adoptive T cell therapy.
Similar to TCR therapy, CAR-T therapy involves the
process of T cell extraction with ex vivo transduction
of the T cell with chimeric antigen receptor (CAR),
which can specifically recognize a defined tumour
antigen. Compared to TCR therapy, the introduction
of CAR transgene in TRAC gene can knock out the
gene encoding TCR simultaneously (Roth, Puig-
Saus, Yu, Shifrut, Carnevale and Li et al 2018).
However, one of the major barriers of CAR-T
therapy is the effect of immunosuppression. Out of
the multiple immunosuppressive pathways, the
hypoxia-adenosine link is relatively prominent in
tumour region, where adenosine binding to the
receptor A2AR reduces the accumulation of
intracellular cAMP and increases the production of
anti-inflammatory factors in immune cells to
suppress the immune response (Raker, Becker and
Steinbrink 2016). Previous studies indicated that
pharmacological blockade of A2AR is able to
enhance the T-cell-mediated antitumour effect
(Halpin-Veszeleiova, Hatfield 2020). Hence, these
findings suggest the potential of targeting the
immunosuppressive pathway via CIRSPR-Cas9 to
improve the efficacy of adoptive T-cell therapy.
In May 2021, an article published in Nature by
Giuffrida et al. It indicated that A2AR deletion can
enhance the efficacy of CAR-T therapy via CRISPR-
Cas9, which further proves the strong potential of the
use of CRISPR technology in improving anticancer
therapies compared with other methodologies
(Giuffrida, Sek, Henderson, Lai, Chen and Meyran et
al 2021). They delivered the recombinant Cas9 and
sgRNA targeting at the gene encoding A2AR into
naïve splenocytes via electroporation, followed by
retroviral transduction of CAR targeting human Her2
cells. With the aim to investigate whether CRISPR-
Cas9-mediated A2AR deletion could enhance CAR-
T cell function, the level of cAMP signalling, in vivo
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
130

antitumour efficacy in mice models, and
transcriptional profile of the engineered T cells were
all assessed and analysed. It was then found the
editing efficiency of CRISPR-Cas9 on the gene
encoding A2AR could achieve more than 75% in
human CAR-T cells and result in potent attenuation
in the level of intracellular cAMP. Because of the
high sensitivity of A2AR towards adenosine, A2AR
knockdown via short hairpin RNA (shRNA) or
pharmacological blockade is not effective enough in
attenuating the conversion of ATP to cAMP. Instead,
the full knockout of A2AR is sufficient to
significantly suppress the immunosuppressive
pathway mediated by adenosine in the function of
CAR-T cells. The survival of the tumour bearing
mice was significantly prolonged due to the
enhanced inhibition of tumour growth by CAR-T
cells after A2AR deletion, and the memory recall
responses was able to get evoked. Through the
analysis of the transcriptional profile, the suppressive
effect on the production of pro-inflammatory
cytokines like IFNγ and TNF by CD4+ and CD8+
mediated by hypoxia-adenosine pathway was also
significantly reduced, indicating the enhanced
therapeutic efficacy. It has been found that the
increased CAR-T cell activation mediated by the
knockdown or knockout of A2AR could enhance the
expression of several effector-related genes
including PD-1, granzyme B and Ki-67, which may
compromise the persistence the T cells. Surprisingly,
the deletion of A2AR by CRISPR-Cas9 had minimal
effect in the persistence of the CAR-T cells compared
with the control group, unlike knockdown or
pharmacological blockade. However, the
mechanisms that full knockout of A2AR by
CRISPR-Cas9 uses to circumvent the reduction in
persistence is unknown yet, and it may be related
with the production of pro-survival factors in
memory T cells. For next step, it would be interesting
to analyse the difference in the expression of the
memory associated genes between knockdown and
knockout CAR-T cells to investigate the underlying
mechanisms of the uncompromised persistence in
CRISPR-modified CAR-T cells.
3 DISCUSSIONS
As discussed above, T-cell exhaustion and
immunosuppression are major technical barriers that
result in reduced efficacy and potency of the adoptive
T cell therapy when the cells are infused back into
the patients. Gene-editing technology like CRISPR-
Cas9 can be an influential tool to overcome these
limitations with increasing editing efficiencies and
precisions over these years. Herein, two latest studies
from 2020 and 2021 were discussed in this review to
demonstrate how CRISPR-Cas9 helps with
improving the effectiveness of CAR-T therapy and
TCR therapy.
In these 2 studies, different pathways were
targeted with similar aims, and both show promising
future for clinical application. The first study used
CRISPR-Cas9 to prevent the engineered T cell
exhaustion through suppressing the apoptosis
pathway via PD-1 knockout and to improve the
expression of the synthetic TCR through disrupting
the expression of endogenous TCR, and it has been
proved safe and effective to improve the cell
persistence in the first-in-human pilot study.
