
Quantity and Composition of Liquid Products from Used Motorcycle
Tire Pyrolysis
Sitti Sahraeni, Ibnu Eka Rahayu and Helda
Department of Chemical Engineering, Polytechnic State of Samarinda, East Kalimantan, Indonesia
Keywords: Used Tires, Pyrolysis, Liquid Products, Yield, Temperature.
Abstract: Used tires are very difficult to degrade naturally. So far, the handling of used motorcycle tires has only been
hoarding or burning directly in the open air. This is less able to reduce used tires because it will
cause
problems with pollutant gases resulting from burning used materials that can pollute the
environment. The
pyrolysis process is one way to minimize waste restrictions. The purpose of this study is to determine the
effect of the temperature of the pyrolysis of used motor vehicle tires on the yield of liquid products and to
determine the composition of the liquid product at the highest yield. The pyrolysis process of used tires was
carried out for 2 hours and 1000 grams of raw material. Pyrolysis was carried out at
various operating
temperatures, namely 450 ° C, 500 ° C, 550 ° C, 600 ° C, and 650 ° C without using a
catalyst. The yield
of liquid products produced at operating temperatures were 450 ° C, 500 ° C, 550 ° C,
600 ° C, and 650 °
C respectively 41.31%, 55.07%, 51.49%, 41 , 99%, and 36.89%. The liquid product
composition test for
used motor vehicle tires at a temperature of 550 ° C was analyzed using GC-MS (Gas Chromathography-
Mass Spectrometer), the hydrocarbon chains were obtained as follows: (C5-C12) as much as 95.12% and
(C13-C20) as much as 4, 88%.
1 INTRODUCTION
Used tires are very difficult to degrade
naturally by
nature. So far, the handling of used motorcycle tires
has only been carried
out by hoarding or burning
directly in the
open air. This is less able to reduce
used tires, because it will cause problems with
pollutant gases resulting from burning used tires that
can pollute the environment
.
Another way to process
used tires is by processing them into handicraft
products. Handling used tires is
usually done using
the already popular method, namely 3R (Reuse,
Reduce, Recycle), but that method has weaknesses. A
better alternative for dealing with waste or used
tires is to convert them into other
forms, namely
liquid fuels as alternative
energy. Rubber-based tires
are one of the
synthetic polymers (polystyrene).
Polystyrene is derived from petroleum so the
best
solution at this time is to return to the
form of oil.
Polystyrine cracking is a way of reducing this
waste. Cracking is the process of breaking polymer
chains into compounds
with lower molecular
weights. Although this method is included in
recycling, the results of recycling do not return to the
form of tires or rubber. This used tire cracking uses
the
pyrolysis method (Handono, M. R. T., 2017). The
tire composition
consists of Styrene-butadiene
rubber (SBR) 62.1%, Carbon black 31%, Extender
Oil 1.9%, Zinc Oxide 1.9%, Stearic Acid 1.2%,
Sulfur 1.1%, Accelerator 0 ,7% (Williams &
Besler,
1995). Where Styrene-butadiene
rubber (SBR) is a
synthetic rubber that will
be converted into another
form, namely
liquid fuel as an alternative energy.
Based on the results of research conducted by (Muis
et
al., 2019) it was found that the compounds
contained in the pyrolysis oil of used tires have the
following hydrocarbon chains: (C1- C5) as much as
0.33%, (C5-C12) as much as 88.96% and (C10-C28)
10.71%. The hydrocarbon compounds found in the
oil
from the pyrolysis of used tires contain a lot of
aromatic compounds, wherein the
aromatic
compounds are derivatives of petroleum
hydrocarbon compounds which function as fuel
components. Many researches on pyrolysis using
used motor vehicle tires have been carried out, one
of which was conducted by Muis et al., (2019) This
researcher used used tires as raw materials with
variations in catalyst mass. Pyrolysis was carried out
at a temperature of 400⁰C within 3 hours, the oil
conversion obtained increased with increasing
1142
Sahraeni, S., Eka Rahayu, I. and Helda, .
Quantity and Composition of Liquid Products from Used Motorcycle Tire Pyrolysis.
DOI: 10.5220/0010961000003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 1142-1145
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
catalyst
mass. The highest yield was found in the
180 gram catalyst weight of 41.073%. Dumilah &
Kholidah, (2019) conducted a
study using used two-
wheeled motorcycle
tires as raw materials with a
temperature
variation of 200⁰C – 300⁰C in an
operating time of 3 hours and using a zeolite catalyst.
The best results obtained were 58.6030% at
a
temperature of 300⁰C. To get maximum and
efficient results, the pyrolysis process is
carried out
without the use of a catalyst, this aims to avoid the
activation of the catalyst
which is quite long so that
it can shorten the
time used for the pyrolysis process.
