
Studies on Calibration of Medical Carbon Dioxide Incubator
Han Luo
1a
, Tingting Ren
2b
, Yang Xu
1c
, Zhongmu Zhou
1d
and Huijuan Wang
1e
1
Center for Medical Metrology, Chongqing Academy of Metrology and Quality Inspection, Chongqing, China
2
Center for Length Metrology, Chongqing Academy of Metrology and Quality Inspection, Chongqing, China
Keywords: Medical Carbon Dioxide Incubator, Calibration, Temperature Deviation, Temperature Fluctuation,
Temperature Uniformity, CO
2
Concentration Indication Error, CO
2
Concentration Control Error, Humidity.
Abstract: Medical carbon dioxide incubator is widely used in medical microbiological and biotechnological laboratories
to simulate the growth environment for in vitro culture of cells or tissues of organisms. This article presents
a novel design of calibration procedure to test the essential parameters of the medical carbon dioxide incubator
such as "temperature deviation", "temperature fluctuation", "temperature uniformity", "CO2 concentration
indication error", "CO2 concentration control error" and "humidity" in order to ensure the accuracy and
reliability of the instrument. The research result can ensure the metrological traceability of medical carbon
dioxide incubator by studying the instrument’s key technical parameters and selecting the suitable
metrological standards.
1 INTRODUCTION
Medical carbon dioxide incubator is widely used in
medical microbiological and biotechnological
laboratories to simulate the growth environment for
in vitro culture of cells or tissues of organisms.
Usually, the temperature inside the chamber is
regulated at 37℃, and the CO
2
content of the
atmosphere in the chamber is regulated at 5%. The
main difference between medical carbon dioxide
incubator and normal incubator is that the former can
provide a certain concentration of CO
2
in order to
meet the growth environment requirements of
microorganism. Medical carbon dioxide incubator is
essential metrological equipment for cell, tissue and
bacterial culture in immunology, oncology, genetics
and bioengineering researches. However, till now,
national metrological verification regulation has not
been issued applicable to the calibration of medical
carbon dioxide incubator, therefore, and the
corresponding traceability system of which has not
been established yet.
The purpose of this paper is to study the
influencing factors on essential technical parameter
such as "temperature deviation", "temperature
a
https://orcid.org/0000-0003-0763-3727
b
https://orcid.org/0000-0002-6320-0179
c
https://orcid.org/0000-0001-8385-9790
fluctuation", "temperature uniformity", "CO
2
concentration indication error", " CO
2
concentration
control error" and "humidity" of medical carbon
dioxide incubator, as well as to determine the
measuring standards and to establish an applicable
calibration procedure, in order to improve research
conditions of medical laboratories, and furthermore,
to establish the traceability system of medical carbon
dioxide incubator.
2 CLIBRATION CONDITIONS
2.1 Environmental Conditions
Temperature: (15~35) ℃;
Humidity: ≤85%RH;
The environmental conditions shall also meet the
requirements for the normal use of measuring
standards and other supporting equipment.
d
https://orcid.org/0000-0002-9082-4681
e
https://orcid.org/0000-0001-6633-6453
Luo, H., Ren, T., Xu, Y., Zhou, Z. and Wang, H.
Studies on Calibration of Medical Carbon Dioxide Incubator.
