
The Effect of Air Flow Rate on the Production of Active Charcoal
from Palm Oil Shells with Partial Oxidation Method
Firman and Sitti Sahraeni
Department of Chemical Engeneering, Polytechnic State of Samarinda, East Kalimantan, Indonesia
Keywords: Activated Charcoal, Oil Palm Shell, Air Flow Rate, Partial Oxidation, Pyrolysis.
Abstract: Palm kernel shell is one of the palm oil processing wastes which is quite large, reaching 6.5% of 1 ton of
palm
oil. This shell can be used as an ingredient for making activated charcoal. Activated charcoal is widely
used as an adsorbent, gas purification, water purification and so on. The palm kernel shell (PKS) is the
hardest part of the components found in oil palm. Oil palm shell contains 26.6% cellulose and 27.7%
hemicellulose which are
good for making activated charcoal. This study aims to determine the effect of air
flow rate on activated charcoal
according to SNI No. 06-3730-1995. Carbonization and activation are
carried out using pyrolysis with the
principle of partial oxidation. The pyrolysis process was carried out at
air flow rates of 20, 25, 30, 35 and 40 L/ min for 5 hours. The best results were shown at an air flow rate of
35 L / min with a product yield of 20%, water content of 5.82%, ash content of 7.51%, volatile matter
content of 8,73%, fixed carbon 77,94%, and
absorption. iodine of 750,1403 mg / g. These results have met
the SNI 06-3703-1995 standards.
1 INTRODUCTION
Oil palm shells contain 26.6% cellulose and
27.7% hemicellulose which are good for making
activated charcoal. The average annual production of
oil palm fruit is 5.6 million tons, which means that
around 364,000 tons of shells are produced. This
number will continue to increase in line with the
increase in palm oil production. With the availability
of this waste, a further process is needed to convert
palm oil shell waste into a product that has high
economic value such as activated charcoal
(Yuliusman, 2015).
The development of industry is increasing
along with the development of science and
technology, so that industry is one of the important
sectors that supports the Indonesian economy.
However, there are several industries that are
developing slowly, in this case the charcoal and
activated charcoal manufacturing industry. Activated
charcoal is widely used as adsorbent, gas purification,
water purification and so on. Activated charcoal can
be made from all materials containing charcoal, both
organic and inorganic, provided that the material has
a porous structure.
Activated charcoal is a porous solid containing
85-95% carbon, produced from carbon-containing
materials by heating at high temperatures. Charcoal
is a porous solid material
which is the result of
combustion of materials
containing carbon
elements, while activated charcoal
is charcoal that
is activated by immersion in
chemicals or by
flowing hot steam into the material,
so that the
pores of the material become more open with a
surface area range from 300 to 2,000 m2/g. The
wider
surface of activated charcoal has an impact on the
higher absorption of gas or liquid materials. The
methodology used includes the process of preparing
activated charcoal, absorption and testing.
Research on the manufacture of activated
charcoal with the pyrolysis method conducted by
Hasan et al., 2020 which the study varied the addition
of the amount of N2 gas, namely without the addition
of nitrogen gas, the addition of nitrogen gas in
batches, and the addition of nitrogen gas continuously
with air flow rates of 0.5 L/min and 1 L/min. The best
pyrolysis conditions are with nitrogen gas flowing
continuously at 1 liter/minute. characteristics of
activated charcoal with a yield value of 42%, water
content 3.23%, ash content 2.73% volatile substances
28.37%, fixed carbon 66.16%. Research on the
pyrolysis mechanism namely the production of
pilot scale
activated charcoal from oil palm shells
1138
Firman, . and Sahraeni, S.
The Effect of Air Flow Rate on the Production of Active Charcoal from Palm Oil Shells with Partial Oxidation Method.
DOI: 10.5220/0010960900003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 1138-1141
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
(cks), the study varied the pyrolysis time and
temperature. In this variation, the yield is 33-52%,
water content 2- 5%, volatile matter 20-70%, ash
content 2-10% and fixed carbon 22-70%.
Yuliusman, 2015 conducted research with the
title of making activated carbon from palm oil shells
with KOH and N2/CO2 as activating ingredients.