Research on the effect of pyrolysis
temperature
without a catalyst using bicycle tire waste raw
materials with
temperature variations of 450 C, 500
C, 550 C, 550 C, and 650 C. The percent yield of
the liquid product produced was 49.6% with
the
optimum temperature of 600 C with a
reaction time
of 39 minutes.
The percent yield generated on research
Debalaxmi is higher as many as 49.5% compared to
the research conducted by Muis., Et al (2019), which
is
only about 41.07%, this is because the
higher
the temperature the more more liquid
product is
produced and can reduce the
reaction time required.
Therefore, the
pyrolysis process of used motorcycle
tires without using a catalyst in temperature
variations is carried out at medium to high
temperatures, it is hoped that it can produce
a
maximum and faster percent. The purpose of this
study was to
determine the effect of the pyrolysis
temperature of used motor vehicle tires on the yield
of liquid products and to determine the composition
contained in the liquid
products at the highest yields.
2 METODHOLOGY
This study uses quantitative and qualitative
analysis.
used motorcycle tires are pyrolyzed in a reactor with
variations in temperature. Quantitative analysis is
carried out by weighing the resulting product. Used
motorcycle tires are cleaned and dried and then
reduced in size by 2 cm. A total of 1000 grams were
put into the pyrolysis apparatus
and the process was
run for 2 hours with
variations in pyrolysis time
(450, 500, 550, 600, 650) °C. The resulting liquid
product is
then weighed and its composition
analyzed using GC-MS
3 RESULT AND DISCUSSION
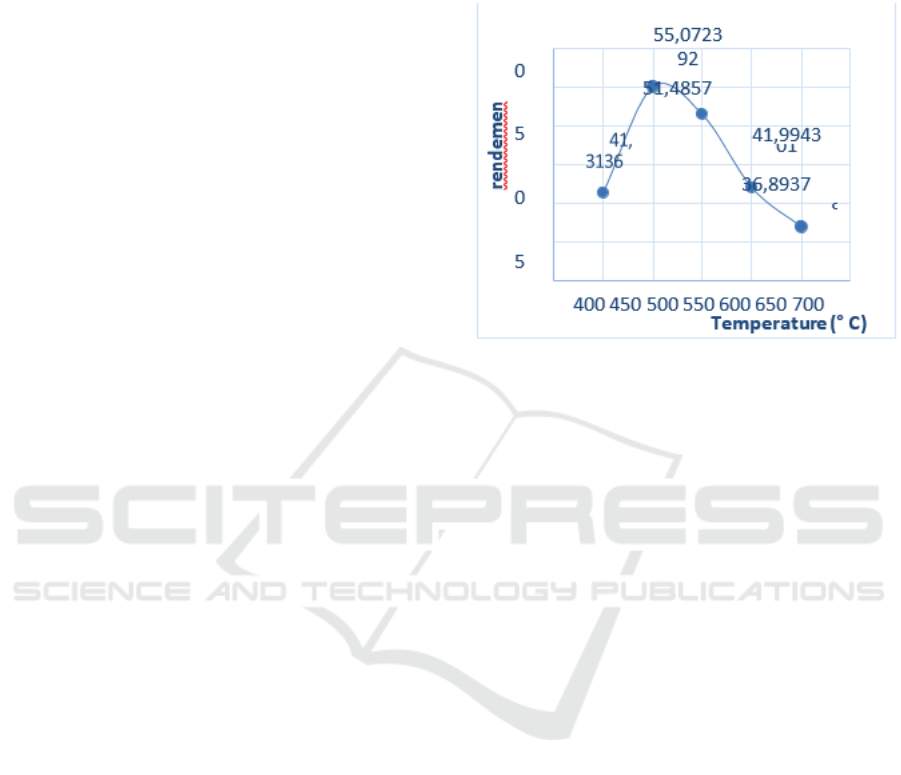
The relationship between temperature and the yield
of liquid products
can be seen in Figure 1
Figure 1: The Effect of Temperature
Relationship on the
Yield of Liquid Products.