DOI: 10.5220/0011186700003444
In Proceedings of the 2nd Conference on Artificial Intelligence and Healthcare (CAIH 2021), pages 91-96
ISBN: 978-989-758-594-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
91
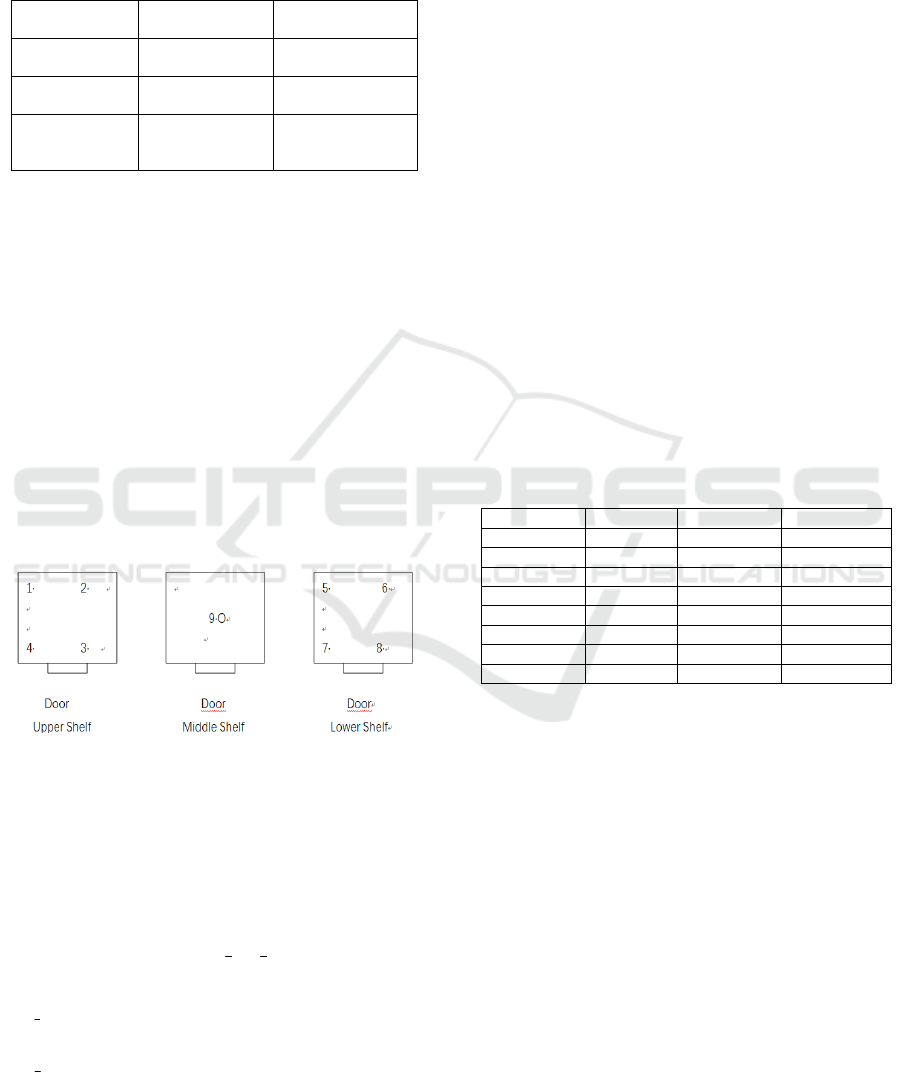
2.2 Measuring Standards and Other
Supporting Equipment
Table 1: Measuring Standards and Other Supporting
Equipment.
Measuring
Range
Technical
Requirements
Standard
Thermomete
r
(0~65) ℃
Definition: ≤0.1℃
MPE: ±0.2℃
Standard CO
2
Senso
r
(0~20)%
Definition: ≤0.1%
MPE: ±0.3%
Standard
Humidity
Senso
r
(10~100)%RH
Definition: ≤0.1%
MPE: ±2.0%RH
3 TEMPERATURE CLIBRATION
3.1 Temperature Calibration
Procedure and Calculation
Equations
Place 9 calibrated standard thermometers in the
empty chamber of medical carbon dioxide with
shelves in place as demonstrated in Figure 1. Locate
standard thermometers (marked as 1, 2, 3, 4, 5, 6, 7,
8) in each of the eight corners of the incubator
approximately 1/10 length of the side from each wall,
and place the ninth standard thermometer (marked as
9) in the geometric center of the chamber.
Figure1: Positioning of Standard Sensors
Set the control temperature at 37°C. Take 16
readings of each standard thermometer as well as the
incubator’s temperature indication value at regular
intervals of 2min in 30 min during steady temperature
condition. Calculate the temperature deviation,
temperature fluctuation and uniformity according to
equation (1), (2), (3):
0
ttt
d
−=Δ
(1)
tΔ
—Temperature deviation, ℃;
d
t
—Average indication temperature of medical
carbon dioxide incubator, ℃;
0
t
—The average temperature measured by
standard thermometer 9 located in the geometric
center, ℃.
2/)(
min0max0
ttt
f
−±=Δ
(2)
f
t
Δ
—Temperature fluctuation, ℃;
max0
t
—The maximum temperature value
measured by standard thermometer 9 located in the
geometric center, ℃;
min0
t
—The minimum temperature value measured
by standard thermometers located in the geometric
center, ℃.
2/)(
minmax
ttt
u
−=Δ
(3)
max
t
—The maximum temperature reading taken
by standard thermometers during 30min, ℃;
mini
t
—The minimum temperature reading taken
by standard thermometers during 30min, ℃;
u
tΔ
—Temperature uniformity, ℃.
3.2 Experimental Results of
Temperature Calibration
Select several typical types of medical carbon dioxide
incubators as the calibrated subjects. The
experimental result is demonstrated in Table 2.