Whereas the second study distinctively targeted the
immunosuppressive pathway via A2AR knockout to
increase the antitumour response, and it has been
proved effective in improving the potency of the
CAR-T cells in animal models. This has shed the
light of utilizing CRISPR-Cas9 to target multiple
immunosuppressive genes to improve the therapeutic
efficacy of CAR-T therapy in the future. In contrast
to the first study, the second study showed that A2AR
deletion via CRISPR-Cas9 in T cells has increased
the expression of PD-1. However, the persistence of
the CAR-T cells is not significantly affected but with
increased cytokine production, and this achieved a
well-balanced trade-off between cell persistence and
therapeutic efficacy. Compared with other
immunosuppressive pathways, the adenosine-
activated pathway is more prominent in hypoxia
tumour microenvironment, which also equips it with
advantageous efficacy profile. In terms of frequency
of editing, the editing efficiencies of TRAC, TRBC,
and PDCD in the first study are 45%, 15%, and 20%
respectively, and it might be due to the limited
progress in the CRISPR-based technology back in
2016 when their clinical trial application was
approved, leading to higher off-target effect.
Whereas the editing efficiency of A2AR reached
over 75% in the latest paper here, suggesting the
strong potential of CRISPR technology in facilitating
anticancer therapies with increasing on-target editing
efficiency over the years.
However, safety considerations are still important
considerations regarding the permanent deletion of
certain genes using CRISPR-Cas9. There are still
limited studies using gene-editing technologies to
target immunosuppressive pathways in CAR-T cells.
Despite the in vivo assessment in mice models
proved that A2AR-edied CAR-T cells are well
tolerated with good safety profile through liver and
Analysis on How CRISPR Technology Facilitates Anticancer Therapeutics
131
kidney toxicity analysis. Whether permanent A2AR
deletion will induce excessive immune response
against the host still needs further assessment and
observation. In addition, PD-1 is not the only
indicator of T-cell exhaustion which is also related
with other immunoregulatory pathways like soluble
factors IL-10 and regulatory T cells (Wherry 2011).
Moreover, the underlying mechanism of increased
persistence and improved potency of the modified T
cells after PD-1 and A2AR knockout are not clear
yet. Hence, despite the positive conclusion in early
clinical trials and incipient in vivo assessment, the
wide feasibility and long-term safety of CRISPR-
modified engineered T cells still needs future
investigations.
Nowadays, CRISPR-based technology has
shown promising future in improving the
effectiveness of immunotherapy to enhance its
efficacy and reduce the toxicity. Immunotherapy is
mainly based on the innate and adaptive immune
system of the host to activate the specific immune
response or reduce the immunosuppressive effect,
including monoclonal antibodies, vaccine therapies,
checkpoint inhibitors, and adoptive T cell therapies.
The general principle of adoptive T cell therapy is to
boost the antitumour response through ex vivo
manipulation of the T cells to increase their ability
and specificity targeting the tumour when they are
infused back into the patient. The modalities include
ex vivo expansion of tumour-infiltrating
lymphocytes (TILs), the introduction of transgenic
TCR that targets the major histocompatibility
complex (MHC) to eradicate tumour cells, and gene
transfer of chimeric antigen receptors that target
specific antigen presented on the surface of the
tumour cells . With the high response rate compared
with conventional chemotherapy, adoptive T cell
therapy has made huge progress in recent years.
Several therapies have been approved for clinical use
with large number of them in the stage of clinical
trials. Notwithstanding such success in its early
clinical application, adoptive T cell therapy are still
facing various challenges. For example, CAR-T
therapy is mainly used in haematological cancers,
because of the high heterogeneity of the solid
tumours leading to challenges for transgenic TCR
targeting the tumours. Moreover, the nature of
adoptive T cell therapy being highly personalized
also make the manufacturing cost unexpectedly high
and difficult to get industrialized. Hence allogeneic
CAR T cells from have become a promising direction
to reduce the manufacturing cost and simplify the
procedure. However, the main barrier of using
allogeneic T cells from healthy donors are the
rejection by the immune system of the recipient and
the toxicity resulted from the non-self antigen grafted
from the recipient to the donor cells (Graham,
Jozwik, Perpper and Benjamin 2018). Thus, in
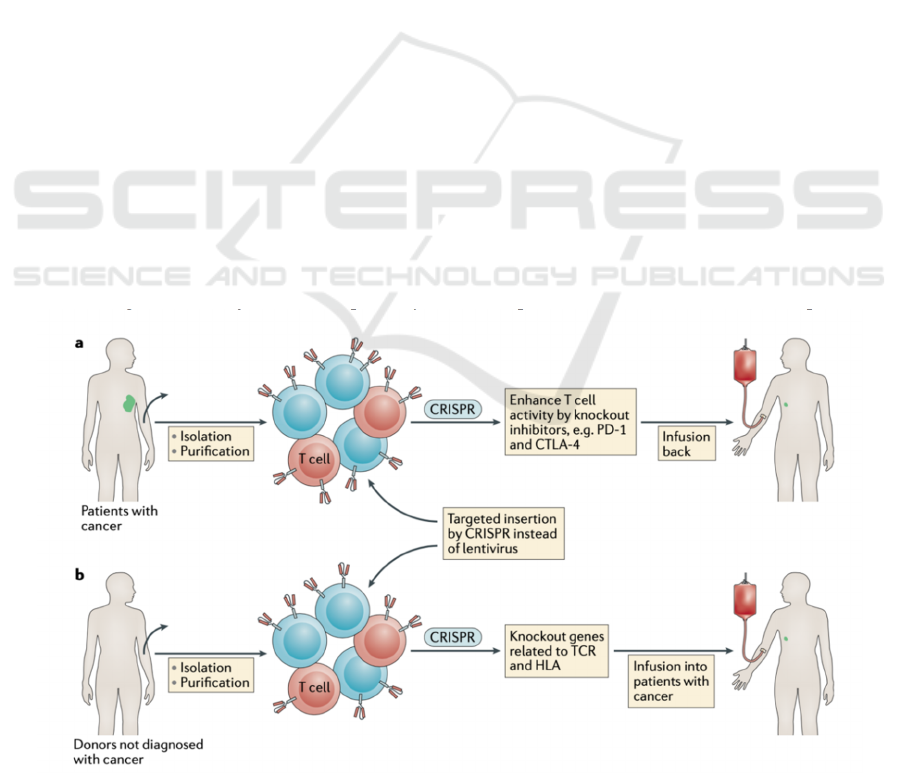
addition to improving the efficacy of the current
adoptive T cell therapies, CRISPR-Cas9 can also be
a useful tool to knock out the related genes expressed
on the surface of the allogeneic T cells to reduce the
rejection and toxicity (see Fig.2) and make the
therapies “off -the-shelf” for most of the patients.