Chemical activation with KOH 75% and physical
activation at a temperature of 850°C using N2 gas for
1 hour followed by CO2 gas for 1 hour.
Characteristics produced by the activation process at
a temperature of 850°C have an iodine number of 884
mg/g with a water content of 13.6%, an ash content
of 9.4% and a concentration lost on heating of 23.1%.
In this study using the pyrolysis method with the
principle of partial oxidation with a pilot plant scale
with a raw material capacity of 5 kg/batch, physically
activated not using pure N2 gas and CO2 but utilizing
excess N2 gas in the air by limiting the air flow rate
which aims to increase the pores. so that activated
charcoal has a high absorption capacity. The
advantage of this method is to use internal heat or heat
generated from the oxidation reaction that arises by
limiting the air flow rate in the raw material
combustion process so that it can reduce combustion
efficiency. In addition, with the air flow rate, biomass
can create new porosity which can affect the
combustion process and the resulting liquid smoke.
The purpose of this study was to determine the
effect of air flow rate on activated charcoal using oil
palm shells with a pyrolysis process using the
principle of partial oxidation in a pilot plant scale with
a raw material capacity of 5 kg/batch, and to
determine the quality of charcoal produced by
pyrolysis of oil palm shells. One of the advantages of
making activated charcoal with the partial oxidation
method is that at the activation stage it does not use
chemicals but utilizes nitrogen gas in the air as an
activator by limiting oxygen entering through the air
flow rate.
2 METHODOLOGY
A total of 5 kg of PKS shells are dried and
cleaned then the pyrolysis process will be carried out
using a pyrolysis device which is assembled
consisting of a raw material sleeve, combustion
chamber, compressor and condenser. The pyrolysis
combustion process uses coals that are inserted
through the bottom hole of the pyrolysis and held for
± 10 minutes. Then the compressor is turned on and
adjusts the air flow rate on the flow meter according
to the air flow rate used. Cooling water is run in the
condenser section. The process runs for 5 hour. The
resulting charcoal is then analyzed for absorption
of
iodine by the SNI method No. 06-3730-1995
Calculate the absorption of activated charcoal against
iodine using the following formula:
Iodine NUmber 𝐼
=
10 −
𝑉
x 𝑁
𝑁
x126,9 x fp × N
W
Keterangan:
V
tio
=
Required volume
of sodium
thiosulfate
solution (ml)
N
tio
= Normality of sodium thiosulfate (N)
solution
N
iod
= Normality of I
2
solution
126,9 = Iodine atomic weight
W
= Sample mass (grams)
3 RESULT AND DISCUSSION
The yield of activated charcoal produced relatively
decreases as air is added or flowed into the
reactor
during the pyrolysis process.
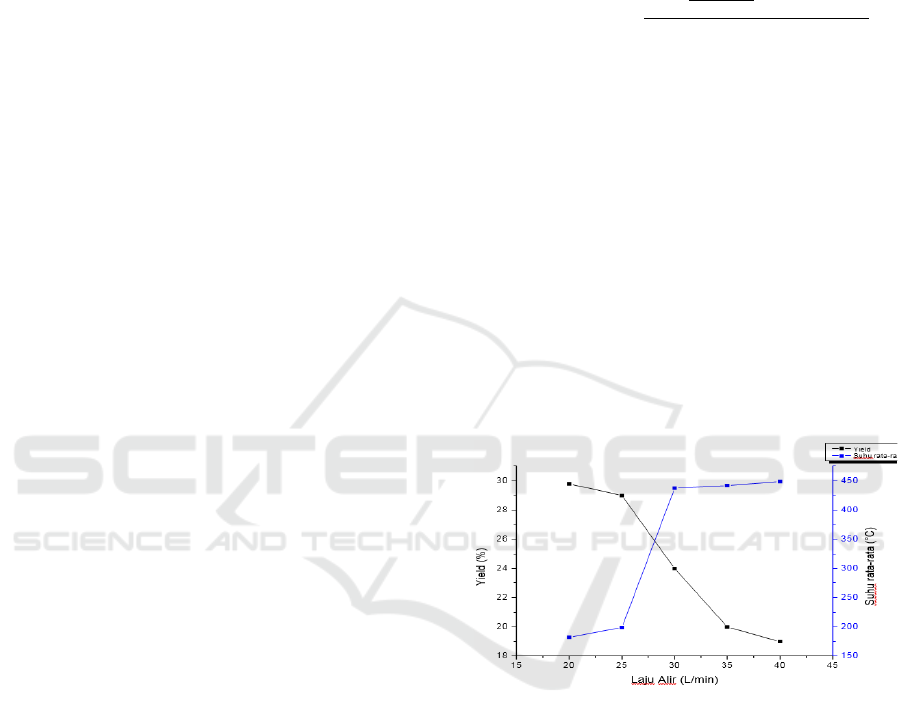
Figure 1: The relationship between the air flow rate and
the average temperature of pyrolysis with the yield of
activated charcoal produced.