Table 2: Temperature Calibration Results (unit:℃).
Type Deviation Fluctuation Uniformity
3111 0.2 ±0.05 0.5
3131 0.2 ±0.05 0.4
371 0.2 ±0.1 0.5
HF240 -0.3 ±0.1 0.4
HF240 0.3 ±0.1 0.5
HF151 -0.2 ±0.2 0.3
HF90 -0.3 ±0.2 0.4
WJ-3-160 0.2 ±0.05 0.3
The temperature deviation and fluctuation
calibration results have met the metrological criterion
set by YY 1621-2018 Medical carbon dioxide
incubator of no more than ±0.3 ℃. The temperature
uniformity calibration results have met the
metrological criterion set by YY 1621-2018 Medical
carbon dioxide incubator of no more than ±0.5 ℃.
4 CO
2
CONCENTRATION CALI-
BRATION
4.1 CO
2
Concentration Calibration
Procedure and Calculation
Equations
Place the standard CO
2
Senor (marked as O) in the
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
92

geometric center of the incubator chamber as
demonstrated in Figure 1.
Set the incubator’s control temperature at 37°C
and the CO
2
concentration value at 5%. Take 10
readings of the standard CO
2
sensor as well as the
incubator’s CO
2
concentration indication value at
regular intervals of 15min 2 hours after the set value
of temperature and CO
2
concentration has reached.
Calculate the CO
2
concentration indication error and
control error according to equation (4), (5):
sdd
CCC −=
(4)
d
C
—CO
2
concentration indication error, %;
d
C
—Average value of 10 CO
2
concentration
indication readings of the incubator, %;
s
C
—CO
2
concentration set value of incubator, %.
sc
CCC −=
(5)
c
C
—CO
2
concentration control error, %;
C
—Average value of 10 readings of CO
2
sensor,
%.
4.2 Experimental Results of CO
2
Concentration Calibration
Select several typical types of medical carbon dioxide
incubators as the calibrated subjects. The
experimental result is demonstrated in Table 3:
Table 3: CO
2
Concentration Calibration Results (unit: %).
Type Indication Erro
r
Control Erro
r
3111 0.1 0.3
3131 -0.2 -0.4
371 0.2 0.4
HF240 0.1 0.2
HF240 0.1 0.1
HF151 0.2 -0.4
HF90 -0.1 -0.2
WJ-3-160 0.1 0.1
The CO
2
concentration indication error
calibration results have met the metrological
criterion set by YY 1621-2018 Medical carbon
dioxide incubator of no more than ±0.2%, and the
CO
2
concentration control error calibration results
have met the metrological criterion set by YY 1621-
2018 Medical carbon dioxide incubator of no more
than ±0.5%.
5 HUMIDITY CALIBRATION
5.1 Humidity Calibration Procedure
Place the standard humidity
Senor (marked as O) in
the geometric center of the incubator chamber as
demonstrated in Figure 1.
Set the incubator’s control temperature at 37°C
and the CO
2
concentration value at 0%. Take the
reading of the standard humidity
sensor 2 hours after
the set value of temperature.
5.2 Experimental Results of Humidity
Calibration
Select several typical types of medical carbon dioxide
incubators as the calibrated subjects. The
experimental result is demonstrated in Table 4.
Table 4: Humidity Calibration Results (unit: %).
Type
Humidity measured by Standard
Senso
r
3111 97.6
3131 98.1
371 99.0
HF240 97.9
HF240 98.2
HF151 99.1
HF90 99.0
WJ-3-160 99.5
The humidity calibration results have met the
metrological criterion set by enterprise standards of
no less than 95%.
6 UNCERTAINTY ANALYSIS
6.1 Uncertainty Analysis on
Temperature Measurement
6.1.1 Standard Uncertainty Introduced by
Temperature Measurement
Repeatability
Select a type 3111 medical carbon dioxide incubator
to conduct the temperature calibration procedure
presented in Chapter 3 at the set value of 37℃. The
obtained values are demonstrated in Table 5:
Table 5: Temperature Repeatability Test Result (unit: ℃).