Figure 1. shows that when the pyrolysis
process
is carried out, the percent yield
increases from a
temperature of 450 °C to
500 °C, this is because at
high temperatures the carbon chain will be more
easily cracked than at low temperatures so that the
yield of liquid products produced will be more and
more (Kholidah , 2018). The yield
percentage
increased with the initial increase
in temperature and
after the temperature of 550oC the percentage yield
decreased. Jung et al., (2013) explained this
phenomenon
that after reaching the optimal
temperature, several secondary reactions such as
polyaromatic formation reactions are
initiated during
the pyrolysis of used tires which reduce the yield
of liquid products. This is also in accordance with
research conducted by Udyani et al (2018), where the
yield produced will decrease with increasing
temperature from pyrolysis. This is because
the
higher the temperature, the more used tires break
down into non-condensable gases (CO, CO2, CH4,
etc.) so that less liquid is
produced. Based on Figure
1 shows that the
temperature of 500 °C is the optimal
temperature because it reaches the highest
yield of
55.07% which when the temperature
is increased to
550 °C the liquid product
decreases to 51.49% with
a pyrolysis
process time of 2 hours, while in the
research conducted by Dumilah and Kholidah
(2019), using temperature
variations where at the
highest temperature
of 300 °C added using a 400
gram catalyst
produced a liquid product of 58.60%,
and in
the research conducted by Muis et al.,
Quantity and Composition of Liquid Products from Used Motorcycle Tire Pyrolysis
1143
(2019) using The variation of the weight of the
catalyst which had previously been activated for 10
hours and the pyrolysis
process for 3 hours at a
temperature of 400°C resulted in the highest
percentage yield,
which was around 41.07%.
From the yield obtained, it shows that the
percent yield obtained between the pyrolysis
of used
tires using a catalyst and the
pyrolysis of used tires
without the use of a
catalyst is only a difference of
about 3.53% when compared to the results of
research conducted by Dumilah and Kholidah (2019),
and higher when compared to with research
conducted by Muis et al., (2019). The results of the
pyrolysis of used motor vehicle tires without using
a catalyst obtained a higher percent yield because
during the process it
uses a higher temperature so
that the
cracking process of used tires becomes faster
and at the optimum temperature produces more liquid
products.
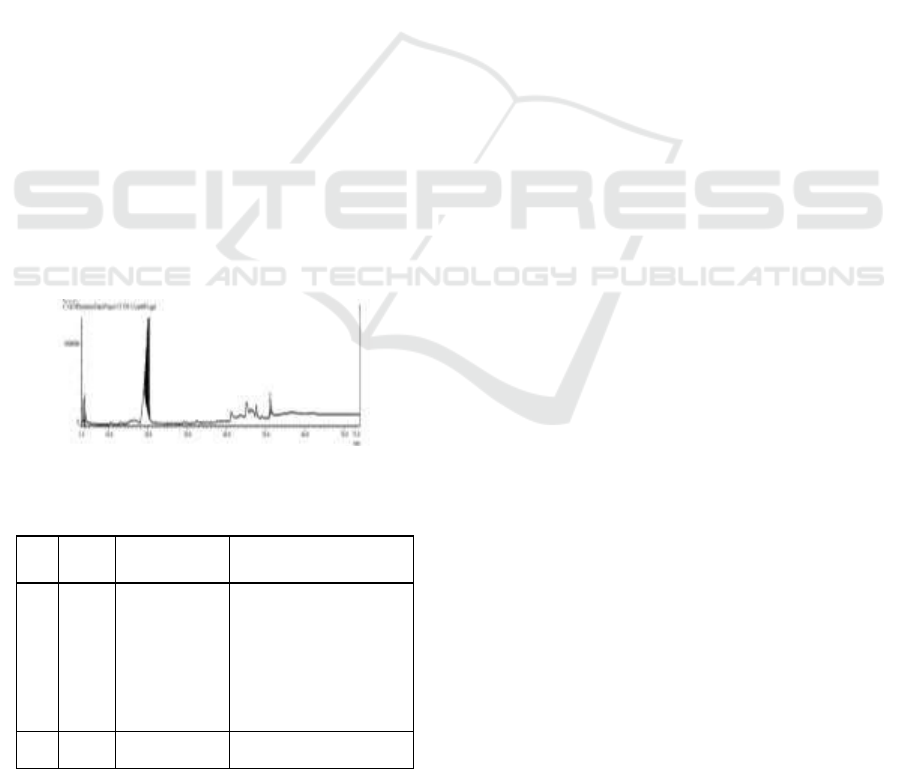
The results of the pyrolysis of used motor
vehicle tires which have the highest percent
yield at
a temperature of 500 °C were
analyzed using GC-
MS (Gas Chromatoghraphy-Mass Spectrometry).
Based on Table 4.2, it shows that the
characteristics of the liquid fuel obtained have the
following hydrocarbon chains: (C5 - C12) as much
as 95.12% and (C13 - C20¬) as much as 4.88%. This
is similar to the
experiment conducted by Andry et
al., (2020) where the results of the GC-MS analysis
showed the highest hydrocarbon compounds (C5 -
C12) and included the
gasoline fraction.
Figure 2: Spectra of GC-MS Pyrolysis Oil of Used Tires.
Table 1: GC-MS analysis at a
temperature of 500 °C.
No %
Area
Molecular
Formula
Compoun
d
1.