In Figure 1 it can be seen that at a flow rate of 20
L/min the activated charcoal product produced is at
the maximum yield, which is 29.8%, while at a flow
rate of 40 L/min the minimum yield of activated
charcoal product is 19%. . It can be seen that the yield
continues to decrease along with the addition or flow
of air into the reactor, namely the more oxygen and
nitrogen gas that is circulated, the yield of activated
charcoal obtained is also relatively decreased (Hasan
et al., 2020). In addition, temperature is also very
influential on the pyrolysis process. The higher the
temperature, the better
the
decomposition/decomposition process, but the less
The Effect of Air Flow Rate on the Production of Active Charcoal from Palm Oil Shells with Partial Oxidation Method
1139
amount of charcoal obtained while the more liquid
and gas results, due to the large amount of
decomposed and evaporated substances. The
maximum yield was obtained at an average
temperature of 182.68 °C at 29.8% and the minimum
yield was obtained at a temperature of 448.98 °C at
19%, this is in accordance with the statement of Haji
et al., 2010 that due to the high temperature some
charcoal turns into ash and volatile gases, so the yield
tends to be low. It can be concluded that the oxygen
and nitrogen that are flowed into the reactor help the
pyrolysis process occur perfectly, the incoming
oxygen reacts with the activated charcoal to become
CO
2
which causes the amount of solids to decrease.
The function of oxygen here is to oxidize the material
while nitrogen is a physical activating agent. The
absorption of iodine (iod adsorption) indicates the
ability of activated carbon to adsorb components with
low molecular weight. Activated carbon with high
Iodine absorption microstructure and pores.
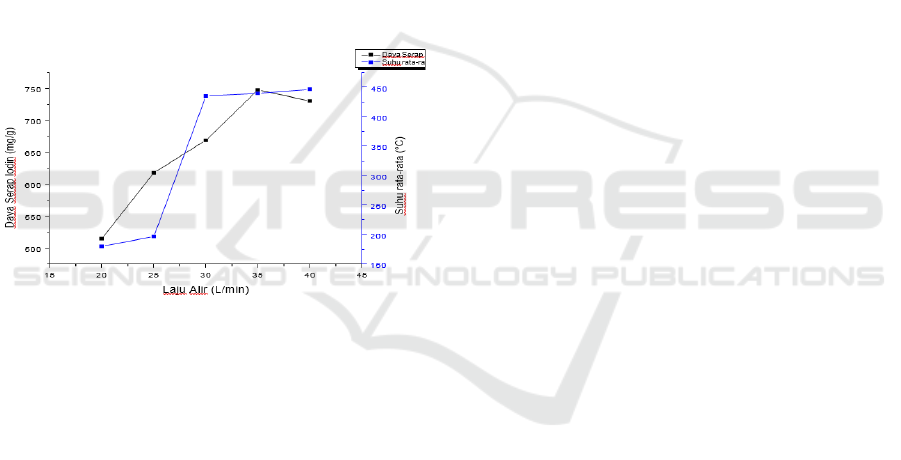
Figure 2: The relationship between the air flow rate
and the
average temperature of pyrolysis on the
absorption of
iodine in activated charcoal.
The absorption of iodine (iod adsorption)
indicates the ability of activated carbon to adsorb
components with low molecular weight. Activated
carbon with high
Iodine absorption means it has a
larger surface area
and also has a larger
microstructure and pores.