No. Measured Temperature
1 36.8
2 36.9
3 36.8
4 36.7
5
36.8
6
36.7
7 36.9
8
36.8
Studies on Calibration of Medical Carbon Dioxide Incubator
93

9
36.8
10
36.7
Sum
367.9
___
t
36.79
Calculates the standard deviation with Bessel
formula:
℃074.0
110
)(
1
)(
)(
10
1
2
___
1
2
=
−
−
=
−
−
=
=− i
i
n
i
i
tt
n
tt
ts
(6)
16 readings shall be taken when the medical
carbon dioxide incubator is calibrated in practical
use. So the standard uncertainty introduced by
measurement repeatability is as follows:
℃
0185.0
16
)(
1
==
t
s
u
(7)
6.1.2 Standard Uncertainty Introduced by
the Resolution of Standard
Thermometer
The resolution of standard thermometer is 0.1℃,
considering uniform distribution, the standard
uncertainty introduced by the resolution is as follows:
℃
029.0
32
1.0
2
==u
(8)
6.1.3 Standard Uncertainty Introduced by
the Maximum Permissive Error of
Standard Thermometer
The maximum permissive error of standard
thermometer is ±0.2℃, considering uniform
distribution, the standard uncertainty introduced by
maximum permissive error is as follows:
℃
05.0
32
2.0
3
==u
(9)
6.1.4 Synthetic Uncertainty of Temperature
Measurement
The uncertainty components analyzed are shown in
Table 6:
Table 6: Summary of Uncertainty Components of
Temperature Measurement (Unit: ℃).
Source of Standard
Uncertaint
y
Uncertainty
T
yp
e
Uncertainty
Measurement
Repeatabilit
y
A 0.0185
Resolution of
Standard
Thermomete
r
B 0.029
The Maximum
Permissive Error of
Standard
Thermomete
r
B 0.058
Since the standard uncertainty introduced by the
resolution of standard thermometer
℃
029.0
2
=u
is
positively correlated with the uncertainty introduced
by measurement repeatability
℃
0.0185
1
=u
,
therefore, only the standard uncertainty introduced by
resolution of standard thermometer
2
u
is considered,
when synthetic uncertainty is calculated as follows:
℃
064.0
32c
=+= uuu
(10)
6.1.5 Extended Uncertainty of Temperature
Measurement
Take the inclusion factor k=2 (confidence probability
is 95%), the extended uncertainty is calculated as
follows:
℃0.130.12820.064
c
≈=×== kuU
(11)
6.2 Uncertainty Analysis on CO
2
Concentration Measurement
6.2.1 Standard Uncertainty Introduced by
CO
2
Concentration Measurement
Repeatability
Select a type 3111 medical carbon dioxide incubator
to conduct the CO
2
concentration calibration
procedure presented in Chapter 4 at the set value of
5%. The obtained values are demonstrated in Table 7:
Table 7: CO
2
Concentration Repeatability Test Result.
No. Measured CO
2
Concentration/%
1 4.8
2 4.7
3 4.8
4 4.7
5 4.9
6 4.6
7 4.7
8 4.8
9 4.8
10 4.7
Sum 47.5
___
t
4.75
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
94

Calculates the standard deviation with Bessel
formula:
%085.0
110
)(
1
)(
)(
10
1
2
___
1
2
=
−
−
=
−
−
=
=− i
i
n
i
i
tt
n
tt
ts
(12)
10 readings shall be taken when the medical
carbon dioxide incubator is calibrated in practical
use. So the standard uncertainty introduced by
measurement repeatability is as follows:
%027.0
10
)(
1
==
t
s
u
(13)
6.2.2 Standard Uncertainty Introduced by
the Resolution of Standard CO
2
Sensor
The resolution of standard CO
2
sensor is 0.1%,
considering uniform distribution, the standard
uncertainty introduced by the resolution is as follows:
%029.0
32
1.0
2
==u
(14)
6.2.3 Standard Uncertainty Introduced by
the Maximum Permissive Error of
Standard CO
2
Sensor
The maximum permissive error of standard CO
2
sensor is ±0.3%, considering uniform distribution, the
standard uncertainty introduced by maximum
permissive error is as follows:
87%0.0
32
3.0
3
==u
(15)
6.2.4 Synthetic Uncertainty of CO
2
Concentration Measurement
The uncertainty components analyzed are shown in
Table 8:
Table 8: Summary of Uncertainty Components of CO
2
Concentration Measurement.