95,12
%
C
5
- C
12
1H-Azepine,
hexahydro;
2H-
Azepine-2-One,
hexahydro;
cyclobutene,
1,2,3,4- tetramethyl;
Hexanoic acid, 6- amino
2. 4,88
%
C
13
- C
20
9-Octadecenal, (Z)
Based on research conducted by Muis., et al
(2019), the results of pyrolysis
analyzed using GC-
MS are the results of pyrolysis that have the highest
conversion,
namely 41.073% at a temperature of 400
°C. The analysis results obtained have the
highest
hydrocarbon chain (C5 - C12) as much as 88.96%.
This shows that the
pyrolysis process using a catalyst
carried out
by Muis., et al (2019) produced the
same
fraction as the study without using a catalyst
but the operating temperature used was lower at
400 °C compared to the pyrolysis
experiment
without using a catalyst, which was 500 °C. . This
is due to the presence of a
catalyst so that in the
Muis., et al. (2019) experiment, the process of
breaking or breaking long chain hydrocarbons is
faster.
4 CONCLUSIONS
1. The higher the temperature, the
lower the
liquid product yield and the percent yield
obtained. The
temperature of 500 °C is the
optimal temperature where the
highest yield
percentage is 55.07%.
2. The results of the GC-MS analysis
show that
the composition of the
liquid product at a
temperature of 500 °C has the most
hydrocarbon chains (C5 - C12) as much as
95.12% which is included in the
gasoline
fraction (gasoline).
ACKNOWLEDGEMENTS
The author would like to acknowledge
the Center
for Research and Community
Service at Polytechnic
State of Samarinda
which has provided funding for
this research as well as to the Chemical Engineering
Laboratory of Polytechnic State of Samarinda as a
place for the research to be
carried out.
REFERENCES
Arahim, A. A., Widayat, dan Hadiyanto. (2020). Liquid
Fuel Production From
Motorized Vehicle
Tires
With
Pirolysis
Process. International
Coference on Science
and Applied Science,
https://doi.org/10.1063/5.0030380.
Arita, S., Assalami. A., Naibaho.D.I. (2015). Proses
Pembuatan Bahan Bakar Cair
Dengan Memanfaatkan
Limbah Ban Bekas Menggunakan Katalis Zeolit.
Teknik Universitas Sriwijaya
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
1144

Damayanthi, Reska dan Martini, R. (2009). Proses
Pembuatan Bahan Bakar Cair
Dengan Memanfaatkan
Limbah Ban Bekas Menggunakan Katalis Zeolit Y
Dan Zsm-5. Institutional Repository, Diponegoro
University, 8.
Dumilah, T. R., & Kholidah, N. (2019). Pengaruh
Temperatur terhadap Hasil
Pirolisis Limbah Ban
menjadi Bahan Bakar Cair menggunakan Katalis
Zeolit.
Juma, M., Koreňová, Z., Markoš, J., Jelemensky, L., &
Bafrnec, M. (2007). Experimental study of pyrolysis
and combustion of scrap tire. Polymers for
Advanced
Technologies, 18(2), 144–148.
Miandad, R., Nizami, A. S., Rehan, M., Barakat, M. A.,
Khan, M. I., Mustafa, A., Murphy, J. D. (2016).
Influence of temperature and reaction time on the
conversion of polystyrene waste to
pyrolysis liquid oil.
Waste
Management.
https://doi.org/10.1016/j.wasman.2016.09.023
Muis, L., Prabasari, I. G., & Suyana, N. (2019). Pengaruh
Berat Katalis Zeolit
Alam terhadap Pencairan Limbah
Ban dalam Bekas Kendaraan Bermotor Roda Dua
Menjadi Bahan Bakar Cair. Jurnal Daur Lingkungan,
2(2), 63. https://doi.org/10.33087/daurling.v2i2.
29
Nisar, J., Ali, G., Shah, A., Iqbal, M., Ali, R., Anwar, F.,
Salim, M. (2019). Fuel
production from waste
polystyrene via
pyrolysis : Kinetics and product
distribution. Waste Management, 88, 236–247.
https://doi.org/10.1016/j.wasman.2019. 03.035
Onwudili, J. A., Insura, N., & Williams, P. T. (2009).
Composition of products
from the pyrolysis of
polyethylene and polystyrene in a closed batch reactor:
Effects of temperature and residence
time.
Journal of
Analytical
and Applied Pyrolysis, 86(2), 293–303.
https://doi.org/10.1016/j.jaap.2009.07. 008
Handono,M.R.T. (2017). Pembuatan Bahan Bakar Cair
Dengan Memanfaatkan
Limbah Ban Bekas
Menggunakan Katalis Limbah Bekas Perengkahan
Minyak Bumi PT. Pertamina RU III
Quantity and Composition of Liquid Products from Used Motorcycle Tire Pyrolysis
1145