Figure 2: Graphical Abstract of using CRISPR-Cas9 on allogeneic T cells for CAR-T cells (Yin, Xue and Anderson 2019).
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
132

4 CONCLUSIONS
To sum up, this paper discusses the clinical feasibility
of CRISPR-Cas9 being applied into adoptive T cell
therapy and addresses the limitations of the current
investigations. Gene-editing technology has overall
showed high clinical translational potential in
overcoming T cell exhaustion and
immunosuppression to improve the therapeutical
effectiveness of adoptive T cell therapy. Moreover, it
is also promising to contribute to the development of
allogeneic CAR-T therapy through reducing the
toxicity and rejection effect via gene knockout and to
further simplify the manufacturing process.
However, safety considerations are still major
concerns regarding the application of gene editing
technologies. The underlying mechanisms of
CRISPR-Cas9 in improving adoptive T cell therapies
are still not fully understood yet, and the clinical
feasibility and safety of CRISPR-Cas9 application in
anticancer therapies still need further investigations,
despite the incipient success.
REFERENCES
C.Graham, A. Jozwik, A. Pepper, R. Benjamin. Allogeneic
CAR-T Cells: More than Ease of Access?. Cells.
2018;7(10):155.
E. Stadtmauer, J. Fraietta, M. Davis, A. Cohen, Weber,
Lancaster E et al. CRISPR-engineered T cells in
patients with refractory cancer. Science. 2020;
367(6481): eaba7365.
E.Wherry. T cell exhaustion. Nature Immunology. 2011;
12(6):492-499.
F. Mojica, C. Ferrer, G. Juez, F. Rodríguez-Valera. Long
stretches of short tandem repeats are present in the
largest replicons of the Archaea Haloferax
mediterranei and Haloferax volcanii and could be
involved in replicon partitioning. Molecular
Microbiology. 1995;17(1):85-93.
H. Yin, W. Xue, D. Anderson. CRISPR–Cas: a tool for
cancer research and therapeutics. Nature Reviews
Clinical Oncology. 2019;16(5):281-295.
J. Hamilton, J. Doudna. Knocking out barriers to
engineered cell activity. Science. 2020;367(6481):976-
977.
K.Halpin-Veszeleiova, S. Hatfield. Oxygenation and
A2AR blockade to eliminate hypoxia/HIF-1α-
adenosinergic immunosuppressive axis and improve
cancer immunotherapy. Current Opinion in
Pharmacology. 2020; 53:84-90.
L.Giuffrida, K. Sek, M. Henderson, J. Lai, A. Chen, D.
Meyran et al. CRISPR/Cas9 mediated deletion of the
adenosine A2A receptor enhances CAR T cell efficacy.
Nature Communications. 2021;12(1).
R. Barrangou, C. Fremaux, H. Deveau, M. Richards, P.
Boyaval, S. Moineau et al. CRISPR Provides Acquired
Resistance Against Viruses in Prokaryotes. Science.
2007;315(5819):1709-1712.
S. Sternberg, S. Redding, M. Jinek, E. Greene, J. Doudna.
DNA interrogation by the CRISPR RNA-guided
endonuclease Cas9. Nature. 2014;507(7490):62-67.
T. Roth, C. Puig-Saus, R.Yu , E.Shifrut , J. Carnevale, Li P
et al. Reprogramming human T cell function and
specificity with non-viral genome targeting. Nature.
2018;559(7714):405-409.
V. Raker, C. Becker, K.Steinbrink. The cAMP Pathway as
Therapeutic Target in Autoimmune and Inflammatory
Diseases. Frontiers in Immunology. 2016;7.
Analysis on How CRISPR Technology Facilitates Anticancer Therapeutics
133