Source of Standard
Uncertainty
Uncertainty
Type
Uncertainty
(%)
Measurement Repeatability A 0.31
Resolution of Humidity
Senso
r
B 0.029
The Maximum Permissive
Error of Humidity
Senso
r
B 0.58
Since the standard uncertainty introduced by the
resolution of standard CO
2
sensor
%029.0
2
=u
is
positively correlated with the uncertainty introduced
by measurement repeatability
7%02.0
1
=u
, therefore,
only the standard uncertainty introduced by
resolution of standard CO
2
sensor
2
u
is considered,
when synthetic uncertainty is calculated as follows:
0.092%
32c
=+= uuu
(16)
6.2.5 Extended Uncertainty of CO
2
Concentration Measurement
Take the inclusion factor k=2 (confidence probability
is 95%), the extended uncertainty is calculated as
follows:
℃
0.20.18420.092
c
≈=×== kuU
(17)
6.3 Uncertainty Analysis on Humidity
Measurement
6.3.1 Standard Uncertainty Introduced by
Humidity Measurement Repeatability
Select a type 3111 medical carbon dioxide incubator
to conduct the humidity calibration procedure
presented in Chapter 5. The obtained values are
demonstrated in Table 9:
Table 9: Humidity Repeatability Test Result.
No.
Humidity measured by Standard Sensor
/%
1 97.8
2 97.9
3 98.0
4 98.2
5 97.6
6 97.9
7 97.7
8 98.5
9 97.5
10 97.6
Sum 978.7
___
t
97.87
Calculates the standard deviation with Bessel
formula:
31%.0
110
)(
1
)(
)(
10
1
2
___
1
2
=
−
−
=
−
−
=
=− i
i
n
i
i
tt
n
tt
ts
( 1 8 )
6.3.2 Standard Uncertainty Introduced by
the Resolution of Standard Humidity
Sensor
The resolution of standard humidity
sensor is 0.1%,
considering uniform distribution, the standard
Studies on Calibration of Medical Carbon Dioxide Incubator
95
uncertainty introduced by the resolution is as follows:
%029.0
32
1.0
2
==u
(19)
6.3.3 Standard Uncertainty Introduced by
the Maximum Permissive Error of
Standard Humidity Sensor
The maximum permissive error of standard humidity
sensor is ±2.0%, considering uniform distribution, the
standard uncertainty introduced by maximum
permissive error is as follows:
58%.0
32
2
3
==u
(20)
6.3.4 Synthetic Uncertainty of Humidity
Measurement
The uncertainty components analyzed are shown in
Table 10:
Table 10: Summary of Uncertainty Components of CO
2
Concentration Measurement.
Source of Standard
Uncertaint
y
Uncertainty
Type
Uncertainty
(%)
Measurement
Re
p
eatabilit
y
A 0.027
Resolution of Standard
CO
2
Senso
r
B 0.029
The Maximum
Permissive Error of
CO
2
Senso
r
B 0.087
Since the standard uncertainty introduced by the
resolution of standard humidity
sensor
%029.0
2
=u
is
positively correlated with the uncertainty introduced
by measurement repeatability
0.31%
1
=u
, therefore,
only the standard uncertainty introduced by
measurement repeatability
1
u
is considered, when
synthetic uncertainty is calculated as follows:
0.66%
31c
=+= uuu
(21)
6.3.5 Extended Uncertainty of Humidity
Measurement
Take the inclusion factor k=2 (confidence probability
is 95%), the extended uncertainty is calculated as
follows:
1.3%1.3220.66
c
≈=×== kuU
(22)
7 CONCLUSION
The article studies the key technical parameters such
as "temperature deviation", "temperature
fluctuation", "temperature uniformity", "CO
2
concentration indication error", " CO
2
concentration
control error" of medical carbon dioxide incubator,
determines the appropriate standards and supporting
instruments for measuring, and performs the novel
calibration procedure presented in this article on
several typical types of medical carbon dioxide
incubators widely used in China. The testing results
meet the requirements of national standard YY1621-
2018.
Therefore, the article presents a feasible
procedure for the periodic calibration of medical
carbon dioxide incubator in order to establish the
metrological traceability system of the instrument.
Further work is worth to be done to improve the
calibration method of medical carbon dioxide
incubator.
ACKNOWLEDGEMENTS
Our work was supported by National Metrology
Verification Regulation Program of China (Grant No.
MTC-2019-163).
REFERENCES
ASTM E1292 (2011) Standard Specification for Gravity
Convection and Forced Ventilation Incubators.
American Society of Testing Materials, West
Conshohoken.
JJF 1101(2019)
Calibration Specification of Environmental
Testing Equipment for Temperature and Humidity
Parameters. State General Administration for Quality
Supervision and Inspection and Quarantine, Beijing.
YY 1621(2018)
Medical carbon dioxide incubator.
National Medical Products Administration, Beijing.
ZHOU A.S., et al.. Evaluation of Uncertainty in
Measurement of CO
2
Incubators[J]. Metrology &
Measurement Techniques, 2011.38(11):55-56
CAIH 2021 - Conference on Artificial Intelligence and Healthcare
96