Figure 2. It can be seen that the higher the air flow
rate used in the pyrolysis process, the higher the
temperature rise and the higher the iodine absorption.
This is because the higher the air flow rate used will
reduce volatile substances and increase the amount of
fixed carbon in activated charcoal, the more iodine
will be adsorbed so that the greater the reduction in
the concentration of iodine solution which causes the
higher the absorption of iodine. From Figure 2. the air
flow rates are 20 L/min, 25 L/min, and 30 L/min with
an average temperature of 182.68 °C, 199.12 °C,
437.50 °C, the results of absorption analysis are
obtained. Iodine of 517,0282 mg/g, 620,1861 mg/g,
and 671,1982 mg/g that have not yet entered the
standard, this is due to the oxygen and nitrogen
entering the pyrolysis process has not been
maximized which causes the pyrolysis process to not
run properly. The best results for the absorption of
iodine by activated charcoal in this study were shown
at the air flow rate of 35 L/min of 750.1403 mg/g,
these results met the quality standard of activated
charcoal according to the SNI 06-3703-1955
standard, which was 750 mg /g.
4 CONCLUSIONS
1. The air flow rate reaches the optimum
condition at a speed of 35 L/min. At the above
optimum conditions, the activated charcoal
has already experienced a saturation point.
2. The best results are shown at the air flow rate
an iodine absorption capacity of 750.1403
mg/g. This result has met the standard of SNI
06-3703-1995.
ACKNOWLEDGEMENTS
The author would like to acknowledge the Center for
Research and Community Service at Polytechnic
State of Samarinda which has provided funding for
this research as well as to the Chemical Engineering
Laboratory of Polytechnic State of Samarinda as a
place for the research to be carried out.
REFERENCES
Ridhuan, K., Irawan, D., Zanaria, Y., & Adi, N. (2018).
Pengaruh Cara Pembakaran Pirolisis Terhadap
Karakteristik Dan Efisiensi Arang Dan Asap Cair
Yang Dihasilkan. Sntt, 141– 150.
Sudradjat, R., & Pari, G. (2011). Arang Aktif :
Teknologi Pengolahan dan Masa Depannya. In Badan
Penelitian dan Pengembangan Kehutanan (pertama).
Badan Penelitian dan Pengembangan Kehutanan.
Turmuzi, M., Tua, A. O. S., & Fatimah. (2015). Pengaruh
Temperatur Dalam Pembuatan Karbon Aktif Dari
Kulit Salak ( Salacca
Sumatrana ) Dengan Aktifator.
Jurnal Teknik
Kimia USU, 4(2), 59–64.
Wahyuni, I., & Fathoni, R. (2019). Pembuatan
Karbon
Aktif Dari Cangkang Kelapa Sawit
Dengan Variasi
Waktu Aktivasi. Jurnal
Chemurgy, 3(1), 11.
https://doi.org/10.30872/cmg.v3i1.2776
Wang, P., Zhang, J., Shao, Q., & Wang, G. (2018).
Physicochemical properties evolution of chars from
palm kernel shell pyrolysis. Journal of
Thermal
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
1140

Analysis and Calorimetry, 133(3), 1271–1280.
https://doi.org/10.1007/s10973-018-7185-z
Yek, P. N. Y., Liew, R. K., Osman, M. S., Lee, C. L.,
Chuah, J. H., Park, Y. K., & Lam, S. S. (2019).
Microwave steam activation, an innovative
pyrolysis
approach to convert waste palm shell into highly
microporous activated carbon. Journal of
Environmental Management, 236(August 2018), 245–
253. https://doi.org/10.1016/j.jenvman.2019.01.010
Yuliusman. (2015). Pembuatan karbon aktif dari
tempurung kelapa sawit dengan bahan
pengaktif KOH
dan gas N
2
/CO
2
. Seminar Teknologi Dan Rekayasa
(SENTRA), 978–979.
Yuliyanti. (2016). Pembuatan arang aktif dari sekam padi
dengan menggunakan aktivator asam phospat.
Politeknik Negeri Samarinda.
The Effect of Air Flow Rate on the Production of Active Charcoal from Palm Oil Shells with Partial Oxidation Method
1141
